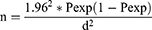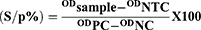Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Animal Brucellosis: Seropositivity rates, Isolation and Molecular Detection in Southern and Central Ethiopia
Authors Wakjira BS, Jorga E , Lakew M, Olani A, Tadesse B, Tuli G, Belaineh R, Abera S, Kinfe G , Gebre S
Received 17 May 2022
Accepted for publication 8 August 2022
Published 27 August 2022 Volume 2022:13 Pages 201—211
DOI https://doi.org/10.2147/VMRR.S372455
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Bayeta Senbata Wakjira,1 Edilu Jorga,2 Matios Lakew,1 Abebe Olani,1 Biniam Tadesse,1 Getachew Tuli,1 Redeat Belaineh,1 Shubisa Abera,1 Getachew Kinfe,1 Solomon Gebre1
1Animal Health Institute, Sebeta, Ethiopia; 2Ambo University, College of Agriculture and Veterinary Science, Ambo, Ethiopia
Correspondence: Bayeta Senbata Wakjira, Email [email protected]
Introduction: Brucellosis is a neglected bacterial zoonosis with serious veterinary and public health importance throughout the world. A cross-sectional study on animal brucellosis was conducted aiming to estimate seroprevalence and molecular detection.
Methods: Blood samples were collected from a total of 4274 individual animals (cattle, small ruminants and camel) from 241 herds/flocks for serology and PCR. Serum samples were tested using multispecies I-ELISA. Blood clots from seropositive animals were also tested for brucellosis via PCR. Additionally, 13 vaginal swab samples were collected from animals (2 from bovine and 11 from small ruminants) with recent abortion history for bacterial isolation and molecular detection.
Results: The overall individual animal and herd level seroprevalence was 3.95% (169/4274) and 18.26% (44/241) respectively. The animal level seroprevalence at species level was 1.58% (47/2982), 8.89% (97/1091) and 12.44% (25/201) in bovine, small ruminants (sheep and goat) and camel, respectively. Herd level seroprevalence were 5.43% (10/184), 52.08% (25/48) and 100% (9/9) in bovine, small ruminant and camel, respectively. The animal level seroprevalence of bovine from intensive and extensive systems was 1.10% (31/2808) and 2.87% (5/174) respectively. Blood clots tested for brucellosis via PCR were negative by RT-PCR. Brucella species was isolated from 6/13 (46.15%) vaginal swab samples cultured on Brucella selective agar, and shown to be B. melitensis using Real-Time PCR.
Conclusion: Overall, seropositivity for camels was higher than what has been reported previously. Also, there was a notable difference in this study in cattle seroprevalence when comparing extensive with intensive systems, with the extensive system having much greater seropositivity.
Keywords: Brucella melitensis, neglected bacterial diseases, camel, zoonosis
Introduction
Brucellosis is a bacterial disease of domestic and wild animals caused by the genus Brucella which has great public health importance globally.12 Currently, 12 species of Brucella are recognized, including B. abortus (cattle), B. melitensis (sheep and goats), B. ovis (sheep), B. suis (pigs), and B. canis (dogs) in addition to seven other species found in various species of wild animals. It is well established that cattle can be infected with B. melitensis and that sheep/goats may harbor B. abortus. Either of these two Brucella species is capable of infecting camelids.17 Classically, detection and identification of Brucella spp. has been based on culture and phenotypic analysis (biotyping) and due to its potential for transmission via aerosol, it must always be handled in laboratories with biosafety level 3.
With the advent of molecular techniques, early PCRs for the genus were based on the 16S rRNA and bcsp31 genes.8 PCR methods based on the 16S rRNA amplify a DNA fragment common to all Brucella species but cross-react with members of the closely related genus Ochrobactrum.31 The IS711 element became the preferred target for general identification purposes due to its restricted occurrence in Brucella spp. and the presence of multiple copies, allowing for unparalleled sensitivity and direct testing on clinical samples.19
In Ethiopia, brucellosis is considered to be endemic. Vaccination of farm animals is not practiced. Reviews of several serological studies done on brucellosis have shown seroprevalence rates in different species ranging from 0% to 26.1%, 0 0.7% to 13.7%, and 0.53% to 9.6%, in bovine, small ruminant and camel, respectively.11,15,16,25,35,41 In pastoral communities, 34.1% human patients with febrile illness from Borana, 29.4% from Hammer and 3% patients from Metema areas were seropositive for Brucella spp. using the IgM/IgG Lateral Flow Assay,29 suggesting high rates of transmission from animals to humans in pastoral areas.
Ethiopia is richly endowed with livestock, most of which are kept by small farmers. Unfortunately, the presence of endemic and transboundary animal diseases limits farmers’ livelihoods as well as their health.
The aim of this study is to conduct a cross-sectional study, to estimate seropositivity of brucellosis in cattle, small ruminants and camels in southern and central Ethiopia and also to isolate and detect the circulating Brucella species in the study area using molecular methods.
Materials and Methods
Description of the Study Area
The study was conducted in southern and central areas of Ethiopia (Figure 1). From southern area, three different zones were sampled, with several districts in each. Specifically, in Wolayita Zone, the districts of Bolso Sore, Damote Sore, Damote Gale, and Soddo were studied. In South Omo Zone, the districts of Nyangatom, Hammer, Benatsemay, and Male were sampled. And in Borana Zone, the districts of Surupa, Arero, and Elowayu were sampled. All of these formed what is termed “southern area” in this paper. For the more central part of Ethiopia, West Shewa was sampled (Ambo district), and in East Shewa, four districts were sampled, including Adama, Lume, Batu, and Dugda. In addition, in the special zone of Oromia surrounding Finfine, the districts of Holeta, Sululta, and Sebeta were sampled. These are considered “central area”.
 |
Figure 1 Map of the study areas and herds. |
Study Population
The study population included cattle, small ruminants and camels, all over six months of age. All sampling was done in 2021, and all animals sampled appeared clinically healthy. From the southern areas, all the target populations were managed under extensive pastoral systems. In Walaita and Central Ethiopia, study populations were primarily dairy, and all were managed under intensive production systems. Extensive rearing refers to those herds and flocks managed primarily without housing and in which animals may move long distances for forage. Intensive production systems refers to herds reared in which animals within the herd are always in close contact. All of the intensive systems in this study were dairy farms.
Sample Size Determination and Sampling Methodology
The sample size was calculated separately for bovine, small ruminants and camels based on the previous reports of seroprevalence for the species in the study areas according to the following formula:42
In this formula, n equals required sample size, Pexp equals expected prevalence, and d equals desired absolute precision. The desired precision was 5%, which allows for a 95% confidence level. Accordingly, the sample size for cattle sampling for the nine districts of the Central regions was calculated as 153 for each district based on previous reports of 11.2% (124/1106) seroprevalence and for the Borana were calculated as 115 based on previous reports of 8.2%.13,51 As there were no published reports from intensive dairy farms from Wolaita zone, the sample size was calculated by considering the expected prevalence of 50% and hence the calculated sample size was 384. Small ruminant sample size was calculated based on previous reports of 8.1% (23/283) and 4.2% (16/384) for Borana (230) and South Omo (124), respectively.5,49 Sample size for camel was calculated as 47 based on a report of 3.1% (12/384) seroprevalence.1
Based on the above calculation, 2144 animals from all districts of the study areas were determined to be included into the study. However, sample size was increased approximately two-fold so as to increase precision and reduce standard error. Accordingly, in the present study, a total of 4274 animals from all districts were selected to investigate brucellosis for this study purpose, as shown in Table 1.
 |
Table 1 Summary of Calculated and Collected Sample Size for This Study |
Multistage sampling was used to get the required animal samples for the southern areas. Zones were selected purposively and districts (woreda) from the selected zones were randomly selected and in turn kebeles and villages were selected randomly from the districts. Accordingly, from each selected district four villages and from the selected villages, households/herd/flocks were selected by simple random sampling. All animals within the selected herds were sampled. Total number of samples required was distributed according to the animal population proportionally for each administrative category. The milk producing districts from the central part were selected purposively and the farms from selected districts (towns) were selected randomly and all cattle from the selected farms were sampled.
Blood Sample Collection
For serological and molecular analysis, blood samples were collected aseptically from the jugular vein of individual animals. Approximately 5–7mL of blood was collected from each study animal using new plain vacuum tubes and then the blood samples were kept overnight at room temperature to allow clotting. The separated sera were then carefully moved into cryovials. Harvested sera and blood clots were transported to the Animal Health Institute serology and molecular laboratory in an insulated box containing ice packs. All were stored at −20℃ in the laboratory until processing.
Bacteriological Sample Collection
Vaginal swab samples were collected using Stuart transport medium from animals that had a history of recent abortion. This included two cows and 11 small ruminants. Swabs were transported under cool conditions to the bacteriology laboratory of the Animal Health Institute and stored at −20℃ until processed.
Laboratory Diagnosis
Serological Tests
Commercial brucellosis serum indirect multi-species ELISA Kit (BRUS-MS-5P ID Screen Brucellosis Serum Indirect, Multispecies, lot number C35) was used to detect antibodies directed against B. abortus, B. melitensis and B. suis from 4274 sera samples and performed as per manufacturer’s instructions. This commercial test is not yet validated for use in camels, and currently there are no serologic tests fully validated for use in camels.47 However, the OIE supports the ELISA for screening of flocks/herds and individual animals in all livestock species, including camels.40 Optical density was measured at 450nm. The kit was verified as per kit instructions and the positive cut-off point was calculated as:
Accordingly, samples with a s/p% less than or equal to 110% were considered negative, greater than 110% and less than 120% were considered doubtful, and greater than or equal to 120% were considered positive.
Bacteriological Test
Media Preparation and Culturing
Brucella selective media was prepared by suspending the required amount of Brucella medium base (CONDA Cat. 1374, Spain), in sterile 5% V/V inactivated horse serum (ie, horse serum held at 56℃ for 30 minutes). Rehydrated contents of Brucella selective supplement (SR083A) were aseptically added to the sterilized Brucella basal medium and homogenized before plating and then 15 to 20 mL of the medium was poured into the Petri dish and allowed to solidify.3 The plates were incubated at 37℃ for 48h for sterility check and no bacterial colony growths were considered as sterile and used for culture.
Thirteen vaginal swab samples were streaked directly from Stuart transport medium to the plate under Biosafety Level (BSL3) facilities with proper personal protections. Inoculated plates were incubated at 37℃ aerobically. Duplicate samples were also incubated in the presence of 5%CO2 (using anaerobic candle jar) for up to two weeks. The colonies were checked every 24h for Brucella species growth. Brucella-suspected colonies were characterized by their typical round, glistening, pinpoint and honey drop-like appearance according to standard methods.3
Microscopic Examination
Brucella suspected colonies were selected using a sterile plastic loop and mixed with a drop of sterile distilled water and smeared on a clean glass slide. The smear was heat fixed on the slide and air-dried. Identification of the organism was done by gram staining technique and Modified Ziehl-Neelsen staining technique.24
Biochemical Test
Subsequent biochemical tests, including urea testing and lack of growth on MacConkey agar were also done.
Molecular Test
Genomic DNA Extraction from Blood Clots of Seropositive Animals
Following the result of serological test using ELISA kit, 169 blood clot samples from the seropositive animals were used for detection of Brucella nucleic acid using real time PCR. Genomic DNA was extracted using QIAMP DNA Mini Kit (QIAGEN GmbH strasse 1.40724 Hilden GERMANY) as per manufacturer’s instructions.
Genomic DNA Extraction from Culture
DNA was extracted from solid media colonies by simple boiling method as described.22 Few colonies were removed and suspended in 500μL of sterile double distilled water in a 1.5mL micro-centrifuge tube and kept in a boiling water bath for 10 minutes. Five microliters of the supernatant were used for the PCR after centrifugation at 12000g for 3 minutes and the rest of the DNA sample was stored at −20℃.
Nanodrop DNA Examination
The extracted DNA was checked using Nanodrop spectrophotometer (THERMO, USA), which checks and measures the purity of DNA by reading the absorbance at (260/280 nm) and ng/μL concentration was calculated before PCR was performed using real time PCR.
Real-Time PCR
Real-Time PCR was performed for detection of Brucella spp. DNA from blood clot and culture samples by using the specific primers and TaqMan probe for IS711, B. abortus and B. melitensis sequence of forward and reverse as described in Table 2.
 |
Table 2 Primers and TaqMan®probes Used in This Study |
The thermocycler was run at 95℃ for 10 min to denature double-stranded DNA, then amplification/extension occurred at 95℃ for 15 second and 60℃ for 1 minute for final extension. This process adjusted to run for 45 cycles. Finally, Brucella species was detected using species specific primers of B. abortus and B. melitensis. When the cycle threshold (CT) value of the samples were <45, it was considered and evaluated as positive. If greater than 45, it was considered as negative.
Results
Serology
The seropositive distribution of Brucella spp. infection was assessed for both extensive and intensive production systems. The samples from the central area were all from bovine species and were all managed under intensive production system (intensive dairy farms). Samples from the southern area consisted of all species (bovine, small ruminant, and camel), and were all managed under extensive production systems except for cattle from Wolayita Zone where cattle were managed under intensive systems. The distribution of seropositive herds is presented in Figure 2.
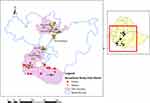 |
Figure 2 Distribution of Brucella seropositive herds. |
The results of bovine seropositivity in the intensive system showed animal and herd level prevalence of 1.10% (31/2808) and 2.87% (5/174).
Individual animal level seroprevalence from extensive management system, for all species, is presented in Table 3 accordingly, the prevalence percentages were 9.19% (16/174), 8.89% (97/1091) and 12.44% (25/201) for cattle, small ruminant and camel, respectively. However, it needs to be noted that the ELISA, although proposed for adequate use in camelids by the OIE, is not a validated test for that group of species, and so these results should be taken as presumptive and not definitive.40
 |
Table 3 Animal and Herd Level Seroprevalance Result from Extensive Management System |
Isolation and Identification of Brucella
There was a total of 13 vaginal swabs collected in the field, all from animals with a recent history of abortion. These animals included 2 swabs from cattle, and 11 swabs from small ruminants. There were no vaginal swabs collected from camels. Brucella spp. were isolated from six of these, including both of the swabs from cattle and four of the 11 samples from small ruminants.
Initially, the isolates were recognized on the basis of colony morphology as having a characteristic Brucella growth with very small, glistening, smooth, round and pin-point, honey-like colonies on Brucella selective agar plates after 4 days of incubation at 37℃, both aerobically, and in the presence of 5% CO2.
Microscopic examination was carried out immediately after the primary isolation of Brucella selective agar and Gram stained cultures showed small gram negative coccobacilli arranged individually and in pairs. With Modified Ziehl-Neelsen (MZN) stain, the organisms of Brucella stained red on a blue background (Figure 3A). The suspected colonies hydrolyzed urea within 2 hours (Figure 3B). No growth was observed on MacConkey agar and the colonies were non-haemolytic on blood agar.
 |
Figure 3 (A) Modified Ziehl-Neelsen (red-pink coccobacilli) stain of Brucella spp and (B) Urea Test Result within 2h in aerobic conditions. |
DNA was extracted from all six cultures that were morphologically and biochemically consistent with Brucella spp. The purity and concentration were measured by Nanodrop spectrophotometer giving 2.06, 2.01, 2.09, 1.7, 1.79 and 2.09 of DNA purity and 464.7ng/μL, 351.0ng/μL, 281.6ng/μL, 369.7ng/μL, 35.6ng/μL and 584.7ng/μL DNA concentration. All of these DNA samples were subjected to real-time PCR screening using the IS711 gene and subsequently for B. melitensis and B. abortus. All six isolates were identified as B. melitensis and none as B. abortus as showed in Figure 4.
 |
Figure 4 Real-time PCR amplification result of bacterial isolate using the B. melitensis primers. |
PCR on Blood Clots
One hundred and sixty-nine blood clots from seropositive animals were subjected to DNA extraction before performing PCR. These samples were screened by using IS711 gene, and all samples were negative. There was no further analysis on these samples and a summary of animal brucellosis based on the three test methods is presented in Table 4.
 |
Table 4 Summary of Animal Brucellosis Using the Three Test Methods |
Discussion
The results of this study have many similarities to previous studies on seroprevalence of brucellosis in Ethiopia. Many previous studies in Ethiopia have recorded individual animal prevalence and are results compare overall with similarity to those previously published results. In our study, overall individual animal level prevalence of 1.58% (47/2982) 8.89% (97/1091) and 12.44% (25/201) of brucellosis in bovine, small ruminant, and camel, respectively.
For individual animal prevalence in cattle, our study yielded 1.58% (47/2982) whereas other studies had ranges from 0.2% to 11.2%.2,7,9,14,17,18,20,21,26,27,38,43,45,50,52 Differences may be due to differing geographic areas or production systems sampled.
In the current study, key differences were observed in bovine brucellosis among individual cattle managed under intensive and extensive systems, with extensive having higher seroprevalence (9.19%) as compared to seroprevalence of individual cattle managed under intensive system (1.10%) as indicated in Table 5. This may be because in extensive management cattle are mixing with sheep and goats. Furthermore, in the intensive system, there is less opportunity for naïve cows to access infective placentas, as the animals are more closely monitored and often confined. Also, the biosecurity measures in the intensive management are higher than in extensive management systems. A previous study of dairy systems in Ethiopia, completed two decades ago, found a 0% seroprevalence for brucellosis.11 The results from this study indicate that brucellosis is in quite a few dairy herds.
 |
Table 5 Sero-Prevalence of Bovine Brucellosis Among Cattle Reared Under Extensive and Intensive Management System |
In the present study, for small ruminants, which were all under the extensive system, and all in the southern part of the country, the overall individual animal seroprevalence of brucellosis in small ruminants was 8.89% (97/1091). This is just in the mid-range of seroprevalences of small ruminant brucellosis reported elsewhere in Ethiopia, with ranges from 1.9% to 13.7%.4–6,16,23,28,33,35,37,39,46,49,50
Regarding camels which were all in the southern part of Ethiopia and all under extensive management, the overall individual animal seroprevalence rate was 12.44% (25/201) which is slightly higher than what has been reported in previous studies, from 0.5% to 9.5%.1,10,13,15,16,30,34,36,44,48 Also, for camels, the herd seropositivity was 100%, but it should be noted that only nine herds were sampled. The results of this study regarding seropositivity in camels may indicate that camel brucellosis is increasing in Ethiopia. However, again, it needs to be noted that this ELISA test is not yet validated for camels. Additionally, none of the previously reported serologic studies for brucellosis in camels in Ethiopia were assessed using validated tests because none exist.
The variation in the animal and herd level prevalence among the reports might be attributable to the agro-ecology as a risk factor for brucellosis, the influence of the agro-ecological zone has a higher prevalence in dry zones.32 Since pasture areas are scarce in dry areas, animals must search for pastures in large areas that imply unrestricted animal-to-animal contact with potential transmissions. Similarly, in extensive farming system infected animals have the highest probability of close contact with healthy animals.
In this study, blood clots from the seropositive animals were assayed by RT-PCR for the presence of Brucella spp. All blood clots were negative. This is an indication that although animals are seropositive, and therefore likely infected at some time in the past, there was no Brucella spp. Circulating at the time of blood collection. At the initial infection, there is a bacteremia that is consistent, however, once infected, animals remain infected for life although the organisms are mostly sequestered within the lymphoid tissue. They do make periodic excursions from the lymph node to infect other areas, eg, placenta, but these potential periods of very low bacteremias are likely sporadic only.
Conclusions
The present study adds to the body of knowledge regarding the extent of Brucella infection in livestock in Ethiopia. In general, population levels of seropositivity were similar to previous studies done throughout the country, with the exception of camels, in which the rate of positivity was higher than seen in any previous study. More attention to public health measures surrounding camels and their products is indicated. Another notable finding was the higher seroprevalence in animals raised extensively, compared to intensive production, such as is seen in the dairy industry in Ethiopia.
Data Sharing Statement
All data generated and analyzed during this study are included in the manuscript. However, the raw data is available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study did not involve human participants. It only involved animal serum and swab samples. Research permit was provided by Animal Research Scientific and Ethics Review Committee of the Animal Health Institute, Sebeta, Ethiopia.
Acknowledgments
We would like to thank the American Society for Microbiology and the US Centers for Disease Control and Prevention (CDC) for funding the sample collection and RT-PCR portions of this work. We would like to thank the Sodo, Jinka and Yabello Regional Veterinary Laboratories for their support and collaboration during sample collection. The authors also would like to extend special thanks to the pastoralists and farm owners for their collaboration during sample collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be
accountable for all aspects of the work.
Funding
The American Society for Microbiology (ASM) in collaboration with Centers for Disease Control and Prevention USA (CDC-USA) provided funding to the Animal Health Institute for sample collection and the serological and molecular diagnostic testing. The bacterial isolation activities were funded entirely by the Animal Health Institute, Sebeta, Ethiopia.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Admasu P, Kaynata G. Seroprevalence of camel brucellosis in Yabello District of Borena Zone, Southern Ethiopia. J Vet Med Res. 2017;4(10):1115.
2. Alemu F, Admasu P, Feyera T, Niguse A. Seroprevalence of bovine brucellosis in Eastern Showa, Ethiopia. Acad J Anim Dis. 2014;3(3):27–32.
3. Alton G, Jones L, Angus R, Verges M. Techniques for the brucellosis laboratory. Institute National de la Recherche Agronomique. J Clin Microbiol. 1988;33(12):3198–3200.
4. Anteneh H. Seroprevalence Of Small Ruminant Brucellosis And Its Public Health Awareness In Selected Two Districts of Afar region, Ethiopia [MSc Thesis]. Debre-zeit, Ethiopia: Addis Ababa University, School of Veterinary medicine; 2014.
5. Ashagrie T, Deneke Y, Tolosa T. Seroprevalence of caprine brucellosis and associated risk factors in South Omo Zone of Southern Ethiopia. Afr J Microbiol. 2011;5(13):1476–1682.
6. Asmare K, Megersa B, Denbarga Y, et al. A study on seroprevalence of caprine brucellosis under three livestock production systems in southern and central Ethiopia. Trop Anim Health Prod. 2012;45:555–560. doi:10.1007/s11250-012-0258-2
7. Asmare K, Asfaw Y, Gelaye E, Ayelet G. Brucellosis in extensive management system of Zebu cattle in Sidama Zone, Southern Ethiopia. Afr J Agric Res. 2010;5:257–263.
8. Baily G, Krahn B, Drasar S, Stoker G. Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg. 1992;95:271–275.
9. Bashitu L, Afera B, Tuli G, Aklilu F. Seroprevalence study of bovine brucellosis and its associated risk factors in Debre Birhan and Ambo Towns. J Adv Dairy Res. 2015;3(131):2. doi:10.4172/2329-888X.1000131
10. Bekele M. Seroepidemiological Study of brucellosis in Camels (Camelus dromedarius), in Borena Lowland Pastoral Areas, Southern Ethiopia [Faculty of Veterinary Medicine, Msc Thesis]. DebreZeit, Ethiopia: Addis Ababa University; 2004.
11. Belihu K. Analysis Of Dairy Cattle Breeding Practices In Selected Areas Of Ethiopia [PhD Thesis]. Berlin: Humboldt University; 2002.
12. Boschiroli M, Foulongne V, O’Callaghan D. Brucellosis: a worldwide zoonosis. Curr Opin Microbiol. 2001;4(1):58–64. doi:10.1016/S1369-5274(00)00165-X
13. Dinka H, Chala R. Seroprevalence study of bovine brucellosis in pastoral and agro-pastoral areas of East Showa zone, Oromia Regional State, Ethiopia. Am Eurasian J Agric Environ Sci. 2009;6:508–512.
14. Geresu M, Ameni G, Kassa T, Tuli G, Arenas A, Kassa M. Seropositivity and risk factors for Brucella in dairy cows in Asella and Bishoftu towns, Oromia Regional State, Ethiopia. Afr J Microbiol Res. 2016;10(7):203–213. doi:10.5897/AJMR2015.7707
15. Gessese A, Mulate B, Nazir S, Asmare A. Seroprevalence of brucellosis in camels (Camelus dromedarius) in Southeast Ethiopia. J Vet Med Sci. 2014;3(1):1–10.
16. Gumi B, Firdessa R, Yamuah L, et al. Seroprevalence of brucellosis and Q-fever in southeast Ethiopian pastoral livestock. J Vet Med Sci. 2013;2(1):1–5. doi:10.4172/2325-9590.1000109
17. Gwida. MA, Rosler U, Rosler U, Rosler U, Neubauer H, Neubauer H. Brucellosis in camels. Res Vet Sci. 2012;92(3):351–355. doi:10.1016/j.rvsc.2011.05.002
18. Haileselassie M. Seroprevalence study of Bovine Brucellosis and its Public Health Significance in Western Tigray [PhD Thesis]. Ethiopia: Addis Ababa University; 2008.
19. Hailu D, Mohamed M, Mussie H, Moti Y. Seroprevalence of bovine brucellosis in agropastoral areas of Jijjiga zone of Somali National Regional State, Eastern Ethiopia. Ethiop Vet J. 2011;15(1):37–47.
20. Halling SM, Tatum FM, Bricker J. Sequence and characterization of an insertion-sequence, IS711, from Brucella ovis. Gene. 1993;133:123–127. doi:10.1016/0378-1119(93)90236-V
21. Ismail A, Wudu T, Wassie M. Seroprevalence and associated risk factors of camel (Camelusdromedarius) brucellosis in and around Dire Dawa, Ethiopia. Glob Vet. 2012;8(5):480–483.
22. Kebede T, Ejeta G, Ameni G. Seroprevalence of bovine brucellosis in smallholder dairy farms in central Ethiopia (Wuchale-Jida district). Revue de Elevage Et Medicine Veterinaire Des Pays Tropicaux. 2008;159:3–9.
23. Khosravi D, Abassi E, Alavi M. Isolation of Brucella melitensis and Brucella abortus from brucellosis patients by conventional culture method and polymerase chain reaction technique. Pak J Med Sci. 2006;22(4):396–400.
24. Lemu D, Mamo H, Deressa A, Pal M. A study on seroprevalence of brucellosis in goats and sheep in East Shewa. Ethiopia. Ethio Int J Multidis Res. 2014;1(4):14–18.
25. Lennette H, Albert Balous J, Hausler J, Jean Shadomy H. Manual of Clinical Microbiology.
26. Megersa B, Biffa D, Niguse F, Rufae T, Asmare K, Skjerve E. Cattle brucellosis in traditional livestock husbandry practice in southern and eastern Ethiopia and its zoonotic implication. Acta Veterinaria Scandinavica. 2011;53(1):24.
27. Megersa B, Biffa D, Abunna F, Regassa A, Godfroid J, Skjerve E. Seroprevalence of brucellosis and its contribution to abortion in cattle, camel, and goat kept under pastoral management in Borana. Trop Anim Health Prod. 2011;43:651–656. doi:10.1007/s11250-010-9748-2
28. Mekonnen H, Shewit K, Moses K, Mekonnen A, Belihu K. Effect of Brucella infection on reproduction conditions of female breeding cattle and its public health significance in western Tigray, northern Ethiopia. SAGE-Hindawi. Vet Med Int. 2011;2011. doi10.4061/2011/354943
29. Negash E, Shihun S, Desta B. Seroprevalence of Small Ruminant Brucellosis and its Public Health Awareness in Selected Sites of Dire Dawa region, Eastern Ethiopia. [MScThesis]. Ethiopia: College of Veterinary Medicine, Haramaya University; 2012.
30. Regassa G, Mekonnen D, Yamuah L, et al. Human brucellosis in traditional pastoral community in Ethiopia. Int J Trop Med. 2009;4(2):59–64.
31. Sayour E, Elbauomy M, El-Kholi K, Shehata E. Brucellosis prevalence and serologic profile of male one-humped camels reared in Somaliland and eastern Ethiopia for meat production. Glob Vet. 2015;14(1):67–76.
32. Scholz C, Al Dahouk S, Tomaso H, et al. Genetic diversity and phylogenetic relationships of bacteria belonging to the Ochrobactrum–Brucella group by recA and 16SrRNA gene-based comparative sequence analysis. Syst Appl Microbiol. 2008a;31:1–16. doi:10.1016/j.syapm.2007.10.004
33. Silva I, Dangolla A, Kulachelvy K. Seroepidemiology of Brucella abortus infection in bovids in Sri Lanka. Prev Vet Med. 2000;46:51–59. doi:10.1016/S0167-5877(00)00136-7
34. Sintayehu G, Melesse B, Abayneh D, et al. Epidemiological survey of brucellosis in sheep and goats in selected pastoral and agro-pastoral lowlands of Ethiopia. Rev Sci Tech. 2015;34(3):881–893. doi:10.20506/rst.34.3.2403
35. Sisay Z, Mekonnen H. Seroprevalence of Brucella infection in camel and its public health significance in selected districts of Afar Region, Ethiopia. J Environ Occup Sci. 2012;1(2):91–98. doi:10.5455/jeos.20120711034013
36. Tadeg M, Gudeta R, Mekonen Y, Asfaw T, Birru L, Reda A. Seroprevalence of small ruminant brucellosis and its effect on reproduction at Tallalak district of Afar region, Ethiopia. J Vet Med Sci. 2015;7(4):111–116.
37. Tassew H, Kassahun F. Sero-epidemiological study of camel brucellosis in Mehoni District, South Eastern Tigray. J Microbiol Res. 2014;4(1):18–23.
38. Tegegn H, Feleke A, Adugna W, Melaku K. Small ruminant brucellosis and public health awareness in two districts of Afar Region, Ethiopia. J Vet Sci Technol. 2016;7(335):2. doi:10.4172/2157-7579.1000335
39. Tesfaye G, Tsegaye W, Chanie M, Abinet F. Seroprevalence and associated risk factors of bovine brucellosis in Addis Ababa dairy farms. Trop Anim Health Prod. 2011;43:1001–1005. doi:10.1007/s11250-011-9798-0
40. Teshale S, Muhie Y, Dagne A, Kidanemariam A. Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of Eastern Ethiopia: the impact of husbandry practice. Revue d Elevag eet Medicine Veterinaire des Pays Tropicaux. 2006;157:557–563.
41. World Organisation for Animal Health (OIE). Terrestrial animal health manual. Brucellosis; 2018: 355–398.
42. Tewodros E, Dawit A. Sero-prevalence of small ruminant brucellosis in and around Kombolcha, Amhara Regional State, North-Eastern Ethiopia. J Vet Med Sci. 2015;4:5.
43. Thrusfield M. Veterinary Epidemiology.
44. Tibesso G, Ibrahim N, Tolosa T. Seroprevalence of bovine and human brucellosis in Adami Tulu, Central Ethiopia. World Appl Sci J. 2014;31(5):776–780.
45. Tilahun B, Bekana M, Belihu K, Zewdu E. Camel brucellosis and management practices in Jijiga and Babile districts, Eastern Ethiopia. J Vet Med Anim Health. 2013;5(3):81–86.
46. Tolosa T, Regassa F, Belihu K. Seroprevalence study of bovine brucellosis in extensive management system in selected sites of Jimma Zone, Western Ethiopia. Bull Anim Health Prod Afr. 2008;56:25–37. doi:10.4314/bahpa.v56i1.32823
47. Tsegay A, Tuli G, Kassa T, Kebede N. Seroprevalence and risk factors of brucellosis in small ruminants slaughtered at DebreZeit and Modjo export abattoirs, Ethiopia. J Infect Dev Ctries. 2015;9(4):373–380. doi:10.3855/jidc.4993
48. Wernery U. Camelid brucellosis: a review. Rev Sci Tech off Int Epiz. 2014;33(3):839–857. doi:10.20506/rst.33.3.2322
49. Woldegebriel S. Prevalence and Risk Factors of Camel and Human Brucellosis in South Afar region, northeast Ethiopia [MSc Thesis]. Debrezeit, Ethiopia: Addis Ababa University, School of Veterinary Medicine; 2011.
50. Wubishet Z, Sadik K, Abdala B, Mokonin B, Getachew T, Getachew K. Small ruminant brucellosis and awareness of pastoralists community about zoonotic importance of the disease in Yabello districts of Borena Zone Oromia Regional State, Southern Ethiopia. Curr Trends Biomed Eng Biosci. 2018;12(1):555827.
51. Yohannes M, Degefu H, Tolosa T, Belihu K, Cutler R, Cutler S. Brucellosis in Ethiopia. Afr J Microbiol Res. 2013;7:1150–1157. doi:10.5897/AJMR12.738
52. Yohannes M, Mersha T, Hailu D, Tolosa T, Woyesa M. Bovine brucellosis: serological survey in Guto–Gida District, East Wollega Zone, Ethiopia. Glob Vet. 2012;8(2):139–143.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.