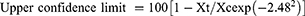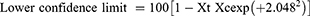Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Anthelmintic Resistance of Gastrointestinal Nematodes of Communally-Grazing Goats in Humbo District, Southern Ethiopia
Authors Alaro T, Dulo F , Wodajo W, Mathewos L
Received 22 September 2023
Accepted for publication 18 November 2023
Published 30 November 2023 Volume 2023:14 Pages 185—194
DOI https://doi.org/10.2147/VMRR.S434584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Tekle Alaro,1 Fitsum Dulo,1 Wondimu Wodajo,2 Lemlem Mathewos3
1School of Veterinary Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 2Sodo Regional Laboratory, Wolaita Sodo, Ethiopia; 3Sodo Town Veterinary Clinic, Wolaita Sodo, Ethiopia
Correspondence: Fitsum Dulo, School of Veterinary Medicine, Wolaita Sodo University, P.O. Box. 138, Wolaita Sodo, Ethiopia, Tel +251 932 292990, Fax +251 46 5515113, Email [email protected]
Background and Aim: Anthelmintic resistance (AR) in gastrointestinal nematodes (GINs) is currently present worldwide and a major challenge to goat production. However, no updated information is available on this topic in the study area. Thus, this study evaluated the efficacy of commonly used anthelmintics on GINs in naturally-infected goats and assessed farmers’ perception of anthelmintic utilization practices in Humbo district, Southern Ethiopia.
Materials and Methods: The field experiments for routinely used anthelmintics, namely, albendazole, ivermectin, and tetramisole, were conducted from September 2022 to April 2023. Sixty naturally-infected goats with nematodes were selected based on egg count (≥ 150 eggs per gram of feces) and allocated randomly into four groups (15 animals per group). Then, fecal samples were collected pre- and post-treatment and examined for fecal egg count reduction (FECRT) to determine the AR status of goat GINs. The modified McMaster technique using standard floatation was used for quantifying the eggs. In addition, a questionnaire survey was conducted to assess anthelmintic utilization practices among goat owners.
Results: The FECR levels for albendazole, ivermectin, and tetramisole were 94.6, 95.9, and 97.3%, respectively. By coproculture, the nematode genera identified before treatment were Haemonchus, Trichostrongylus, Teladorsagia, Oesophagostomum, Bunostomum, and Chabertia Species. However, post-treatment fecal cultures showed that some Haemonchus, Trichostrongylus, and Strongyloides spp. did not respond to the treatments. The questionnaire survey revealed that albendazole was the most commonly used anthelmintic to treat nematode infection in goats. Respondents expressed that anthelmintic treatment was utilized based on veterinarian prescription (59%), availability (32%), efficacy (4%), and affordability (5%).
Conclusion: Tetramisole should be used cautiously to prevent the development of resistant strains, as it was still effective in the study area. Additionally, regular monitoring of anthelmintic effectiveness is necessary.
Keywords: drug resistance, fecal egg count, coproculture, parasites, small ruminants, Southern Ethiopia
Introduction
Goat production has a significant role in the livestock industry worldwide, especially in poorer nations, where it helps ensure food and nutritional security, incomes, and sustainable agriculture.1 However, diseases caused by parasites, particularly gastrointestinal nematodes (GINs) can greatly affect their productivity and profitability.2,3 Their control is mainly based on the use of three chemical classes of anthelmintics: benzimidazoles (BZ), macrocyclic lactones (ML), and imidazothiazoles including levamisole (LEV). Nevertheless, anthelmintic resistance (AR) has developed as a result of unimpeded and widespread usage. In some nations, the proportion of GIN strains that are currently resistant is so high that effective control of parasitic infection is impossible.4,5
High treatment frequency, inadequate anthelmintic dosage, and prolonged use of the same anthelmintic class are the main risk factors for the emergence of AR in parasites.6–8 Consequently, the application of such a suppressive regime along with improper management techniques favors the development and selection of parasites that are resistant to anthelmintics.9
There may be an increase in the number of parasites that can withstand drug treatment with each successive generation due to the hereditary nature of parasite anthelmintic resistance.10 Investigation of AR status and identification of factors related to the development of AR are warranted as they are important components of sustainable GIN control.11
In recent times, many studies have demonstrated the existence of AR in small ruminant nematodes in several countries, including Ethiopia.3,5,12–16 Similarly, a recent study at a goat farm in Haramaya in Eastern Ethiopia also reported the presence of multidrug-resistant gastrointestinal nematodes against commonly used anthelmintics.17
Despite the use of these anthelmintics for a considerable period of time, there is limited information on their efficacy (albendazole, tetramisole, and ivermectin) in Southern Ethiopia. Thus, the present study’s objectives were to assess goat owners’ perception of their anthelmintic utilization and to evaluate the efficacy of the most commonly used anthelmintics against GINs in naturally-infected goats at the field level in Humbo district, Southern Ethiopia.
Materials and Methods
Ethical Approval and Consent to Participate
The study protocol was reviewed and approved by the institutional Animal Care and Use for Scientific Research Committee of Wolaita Sodo University (Ref. No: WSU-IRRC/022/2023). During research work, no animals were harmed or unethically injured/killed. Moreover, goat owners enrolled in this study were those who gave their consent after the purpose of the study was explained to them.
Study Area
A field evaluation of AR to albendazole (ABZ), levamisole (LEV), and ivermectin (IVM) was conducted from September 2022 to April 2023 in Humbo district (Figure 1), Southern Ethiopia. The district is located in the Wolaita zone and approximately 348 km south of the capital city, Addis Abeba. The agro-ecological zone is classified as “Kolla” or lowland, and “Weinadega” or midland, accounting for 22% and 78%, respectively. It lies at a latitude of 6° 43’N and longitude of 37° 45’E, with an altitude of 1100–1920 meters above sea level and irregular topography of mountains with steep slopes. The mean annual rainfall and temperature of the area is 840–1400 mm and 15–29°C, respectively. The vegetation is savannah grassland and the soil type is sandy and clay sandy. Almost all people in the area are engaged in mixed crop-livestock farming. The total livestock population of the district is estimated as 82,839 cattle, 28,045 sheep, 44,371 goats, 7183 equines, and 168,601 chickens.18
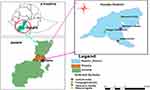 |
Figure 1 Map showing the location of the study area. |
Study Animals
The study animals were indigenous goats, kept under an extensive husbandry system, and maintained on communal grazing land with access to the same watering points. Goats were kept in pens at night at their owners’ respective houses. A total of 384 goats (274 female and 110 male) were screened and sampled, and only goats with a mean excretion of nematode eggs per gram of feces (EPG) of ≥150 at time of treatment were included in the survey. In the study, each goat for inclusion was tagged in the left/right ear and bore a unique identification number. Criteria for inclusion included the following: the goat that had not received any anthelmintic in the previous 8 weeks, farmers’ willingness to participate, history of anthelmintic usage, and a fecal egg count (FEC) of ≥150 eggs per gram of feces.11,19
Determination of Nematode Egg Count
The modified McMaster counting technique was employed for each fecal sample in order to determine the number of eggs per gram of feces (EPG).20–22 Briefly, 3 g of the fecal pellet was mixed in 42 mL of saturated NaCl solution with a sensitivity of 50 EPG of feces.21 A flotation fluid (especially saturated sodium chloride, 400 grams in 1000mL of water, ie 40%) was used to separate eggs from fecal material in a counting chamber (McMaster) with two compartments. The technique was to detect 50 or more eggs per gram of feces so that the count of all eggs within the engraved area of both chambers, and the number of eggs per gram of feces could be calculated by adding the egg counts of the two chambers together and multiplying the total by 50.22
Coproculture and Larval Identification
Fecal samples were collected from 60 experimental goats and cultures were taken to identify the genera of gastrointestinal nematodes, which provide a suitable environment for the hatching and development of eggs in the infective stage (L3), based on Hansen and Perry.23 Pooled coprocultures obtained from the pre- and post-treatment fecal samples were prepared and incubated at 25°C in moist conditions for 7–14 days to obtain infective third stage larvae (L3) that were harvested using the Baermann technique. The GIN L3 in the coprocultures was then differentiated according to keys in Taylor20 and MAFF24 to the generic level as Haemonchus, Trichostrongylus, Teladorsagia, Oesophagostomum, Bunostomum and Chabertia species. Larval composition was determined through microscopic examination of 100 randomly selected L3.
Experimental Design for FECRT
The therapeutic efficacy of commonly used anthelmintic drugs, including albendazole, tetramisole, and ivermectin, was determined using the FECRT as per the guidelines of the World Association for Advancement of Veterinary Parasitology (WAAVP).22 Before the start of the study, the nematode egg excretions in feces in most of the selected flocks were determined with 15 individual fecal samples to ensure a level equal to or above 150 eggs per gram of feces. The experimental goats were naturally parasitized mixed species of gastrointestinal nematodes. A total of 60 goats were randomly selected and divided into four groups (groups 1, 2, 3, and 4). Group 1 was kept as the untreated infected control; group 2, group 3 and group 4 were treated with albendazole at the dosage rate of 7.5 mg/kg body weight orally, tetramisole at the dosage rate of 15 mg/kg body weight orally, and IVM subcutaneous injection at the dosage rate of 200 μg/kg body weight, respectively (Table 1). At day 0, a fecal sample was taken from the rectum of each animal. On days 7 and 14 post-treatment, individual fecal samples were again taken rectally from treated and untreated infected animals. All fecal samples were placed in universal bottles and transported on the same day in an ice-cooled box to Sodo Regional Veterinary Laboratory. In the laboratory, samples were kept at 4°C and processed within 24 hours of collection.
 |
Table 1 Anthelmintic Drugs Used in the FECRT for the Field Efficacy Trial |
Questionnaire Survey
A semi-structured questionnaire was prepared and 100 goat owners were interviewed in order to get information on anthelmintic utilization and perceived efficacy. The sample size of the respondents was determined using the formula (n = 0.25/SE2) proposed by Arsham25 at the standard error (SE) of 0.05 with a 95% confidence interval. The study kebeles were purposively selected based on a relatively large goat population. Afterwards, the sample kebele households were stratified according to their goat ownership and then goat farmers were randomly selected for interview. Animal health workers and community leaders were involved in the selection of goat owners. The questionnaire was designed to obtain information from respondents on drenching practices against GINs, such as type of anthelmintic (AH) used, frequency of administration, dosage rate determination, source of AH, criteria for AH selection, and observations on the responses to treatment. Before the in-person interview, the objective of the research was explained to each respondent, and their full consent was obtained.
Statistical Analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS) V-20 statistical software.26 Descriptive statistics (percentages) were used to measure respondents’ responses to the questionnaire. Results are presented as percentages and the absolute numbers on which these percentages are based are in parentheses. The reduction in FEC post-treatment was calculated using 100 (1- Xt/Xc), that is, the Xt arithmetic mean of the post-treatment egg count on the 14th day and the Xc arithmetic mean of the control group on the 14th day.22 The log transformation of the values of EPG [using log (x + 1)] was performed to minimize and stabilize the variance. A 95% confidence interval was calculated as follows:
where Y2 denotes the variance of the reduction. Then, ANOVA was used to compare the mean EPG within each risk treatment group.
The efficacy of albendazole, tetramisole, and ivermectin for the goats was tested and interpreted according to the WAAVP recommendations for efficacy evaluations of anthelmintics.21,22 Fecal egg count reduction percentage (FECR%) was determined using the formula: 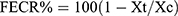 , where Xt and Xc are the arithmetic mean EPG in the treated (t) and untreated control (c) groups respectively, at day 14 post-treatment. Anthelmintic resistance is considered to exist if the FECR% is less than 95% and the lower 95% confidence limit for the reductions is less than 90%. Anthelmintic resistance is suspected if either of the two criteria is met.
, where Xt and Xc are the arithmetic mean EPG in the treated (t) and untreated control (c) groups respectively, at day 14 post-treatment. Anthelmintic resistance is considered to exist if the FECR% is less than 95% and the lower 95% confidence limit for the reductions is less than 90%. Anthelmintic resistance is suspected if either of the two criteria is met.
Results
Questionnaire Survey
All respondents (100%) indicated that they practiced anthelmintic treatment to control the internal parasites of the goat. About 82% of the animal owners interviewed could read as well as understand information on leaflets. Most of the respondents (47%) used albendazole as an anthelmintic drug in goats followed by ivermectin (30%), and tetramisole (23%). Respondents recognized these drugs by color: green, colorless, and white.
The majority of interviewed farmers were buying anthelmintic drugs from government-owned veterinary clinics or animal health posts (58%) and legal private drug shops (34%). However, a few respondents confirmed that one of their sources of anthelmintics was an open market (8%). Farmers selected anthelmintics mainly based on prescription by veterinarians (59%) and availability (32%). A small number of participants stated that they chose drugs by price affordability (5%) and efficacy (4%).
A large proportion of the respondents indicated that their animals were treated with one of the anthelmintics once (55%) or twice (33%) per year, while a smaller proportion said that they treated them three times (12%) per year. Most of the respondents (60%) indicated that they determined the dosage rate for their goats based on the prescribing advice of the animal health professionals, while the remainder (40%) determined the dosage rate based on a visual estimation of the weight of the animal (Table 2).
 |
Table 2 Respondents’ Information on Demographics, Anthelmintic Utilization and Practices |
There were various justifications given by farmers for giving anthelmintics to their goats.The majority of the respondents (90%) stated they have given them because of general symptoms of disease (coughing, emaciation, loss of appetite, rough coat, weakness, and loss of condition), and some gave them simply for deworming. A large proportion of respondents (66%) had bought and administered anthelmintics to their goats directly themselves, whereas the remainder had veterinary support. Following anthelmintic treatment, most of the participants (82%) declared an improvement in the clinical signs and bodily condition of their goats, whereas others (18%) claimed that there was little or no improvement in the goats’ health.
Fecal Egg Counts (FEC) and FECR%
For the screening of AR against GINs, in this study the FECR test was conducted in naturally-infected goats using three brands, namely, albendazole, ivermectin, and tetramizole, which are commonly available on the local markets. The results were determined according to the WAAVP recommendations.22
The details of the drugs used in the tests are summarised in Table 1. FEC and FECR results from the 4 study sites pre- and post-treatment using the three anthelmintics are shown in Table 3. All anthelmintics resulted in a significant reduction in nematode egg counts post-treatment, on day 14 (Table 3). In the present study, the results of FECRT for albendazole, ivermectin, and tetramisole were 94.6, 95.3, and 97.3%, respectively. The lower limit of the 95% confidence level for albendazole and ivermectin was less than 90%.
 |
Table 3 Mean Fecal Egg Counts Pre- and Post-Treatment in Goats and FECR% Studied in Humbo District of Southern Ethiopia |
Third-Stage Larvae Identification
The fecal culture of eggs to third-stage larvae was undertaken parallel to fecal egg counting to differentiate the type of nematodes before and after treatment, for each anthelmintic treatment group and the control group. Before treatment (at day 0), the fecal culture of pooled samples revealed Haemonchus spp. as the predominant parasite, with an occurrence rate of 46%, followed by Trychostrongylus (32%), Teladorsagia (12%), Oesophagostomum (5%), Bunostomum (3%), and Chabertia (2%) (Figure 2). Post-treatment (day 14) fecal cultures showed no infective larvae in the tetramisole treatment group, while Haemonchus spp., Trichostrongylus spp., and Strongyloides escaped treatment in the albendazole and ivermectin groups.
 |
Figure 2 Proportion of GIN larvae (L3) recovered from the pooled fecal sample. |
Discussion
Investigation of the status of anthelmintic resistance is perhaps the most important step in establishing and maintaining effective parasite control of nematode parasites in livestock. At the dosage given, the fecal egg count reduction in albendazole was below 95%, which may suggest the existence of anthelmintic resistance to this drug. Albendazole is widely available and extensively used in the country, including in the study area. Thus, the most likely explanation for the inefficacy of this drug might be its long-term use and the lack of rotation with other drugs in goats maintained under the extensive system by smallholder farmers in this region. Our finding of AR in albendazole concurs with previous studies conducted in other parts of Ethiopia,27,28 and elsewhere in the world, including the Sing Buri Province of Thailand,11 Uganda,29 Northern Italy,30 and India.31 In contrast to our findings, the high efficacy of albendazole against nematodes in goats has been reported on research farms32 in the eastern33 and central highlands34 of Ethiopia.
In the current study, the presence of anthelmintic resistance was suspected in ivermectin against gastrointestinal nematodes, which is consistent with previous studies.28 Nevertheless, our finding is not consistent with other reports of a 100% reduction in egg count in goats in different parts of Ethiopia32,35 and elsewhere in Europe.36 On the other hand, Ratanapob et al,11 Gelot et al,31 and Eysker et al37 reported the presence of ivermectin resistance against the nematode population in goats in Thailand, India, and the Netherlands. The suspected resistance of ivermectin might be attributed to the frequent use of this drug by professionals when controlling gastrointestinal helminths in ruminants because of their broad spectrum and endectocide activity; this encourages excessive use and may lead to lower efficacy over time. As ivermectin was available in the study area in its injectable form and hence animal health professionals administer it, suspected resistance might be attributable to the same causes. Moreover, the treatment was often based on guessed estimation of animal weight, which could sometimes lead to under- or over-dosing of the animals and contribute to the emergence of AR.
The relatively higher efficacy of tetramisole among the drugs tested in this study was probably due to the low frequency of treatment in the study area. Therefore, the majority of nematode parasite populations in goats remained unexposed to anthelmintic treatment and thus remained susceptible. The findings in this study were also in line with those of previous authors, who reported tetramisole as an efficacious drug against gastrointestinal nematodes.28,38 However, the result obtained in this study contrasts with a recent study conducted by Wondimu and Bayu,17 who reported low efficacy of tetramisole in goats in Ethiopia, with a 95.7% FECR and a 87.4% lower limit of the 95% confidence interval. The finding is also in contrast with the findings of Sissay et al33 and Regassa et al.35
The observation of the predominance of L3 of Haemonchus sp. from pretreatment coprocultures is consistent with previous reports from other Ethiopian-based studies and other parts of the world.17,27,39 Likewise, post-treatment fecal cultures further emphasize the predominance of Haemonchus spp., followed by Trichostrongylus spp., and Strongyloides, suggesting AR against single or multiple anthelmintics for these parasites. Haemonchus and Trichostrongylus species have been identified as resistant parasites in sheep and goats, primarily in tropical and subtropical areas.20 However, resistance to various groups of anthelmintics is becoming increasingly common among GINs worldwide.3 Many parasitic nematodes that are of veterinary importance possess genetic traits that promote the development of resistance to anthelmintic drugs.3,40 This finding supports previous studies in Ethiopia32, India,39 and Bangladesh,3 which reported the recovery and predominance of Haemonchus species principally from fecal culture post-treatment. This might be due to the parasite’s higher ecological and biological adaptability.27,33
The questionnaire survey demonstrated that albendazole bolus was the most commonly used anthelmintic in the study area compared to ivermectin and tetramisole, whether by prescription or otherwise, suggesting the widespread availability of the drug. This is in agreement with previous findings which reported that benzimidazoles and macrocyclic lactones were the anthelmintic classes of choice.3,12,41 It also showed that farmers in the study area perform several practices that may be responsible for promoting the development of AR, which is in line with previous studies conducted in different parts of the world.6,11,32
The majority of interviewed farmers were buying anthelmintic drugs from government-owned veterinary clinics or animal health posts and legal private drug shops. It was noticed during the survey that the farmers predominantly chose to buy the anthelmintic drugs from government veterinary clinics or animal health posts because of the sufficient supply and accessibility of them in their locality. However, a few bought anthelmintics from the open market, which might have expired and thus contributed to AR. The majority of the respondents used anthelmintic treatments for their goats when prescribed them by animal health personnel, according to availability. Usually, the majority of farmers select and administer anthelmintics to their goats with supervision by veterinarians. Misuse of drugs, such as inaccurate calculation of doses or improper administration methods, is less likely.
Most of the interviewed farmers in the study area have received formal education and, as a result, carried out treatment on the basis of mainly clinical symptoms such as declining bodily condition, rough coat, and diarrhoea, as reported by Dey et al.3 Only a few farmers reported anthelmintic utilization for simple deworming.
Moreover, the majority of the respondents declared that the treatment was often based on a guess estimate of animal weight. As a consequence, this could sometimes lead to under- or over-dosing, which favors the development of anthelmintic resistance.3 In this survey, a large proportion of the farmers reported the presence of improvement in both clinical signs and bodily condition in their animals after treatment. This result is consistent with the report of Datiko et al,41 who stated that 81% of the respondents indicated that their animals showed improvement in both clinical signs and bodily condition post-treatment. However, some owners claimed that there was little or no improvement in their goats’ health after treatment, which might be associated with the observed AR. This finding is corroborated by a study conducted in Sing Buri Province, Thailand, by Ratanapob et al.11 They reported that farmers thought AR parasites existed in their herds, based on an absence of clinical improvement post-treatment, even after increased doses of anthelmintics above recommended prescription levels.
Conclusions
The present study established a high prevalence of gastrointestinal nematode infections and the presence of anthelmintic resistance to albendazole, while tetramisole was found to be effective against GIN parasites in goats. Moreover, suspected AR to ivermectin was also recorded. The main resistant GINs turned out to be Hemonchus spp., Trichostrongylus spp., and Strongyloides based on the morphological feature of the infective stage three larvae. The AR results highlight the need for regular monitoring and management of anthelmintic resistance in the region to ensure effective parasite control and maintain the health of small ruminants. Moreover, a questionnaire survey indicated that farmers in the study area apply many practices that may lower the efficacy of anthelmintics and favor the development of anthelmintic resistance. Therefore, farmers/practitioners should avoid misuse of anthelmintics and further studies need to be done to clarify the state of the efficacy of the commonly used anthelmintics covering different agro-ecological zones and species of livestock in Ethiopia.
Acknowledgments
We are grateful to Sodo Regional Laboratory for logistic support during the entire research work. We also acknowledge smallholder farmers in the study area for granting permission to collect feces from their goats and conduct experiments on their animals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Namutosi W, Higenyi J, Kizito E, Omodo M. Prevalence and risk factors of gastrointestinal parasite infection in Goats in Sironko District, Eastern Uganda. Uganda J Agric Sci. 2019;19(1):1–14. doi:10.4314/ujas.v19i1.1
2. Terefe G, Faji U, Tolossa YH. Field investigation of anthelmintic efficacy and risk factors for anthelmintic drug resistance in sheep at Bedelle District of Oromia Region, Ethiopia. Ethiop Vet J. 2013;17(2):37–49. doi:10.4314/evj.v17i2.3
3. Dey AR, Begum N, Alim MA, Malakar S, Islam MT, Alam MZ. Gastro-intestinal nematodes in goats in Bangladesh: a large-scale epidemiological study on the prevalence and risk factors. Parasite Epidemiol Control. 2020;9:e00146. doi:10.1016/j.parepi.2020.e00146
4. Várady M, Papadopoulos E, Dolinská M, Königová A. Anthelmintic resistance in parasites of small ruminants: sheep versus goats. Helminthologia. 2011;48(3):137–144. doi:10.2478/s11687-011-0021-7
5. Mickiewicz M, Czopowicz M, Moroz A, et al. Prevalence of anthelmintic resistance of gastrointestinal nematodes in Polish goat herds assessed by the larval development test. BMC Vet Res. 2021;17(1):19. doi:10.1186/s12917-020-02721-9
6. Chandrawathani P, Yusoff N, Wan LC, Ham A, Waller PJ. Total anthelmintic failure to control nematode parasites of small ruminants on government breeding farms in Sabah, East Malaysia. Vet Res Commun. 2004;28(6):479–489. doi:10.1023/b:verc.0000040240.69004.dc
7. Jabbar A, Iqbal Z, Kerboeuf D, Muhammad G, Khan MN, Afaq M. Anthelmintic resistance: the state of play revisited. Life Sci. 2006;79(26):2413–2431. doi:10.1016/j.lfs.2006.08.010
8. Saddiqi HA, Jabbar A, Iqbal Z, Babar W, Sindhu Z-U-D, Abbas RZ. Comparative efficacy of five anthelmintics against trichostrongylid nematodes in sheep. Can J Anim Sci. 2006;86(4):471–477. doi:10.4141/A06-036
9. Almeida GD, Feliz DC, Heckler RP, et al. Ivermectin and moxidectin resistance characterization by larval migration inhibition test in field isolates of Cooperia spp. in beef cattle, Mato Grosso do Sul, Brazil. Vet Parasitol. 2013;191(1–2):59–65. doi:10.1016/j.vetpar.2012.08.012
10. Gárcia CM, Sprenger LK, Ortiz EB, Molento MB. First report of multiple anthelmintic resistance in nematodes of sheep in Colombia. An Acad Bras Cienc. 2016;88(1):397–402. doi:10.1590/0001-3765201620140360
11. Ratanapob N, Thuamsuwan N, Thongyuan S. Anthelmintic resistance status of goat gastrointestinal nematodes in Sing Buri Province, Thailand. Vet World. 2022;15(1):83–90. doi:10.14202/vetworld.2022.83-90
12. Bersissa K, Ajebu N. Comparative efficacy of albendazole, tetramisole and ivermectin against gastrointestinal nematodes in naturally infected sheep in Hawassa, Southern Ethiopia. Rev Med Vet. 2008;159(12):593–598.
13. Seyoum Z, Demessie Y, Bogale B, Melaku A. Field evaluation of the efficacy of common anthelmintics used in the control of gastrointestinal nematodes of sheep in Dabat district, Northwest Ethiopia. Ir Vet J. 2017;70(1):18. doi:10.1186/s13620-017-0097-6
14. Potarniche AV, Mickiewicz M, Olah D, et al. First report of anthelmintic resistance in gastrointestinal nematodes in Goats in Romania. Animals. 2021;11(10):2761. doi:10.3390/ani11102761
15. Mphahlele M, Tsotetsi-Khambule AM, Moerane R, Komape DM, Thekisoe OMM. Anthelmintic resistance and prevalence of gastrointestinal nematodes infecting sheep in Limpopo Province, South Africa. Vet World. 2021;14(2):302–313. doi:10.14202/vetworld.2021.302-313
16. Nabukenya I, Rubaire-Akiiki C, Olila D, Muhangi D, Höglund J. Anthelmintic resistance in gastrointestinal nematodes in goats and evaluation of FAMACHA diagnostic marker in Uganda. Vet Parasitol. 2014;205(3):666–675. doi:10.1016/j.vetpar.2014.07.019
17. Wondimu A, Bayu Y, López-Arellano ME. Anthelmintic drug resistance of gastrointestinal nematodes of naturally infected goats in Haramaya, Ethiopia. J Parasitol Res. 2022;2022:4025902. doi:10.1155/2022/4025902
18. office HDLaFD. Annual report from Humbo District Livestock and Fishery Development office, Wolaita zone, SNNPR state, Ethiopia (Annual report, Unpublished); 2022.
19. Domke AV, Chartier C, Gjerde B, et al. Prevalence of anthelmintic resistance in gastrointestinal nematodes of sheep and goats in Norway. Parasitol Res. 2012;111(1):185–193. doi:10.1007/s00436-012-2817-x
20. Taylor MA, Coop RL, Wall RL. Veterinary Parasitology. Blackwell Sciences Limited; 2007.
21. Coles GC, Jackson F, Pomroy WE, et al. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136(3–4):167–185. doi:10.1016/j.vetpar.2005.11.019
22. Coles GC, Bauer C, Borgsteede FH, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44(1–2):35–44. doi:10.1016/0304-4017(92)90141-u
23. Hansen J, Perry B. The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants. Ilrad; 1994.
24. MAFF (Ministry of Agriculture FaF). Manual of Veterinary Parasitological Laboratory Techniques. UK: ADAS, Her Majesty’s Stationary Office (HMSO); 1986.
25. Arsham H. Descriptive sampling data analysis. Statistical thinking for managerial decision making; 2002.
26. SPSS-20. Statistical Package for Social Sciences. SPSS Inc: Version 20; 2011.
27. Kumsa B, Abebe G. Multiple anthelmintic resistance on a goat farm in Hawassa (southern Ethiopia). Trop Anim Health Prod. 2009;41(4):655–662. doi:10.1007/s11250-008-9237-z
28. Desie Sheferaw MG, Abduro K, Chaka L, Debela E, Abera M. Survey of gastrointestinal nematodes and anthelmintic resistance in sheep and goats in communal grazing pastoral area, Yabello District, southern Ethiopia. Ethiop Vet J. 2015;19(1):35–47. doi:10.4314/evj.v19i1.5
29. Byaruhanga C, Okwee-Acai J. Efficacy of albendazole, levamisole and ivermectin against gastro-intestinal nematodes in naturally infected goats at the National Semi-arid Resources Research Institute, Serere, Uganda. Vet Parasitol. 2013;195(1–2):183–186. doi:10.1016/j.vetpar.2013.01.007
30. Zanzani SA, Gazzonis AL, Di Cerbo A, Varady M, Manfredi MT. Gastrointestinal nematodes of dairy goats, anthelmintic resistance and practices of parasite control in Northern Italy. BMC Vet Res. 2014;10(1):114. doi:10.1186/1746-6148-10-114
31. Gelot I, Singh V, Shyma K, Parsani H. Emergence of multiple resistances against gastrointestinal nematodes of mehsana-cross goats in a semi-organized farm of semi-arid region of India. J Appl Animal Res. 2016;44(1):146–149. doi:10.1080/09712119.2015.1021809
32. Kumsa B, Debela E, Megersa B. Comparative efficacy of albendazole, tetramisole and ivermectin against gastrointestinal nematodes in naturally infected goats in Ziway, Oromia Regional State (Southern Ethiopia). J Anim Vet Adv. 2010;9(23):2905–2911. doi:10.3923/javaa.2010.2905.2911
33. Sissay M, Asefa A, Uggla A, Waller P. Assessment of anthelmintic resistance in nematode parasites of sheep and goats owned by smallholder farmers in eastern Ethiopia. Trop Anim Health Prod. 2006;38(3):215–222. doi:10.1007/s11250-006-4346-z
34. Regassa F, Sori T, Dhuguma R, Kiros Y. Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Int J Appl Res Vet Med. 2006;4(1):51–57.
35. Regassa F, Kebede L, Mamo G, Kumsa B, Beyene T. Efficacy of commonly used anthelmintic drugs in naturally infected sheep and goats in Central Oromia, Ethiopia. Res J Pharmac. 2013;7(4):48–53.
36. Paraud C, Pors I, Rehby L, Chartier C. Absence of ivermectin resistance in a survey on dairy goat nematodes in France. Parasitol Res. 2010;106(6):1475–1479. doi:10.1007/s00436-010-1781-6
37. Eysker M, van Graafeiland AE, Ploeger HW. [Resistance of Teladorsagia circumcincta in goats to ivermectin in the Netherlands] Ivermectineresistentie bij teladorsagia circumcincta bij geiten in Nederland. Tijdschr Diergeneeskd. 2006;131(10):358–361. Dutch.
38. Asmare K, Gelaye E, Ayelet G. Anthelmintic resistance test in gastrointestinal nematodes of small ruminants in southern Ethiopia. Bull Anim Health Prod Afr. 2005;53(2):89–95.
39. Bihaqi SJ, Allaie IM, Banday MAA, Sankar M, Wani ZA, Prasad A. Multiple anthelmintic resistance in gastrointestinal nematodes of caprines on mountain research centre for sheep and goat at Kashmir Valley, India. Parasite Epidemiol Control. 2020;11:e00163. doi:10.1016/j.parepi.2020.e00163
40. Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20(10):477–481. doi:10.1016/j.pt.2004.08.001
41. Datiko J, Terefe G, Bekele J. Anthelmintic utilisation practices and prevalence of gastrointestinal helminth infections in sheep kept in the urban and peri-urban areas of Bishoftu Town. Trop Anim Health Prod. 2013;45(2):633–639. doi:10.1007/s11250-012-0270-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.