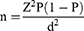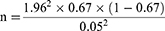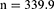Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Anemia in HIV Patients Attending Highly Active Antiretroviral Therapy Clinic at Hoima Regional Referral Hospital: Prevalence, Morphological Classification, and Associated Factors
Authors Kaudha R, Amanya R, Kakuru D, Muhumuza Atwooki R, Mutebi Muyoozi R, Wagubi R , Muwanguzi E, Okongo B
Received 5 July 2023
Accepted for publication 7 October 2023
Published 12 October 2023 Volume 2023:15 Pages 621—632
DOI https://doi.org/10.2147/HIV.S425807
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Rose Kaudha, Richard Amanya, Demiano Kakuru, Roggers Muhumuza Atwooki, Ronald Mutebi Muyoozi, Robert Wagubi, Enoch Muwanguzi, Benson Okongo
Department of Medical Laboratory Science, Mbarara University of Science and Technology, Mbarara City, Uganda
Correspondence: Benson Okongo, Department of Medical Laboratory Science, Mbarara University of Science and Technology, P.O. BOX 1410, Mbarara City, Uganda, Tel +256 778 557 867, Fax +256-485-20782, Email [email protected]
Purpose: To determine the prevalence of anemia, the morphological classification and to assess the factors associated with anemia among HIV patients attending Highly Active Antiretroviral Therapy (HAART) clinic at Hoima Regional Referral Hospital.
Methods: This was a cross-sectional study among 340 participants attending the HAART clinic at Hoima Regional Referral Hospital. Participants were recruited using a simple random sampling technique. A complete blood count (CBC) was performed using the Sysmex XN-550 hematology analyzer. Thick films were made and examined for malaria parasites, while thin films were examined for the morphological classification of anemia. Bivariate and multivariate logistic analyses were conducted using SPSS (version 23).
Results: Out of the 340 study participants, 255 (75%) were females, and the median age was 39 years (range: 6– 76 years). The overall prevalence of anemia among the study participants was 16.8% (95% CI 13.1– 21.1). Normocytic normochromic anemia was the most prevalent form of anemia (47.4%). The logistic regression at multivariate analysis showed that age groups (18– 27 years, p = 0.017; 28– 37 years, p = 0.005; and ≥ 38 years, p = 0.009), divorced marital status (p = 0.024), the presence of chronic disease (p = 0.010), a family history of anemia (p = 0.007), and the presence of malaria in the past one month (p = 0.001), presence of opportunistic infection (OR = 58, p = 0.000), use of antihelminthic drug in the past 3 months (OR = 0.10, p = 0.003) and unsuppressed viral load (OR = 10.74, p = 0.000) had a significant association with anemia.
Conclusion: Anemia is prevalent in HIV/AIDS patients who receive treatment at Hoima Regional Referral Hospital. Age, marital status, the presence of chronic illnesses, a family history of anemia, experiencing malaria in the past 3 months, the presence of opportunistic infections, the use of antihelminthic drugs in the past 3 months, and an unsuppressed viral load were significantly associated with anemia.
Keywords: anemia, prevalence, HIV/AIDS, antiretroviral therapy
Introduction
Anemia is defined by the WHO as a hemoglobin level of less than 13.0 g/dl for male adults and less than 12.0 g/dl for female adults.1 Anemia still remains one of the major public health burden. In the year 2021, the prevalence of anemia among individuals of all age groups was 24.3%, representing a total of 1.92 billion cases worldwide.2 An estimated 63–95% of HIV patients get anemic at some point throughout their illness.3 Anemia affects both developing and developed countries, making it a worldwide public health issue.
Despite a steady but slow decline in the incidence of HIV infection rate over the last two decades, with improved survival due to the expansion of antiretroviral therapies (ART), the majority of people are living with HIV than ever before.4 However, with increases in life expectancy following the introduction of ART, hematological changes are one of the most common complications among people living with HIV and could impact both the length and quality of their lives.5 As the most frequent hematologic abnormality in people living with HIV, anemia has been identified as a prognostic marker for HIV disease progression and has been linked with lower risk of survival.6,7
Anemia among HIV-infected patients has many underlying causes. HIV could directly and indirectly impact the survival and functioning of hematopoietic stem/progenitor cells (HSPCs) that reside in the bone marrow.8,9 Furthermore, ART medications, inflammatory mediators released by HIV infection, co-infections, or opportunistic infections could all have an impact on how HSPCs proliferate and differentiate during hemopoiesis.9 Hematologic abnormalities like anemia, thrombocytopenia, and neutropenia may arise from either the progressive depletion of HSPCs or the suppression of their function.8 High plasma levels of HIV-1 RNA and low CD4 cell counts (<200 cells/l) have both been linked to an increased risk of anemia.10,11
The bone marrow, where blood is produced, experiences a combined impact from various factors, including HIV viral infection, AIDS treatment medications, inflammatory agents released during infections, and potential opportunistic pathogens. HIV infection has both direct and indirect effects on hematopoietic progenitor cells, disrupting the equilibrium of the bone marrow and impairing the processes of cell proliferation and differentiation in hematopoiesis. This leads to significant outcomes such as altered cell counts across all blood cell lineages, abnormal changes in erythroid and granulocytic series, megaloblastic irregularities in the erythroid series, and an impediment in reticulum endothelial iron utilization. Additionally, because hematopoietic progenitor cells express CD4 receptors, type 4 C-X-C chemokine receptors, and type 5 chemokine receptors, they are susceptible to HIV infection.12
Several studies have reported variations in morphological types of anemia. In a study in Nigeria, Esan et al reported a normochromic normocytic type of anemia as a major type of anemia in HIV patients,13 and in Kenya, microcytic hypochromic anemia was the commonest type of anemia, followed by normocytic hypochromic anemia,14 while in another similar study in Kenya, hypochromic and normochromic anemia were the commonest.15 In Tanzania, Makubi et al reported microcytic anemia as the most predominant morphological type of anemia,16 while in Uganda,17 microcytic hypochromic anemia was reported as the commonest type of anemia.
This study therefore determined the prevalence, morphological type anemia and associated factors in HIV patients attending the ART clinic at Hoima Regional Referral Hospital.
Materials and Methods
Study Area
The study was conducted at the ART clinic at Hoima Regional Referral Hospital in Hoima District. It is located in the Bunyoro sub region of western Uganda. The district is located approximately 230 kilometers (140 miles) by road northwest of Kampala, the capital city of Uganda. Its GPS coordinates are: latitude 1.428578, longitude 31.35488. Hoima Regional Referral Hospital has a bed capacity of 280 and mostly serves the districts of Buliisa, Hoima, Kibaale, Kiryandongo, Kagadi, Kakumiro, Kikuube, and Masindi.
Study Design and Period
This was a hospital-based cross-sectional study conducted between the month of March and September 2022.
Sample Size Calculation
The sample size was calculated using the Kish-Leslie formula (1965). This study used the prevalence of anemia (67%) reported at Mbarara Regional Referral Hospital HIV clinic.18
Where n is the sample size of our study
Z = is the critical value for a 95% confidence interval which is 1.96
P = is the estimated proportion of HIV patients with anemia in our study population (0.67).
d = is the desired level of precision
Sample size = 340
Sampling Procedure
A simple random sampling technique was used to enroll HIV patients attending the ART clinic at Hoima Regional Referral Hospital. A total of 340 study participants were randomly selected and subjected to structured questionnaires to obtain information on socio-demographic characteristics.
Participants were selected through simple randomization method as previously described by Liu et al.19 In summary, each HIV patient, a parent or legal guardian of those below 18 years were tasked with selecting a number from cards and depositing it into a container during the recruitment process. If a patient, or a parent/guardian chose an even number, they were granted permission to provide consent, and the patient or a child was included in the study. The cards were rearranged each time a selection was made.
Selection Criteria
All HIV positive patients attending the Art Clinic at Hoima Regional Referral Hospital who consented to participate in the study were enrolled in the study, while all HIV patients who were critically ill, those that were anemic and on anemia treatment, and all pregnant women in HIV care were excluded too.
Data Collection
The socio-demographic characteristics (sex, marital status, age, level of education, alcohol intake, employment, level of income, whether they were urban or rural residents) were collected using a structured questionnaire.
Clinical Data
Clinical data, eg, duration on ART, presence of chronic disease, presence of malaria in the last 1 month, family history of anemia, antibiotic use in the last 3 months, defaulted from ART for >3 months, presence of opportunistic infections (Tuberculosis, Cryptococcal meningitis and oral Candidiasis), HIV clinical stage, history of anti-tuberculosis (TB) drug use, history of antiviral drug use, history of antifungal use, history of antihelminthic use, Body Mass Index (BMI), a BMI was defined as <18.5 underweight, 18.5–24.5 normal, 25–29.9 overweight and >30 obese), and viral load performed within the last 6 months were extracted from patient files.
4 mL of venous blood samples were collected into EDTA tubes. A complete blood count (CBC) was done using the Sysmex XNL-550 hematology analyzer (22,848, Bornbarch Norderstedt, Germany). Thin blood films for those with low hemoglobin levels (Hb <12.0g/dl in females and <13.0 g/dl in males) were made and stained using Giemsa stain for the morphological classification of anemia, Thick smears were made, stained using Giemsa stain, and examined for hemoparasites. CD4 counts were run using BD FACSPresto™ (USA).
Quality Control
The reagents were standardized and quality-controlled before use. Hematology and CD4 analyzers were calibrated, and control samples were run to establish the accuracy and reproducibility of the results. The generated data was double-entered into an Excel spreadsheet, double-checked for completeness, cleaned, and then analyzed.
Data Analysis
The data were entered into an Excel spreadsheet and then exported to SPSS statistics software version 23 (IBM Inc., USA) for analysis. Categorical data and continuous variables were presented in the form of percentages and frequencies. Factors associated with anemia were studied using logistic regression. A p-value of ≤0.05 was considered statistically significant.
Ethical Consideration
The approval was obtained from the Faculty of Medicine Research Committee (FRC) of MUST (Approval No. MUST/MLS/30). Permission to conduct the study was also sought from the director of Hoima Regional Referral Hospital.
Written informed consent was obtained from the study participants above 18 years and from parents/guardians of study participants less than 18 years. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki (1964). Privacy and confidentiality was followed all through the study process and those with anemia were referred to physicians for management.
Results
Socio-Demographic Characteristics of Study Participants
We recruited 340 study participants into this study. Majority were females (75%), were aged 38 years and above (48.8%), were unemployed (40.9%), had income level less than Ugandan shillings (UGx). 100,000/= (35.6%), had attained secondary education (55%) and were married (79.7%) as shown in (Table 1).
 |
Table 1 Demographic Characteristics of Study Participants |
Prevalence of Anemia Among HIV Patients Attending HAART Clinic at Hoima Regional Referral Hospital
The overall prevalence of anemia among the HIV patients attending HAART clinic at Hoima regional referral hospital was 16.8% (57/340) as presented in (Figure 1).
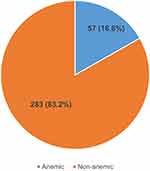 |
Figure 1 Pie chart showing prevalence of anemia among HIV patients attending HAART clinic at Hoima Regional Referral Hospital. |
Morphological Classification of Anemia Among HIV Patients Attending HAART Clinic at Hoima Regional Referral Hospital
The major morphological type of anemia among the HIV patients attending HAART clinic at Hoima regional referral hospital was normocytic normochromic anemia 27 (47.4%) followed by microcytic hypochromic anemia 24 (42.1%), Normocytic hypochromic anemia 4 (7%) and Macrocytic normochromic anemia 2 (3.5%) as shown above (Figure 2).
 |
Figure 2 Bar graph showing morphological classification of anemia among HIV patients attending HAART clinic at Hoima Regional Referral Hospital. |
Factors Associated with Anemia Among Patients Attending ART Clinic at Hoima Regional Referral Hospital
Bivariate Analysis of Factors Associated with Anemia Among Patients Attending ART Clinic at Hoima Regional Referral Hospital
In the bivariate analysis, age (28–37 years, OR = 0.01, p = 0.013; ≥38 years, OR = 0.01, p = 0.008), divorced marital status (OR = 454, p = 0.005), a history of malaria in the past 3 months (OR = 6.44, p = 0.007), a family history of anemia (OR = 14.84, p = 0.007), the presence of opportunistic infections (OR = 65.19, p = 0.000), the use of antihelminthic drugs in the past 3 months (OR = 0.06, p = 0.006), and an unsuppressed viral load (OR = 17.62, p = 0.001) were found to be associated with anemia among HIV patients, as shown in (Table 2).
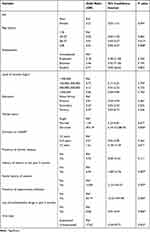 |
Table 2 Bivariate Analysis of Factors Associated with Anemia Among HIV Patients |
Multivariate Analysis of Factors Associated with Anemia Among Patients Attending ART Clinic at Hoima Regional Referral Hospital
All the factors that showed an association at the bivariate level were taken for multivariate analysis, as well as other factors with p-values ≤0.1. In the multivariate analysis, age groups (18–27 years, OR = 0.01, p = 0.017; 28–37 years, OR = 0.01, p = 0.005; and ≥38 years, OR = 0.01, p = 0.009), divorced marital status (OR = 98.96, p = 0.024), the presence of chronic disease (OR = 12.10, p = 0.010), a history of malaria in the past 3 months (OR = 7.11, p = 0.001), a family history of anemia (OR = 8.54, p = 0.007), the presence of opportunistic infections (OR = 58, p = 0.000), the use of antihelminthic drugs in the past 3 months (OR = 0.10, p = 0.003), and an unsuppressed viral load (OR = 10.74, p = 0.000) were found to be associated with anemia, as indicated in (Table 3).
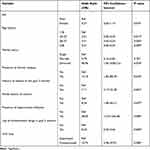 |
Table 3 Multivariate Analysis of Factors Associated with Anemia Among HIV Patients |
Discussion
Anemia is a major concern for individuals suffering from HIV/AIDS, as it is a significant determinant of disease progression. In this study, the overall prevalence of anemia was 16.8%, as defined by the WHO (with a threshold of 12 g/dl for females and 13 g/dl for males). This prevalence is significantly lower than the 47.8% rate of anemia observed among HIV antiretroviral therapy (ART) naive clients attending an immune suppression syndrome clinic at Mbarara Regional Referral Hospital in southwestern Uganda.20 However, the variations in findings could be explained by the fact that the previous study was conducted on HIV-naive clients, whereas the current study was conducted on HIV patients on ART. It is unclear how HIV affects the bone marrow microenvironment in vivo, inhibiting hematopoiesis and directly leading to cytopenia.20
The prevalence of anemia in this current study was lower than studies in Nigeria at 24.3%,21 South Africa at 25.8%,22 Tanzania at 56%,23 Bayamon, Puerto Rico at 41%,24 and China at 39.2%.25 This could be related to geographical differences and food disparities. These disparities in the burden of anemia among HIV-positive patients may be attributable to differences in socio-demographic disparities, study participant characteristics, sample size, and changes in treatment modalities.
In addition, all the study participants were HIV-positive individuals on ART, this may demonstrate the efficacy of HAART in reducing HIV-associated anemia indirectly by lowering the incidence of opportunistic infections and chronic diseases and increasing patients’ nutritional status.
The prevalence of anemia among HIV-positive individuals, on the other hand, was marginally equivalent to the finding from the Ethiopian study, with 11.4% prevalence of anemia.24 Similarly, the findings of this study are comparable to the 16.2% prevalence reported at Jimma University among ART-experienced individuals.25,26
According to the current study’s findings, normocytic normochromic anemia was the most common type of anemia among HIV patients at HRRH (47.4%), followed by microcytic hypochromic anemia (42.1%) and macrocytic hypochromic anemia being the least common (3.5%). Our finding was comparable with studies in 46.5% in Ethiopia,26,27 Panwar et al who reported that 46% of the respondents in their study had Normochromic normocytic anemia, followed by 42.85% microcytic hypochromic anemia in New Delhi, India.28 Our finding defers from two studies, one in Uganda by Nyesigire Ruhinda et al that reported that in HIV-infected children, microcytic-hypochromic anemia (44.9%) was the commonest type of anemia17 and another in Kenya where microcytic hypochromic anemia was the predominant type of anemia among HIV patients on ART in Kenya.29
Factors Associated with Anemia Among Patients Attending ART Clinic at Hoima Regional Referral Hospital
In this study, age groups <18 was found to have increasing odds of developing anemia. This finding is not consistent with studies in China by Shen et al, and Yantao et al, which reported that the prevalence of anemia increases with age (49.6%, 53.5%, and 60.1% among patients aged 18 to 39, 40 to 59, and 60 years, respectively).30,31 Similar findings were as well reported by Wolde et al, where age, persistent diarrhea, the initial ART treatment, the initial CD4 count, and the initial ALT level all served as independent predictors of incident anemia.32
In this study, being divorced was significantly associated with anemia among the study participants, the divorced were 98.96 times likely to develop anemia. This finding is comparable with a study by Guiying et al, in southwestern China that reported that patients who were widowed or divorced had higher odds of having severe anemia than married PLHIV patients.33 This study is in agreement with a study done in Tanzania that reported that being divorced was associated with anemia in people living with HIV/AIDS.34 Our results are also comparable to those of a US study that discovered anemia in divorced HIV patients.35
In this study, there was an association between chronic infection in HIV and anemia, with a 12.1-fold increase in the odds of anemia among patients with chronic illness. This finding is consistent with a study conducted by Araújo-Pereira et al in 18 outpatient research clinics across 10 low- and middle-income countries, which also observed an association in line with our findings.36 In a meta-analysis that combined cross-sectional and case-control studies, it was found that the likelihood of a connection between anemia and tuberculosis (TB) increased as the severity of anemia became more pronounced, with a odds ratio of 3.56.37
Consistent findings were observed in Ethiopia where the likelihood of developing anemia in patients with a prior history of chronic diarrhea increased significantly compared to patients who did not have chronic diarrhea.32 In a study conducted across the United States, it was observed that HIV patients co-infected with the hepatitis C virus showed a correlation with the presence of anemia.38
Our study observed a significant association between a history of malaria in the past month and anemia in the study participants. This finding is comparable with a study in Tanzania among HIV-positive women on ART that reported current malaria infection and a history of episodes of malaria illness during the index pregnancy, where there was an association between a history of malaria and anemia.39 The study found higher odds of anemia among patients who reported malaria in the past one month. Malaria parasites tend to destroy red blood cells and therefore deplete the body’s iron stores. This could be the reason for the higher odds of anemia in HIV patients who have a history of malaria. These findings are comparable to a study by Menon et al in Uganda who noted that the risk factors for anemia included having malaria parasitemia.40
In our present investigation, we found that having a family history of anemia predicts the occurrence of anemia in individuals living with HIV. Interestingly, while most previous research has not specifically emphasized the connection between a family history of anemia and anemia in HIV patients, studies involving different populations have indeed documented associations between a family history of anemia and the development of anemia.41,42
In this current study, the presence of opportunistic infections was associated with anemia after initiation of ART; this is consistent with a study in Ethiopia.43 Opportunistic Infections (OIs) are reported to attack the bone marrow in patients with HIV. These infections may cause marrow changes either directly by the organism itself or indirectly by causing reactive changes.44 In another study in Ethiopia, women with baseline opportunistic infections and women who were on ART for a long duration were significantly associated with anemia among women living with HIV/AIDS.45 The above findings are comparable to a study by46 who reported that factors that often contributed to the risk of developing anemia in HIV infection include the presence of opportunistic infections, sex, and a low CD4+ T-lymphocyte count.47
This current study reported unsuppressed viral load and CD4-count less than 200 cells/mm3 had a significant association with anemia. This finding is consistent with a finding in a study in Tanzania that reported that, the prevalence of anemia increased as the HIV/AIDS advanced from lower to higher WHO clinical stages.39 This is also consistent with a previous study by Nagawa et al that reported that advanced HIV/AIDS clinical stages (clinical stage III or IV) or CD4 Count of <200 had a strong association with anaemia.11 The findings are similarly consistent with studies conducted in Kisumu Kenya and Tanzania,10,48 in Ethiopia,49 and in Ghana50 which found that CD4 cell counts of <200 cells per mm3 and individuals with HIV advanced disease stage III / IV were more likely to develop anemia.
Study Limitations
This study did not collect information on intestinal parasite infections, which are a leading cause of anemia in low- and middle-income nations. Because this was a hospital-based, cross-sectional investigation, the relationship between HIV infection and anemia could not be demonstrated conclusively.
Conclusion
Anemia burden exists among HIV patients at Hoima regional referral hospital, and normochromic normocytic anemia was the commonest type of anemia followed by microcytic anemia among patients. Anemia is prevalent in HIV/AIDS patients who receive treatment at Hoima Regional Referral Hospital. Age, marital status, the presence of chronic illnesses, a family history of anemia, experiencing malaria in the past 3 months, the presence of opportunistic infections, the use of antihelminthic drugs in the past 3 months, and an unsuppressed viral load were significantly associated with anemia. Hemoglobin levels should be monitored routinely, especially among PLWHIV who have one or more of the risk factors for anemia, so that treatment can be initiated if deemed necessary. Further longitudinal studies are needed to determine the association between HIV infection and anemia.
Acknowledgments
The authors would like to acknowledge the study participants, the Director of Hoima Regional Referral Hospital, the medical staff at the ART clinic, and the laboratory staff for their invaluable support during data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. World Health Organization; 2008.
2. Collaborators GA. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: findings from the global burden of disease study 2021. Lancet Haematol. 2023;10(9):e713–e34.
3. Mehta S, Jutur S, Gautam D. Hematologic manifestations of HIV/AIDS. Med Update. 2011;9:484–490.
4. UNAIDS U. UNAIDS data 2017. Jt United Nations Program HIV/AIDS; 2017:1–248.
5. Ezeamama AE, Sikorskii A, Bajwa RK, et al. Evolution of anemia types during antiretroviral therapy—implications for treatment outcomes and quality of life among HIV-infected adults. Nutrients. 2019;11(4):755. doi:10.3390/nu11040755
6. Albrecht S, Franzeck FC, Mapesi H, et al. Age-related comorbidities and mortality in people living with HIV in rural Tanzania. Aids. 2019;33(6):1031–1041. doi:10.1097/QAD.0000000000002171
7. Haider BA, Spiegelman D, Hertzmark E, et al. Anemia, iron deficiency, and iron supplementation in relation to mortality among HIV-infected patients receiving highly active antiretroviral therapy in Tanzania. Am J Trop Med Hyg. 2019;100(6):1512. doi:10.4269/ajtmh.18-0096
8. Durandt C, Potgieter J, Khoosal R, et al. HIV and haematopoiesis. South Afr Med J. 2019;109(8 Supplement 1):S41–S6. doi:10.7196/SAMJ.2019.v109i8b.13829
9. Marchionatti A, Parisi MM. Anemia and thrombocytopenia in people living with HIV/AIDS: a narrative literature review. Int Health. 2021;13(2):98–109. doi:10.1093/inthealth/ihaa036
10. Odhiambo C, Zeh C, Angira F, et al. Anaemia in HIV‐infected pregnant women receiving triple antiretroviral combination therapy for prevention of mother‐to‐child transmission: a secondary analysis of the Kisumu breastfeeding study (Ki BS). Trop Med Int Health. 2016;21(3):373–384. doi:10.1111/tmi.12662
11. Nagawa C. Predictors of Anemia Among HIV Patients in Uganda; 2017.
12. Tsukamoto T. Hematopoietic stem/progenitor cells and the pathogenesis of HIV/AIDS. Frontiers in cellular and infection microbiology. Front Cell Infect Microbiol. 2020;10:60. doi:10.3389/fcimb.2020.00060
13. Esan A, Osime E, Titilayo E. Degree of anaemia and severity of HIV Infection in HIV patients on art and art-naïve. J AIDS Clin Res. 2020;11(5):1–5.
14. Abonyo C, Shaviya N, Budambula V, Were T. Anemia burden, types and associated risk factors among Kenyan human immunodeficiency virus-1 and mycobacterium tuberculosis co-infected injection substance users. Ethiop J Health Sci. 2020;30(5):661. doi:10.4314/ejhs.v30i5.4
15. Khazalwa EM, Were T, Mulama DH, Budambula V. The burden and types of anaemia among HIV infected, ART-naive injection substance users in Kenya. Afr Health Sci. 2022;22(1):431–442. doi:10.4314/ahs.v22i1.52
16. Makubi A, Okuma J, Spiegelman D, et al. Burden and determinants of severe anemia among HIV-infected adults: results from a large urban HIV program in Tanzania, East Africa. J Int Assoc Provid AIDS Care. 2015;14(2):148–155. doi:10.1177/2325957413488195
17. Ruhinda EN, Bajunirwe F, Kiwanuka J. Anaemia in HIV-infected children: severity, types and effect on response to HAART. BMC Pediatr. 2012;12(1):1–6. doi:10.1186/1471-2431-12-1
18. Katemba C, Muzoora C, Muwanguzi E, Mwambi B, Atuhairwe C, Taremwa IM. Hematological abnormalities in HIV-antiretroviral therapy naïve clients as seen at an immune suppression syndrome clinic at Mbarara Regional Referral Hospital, southwestern Uganda. J Blood Med. 2018;105–110. doi:10.2147/JBM.S157148
19. Liu W, Wang L, Yi M. Simple-random-sampling-based multiclass text classification algorithm. Scientific World J. 2014;2014:1.
20. Kyeyune R, Saathoff E, Ezeamama AE, Löscher T, Fawzi W, Guwatudde D. Prevalence and correlates of cytopenias in HIV-infected adults initiating highly active antiretroviral therapy in Uganda. BMC Infect Dis. 2014;14(1):1–10. doi:10.1186/1471-2334-14-496
21. Denue BA, Kida IM, Hammagabdo A, Dayar A, Sahabi MA. Treatment. Prevalence of anemia and immunological markers in HIV-infected patients on highly active antiretroviral therapy in Northeastern Nigeria. Infect Dis Res Ther. 2013;6:S10477.
22. Takuva S, Maskew M, Brennan AT, Sanne I, MacPhail AP, Fox MP. Anemia among HIV-infected patients initiating antiretroviral therapy in South Africa: improvement in hemoglobin regardless of degree of immunosuppression and the initiating ART regimen. J Trop Med. 2013;2013:1–6. doi:10.1155/2013/162950
23. Petraro P, Duggan C, Spiegelman D, et al. Determinants of anemia among human immunodeficiency virus-positive adults at care and treatment clinics in Dar es Salaam. Tanzania. 2016;94(2):384.
24. Santiago-Rodríguez EJ, Mayor AM, Fernández-Santos DM, Ruiz-Candelaria Y, Hunter-Mellado RF. Anemia in a cohort of HIV-infected Hispanics: prevalence, associated factors and impact on one-year mortality. BMC Res Notes. 2014;7(1):1–8. doi:10.1186/1756-0500-7-439
25. Tiewsoh JBA, Antony B, Boloor R. Seroprevalence of HIV-2 and dual infection among HIV-infected individuals with clinical and laboratory features at a tertiary care teaching Hospital, Mangalore: the present scenario. Ann Afr Med. 2019;18(2):70. doi:10.4103/aam.aam_23_18
26. Berhane Y, Haile D, Tolessa T. Anemia in HIV/AIDS patients on antiretroviral treatment at ayder specialized hospital, Mekelle, Ethiopia: a case-control study. J Blood Med. 2020;379–387. doi:10.2147/JBM.S275467
27. Fenta DA, Nuru MM, Yemane T, Asres Y, Wube TB. Anemia and related factors among highly active antiretroviral therapy experienced children in Hawassa comprehensive specialized hospital, southern Ethiopia: emphasis on patient management. Drug Healthc Patient Saf. 2020;Volume 12:49–56. doi:10.2147/DHPS.S230935
28. Panwar A, Sharma S, Kumar S, Sharma A. A study of anemia in human immunodeficiency virus patients: estimating the prevalence, analyzing the causative effect of nutritional deficiencies, and correlating the degree of severity with CD4 cell counts. Med J Dr DY Patil Univ. 2016;9(3):312–318. doi:10.4103/0975-2870.182499
29. Wafula P, Were T, Barasa MJ. Anaemia phenotypes in highly active antiretroviral treatment defaulting adults at the comprehensive care clinic at the Siaya county teaching and referral hospital. J Aids HIV Inf. 2019;5(1):103.
30. Shen Y, Wang Z, Lu H, et al. Prevalence of anemia among adults with newly diagnosed HIV/AIDS in China. PLoS One. 2013;8(9):e73807. doi:10.1371/journal.pone.0073807
31. Jin Y, Li Q, Meng X, et al. Prevalence of anaemia among HIV patients in rural China during the HAART era. Int J STD AIDS. 2017;28(1):63–68. doi:10.1177/0956462415622866
32. Wolde HM. Incidence and risk factors of anemia among HIV/AIDS patients taking anti-retroviral therapy at tertiary hospitals in Addis Ababa, Ethiopia: a retrospective cohort study. J HIV AIDS Infect Dis. 2014;2:1–06.
33. Cao G, Long H, Liang Y, et al. Prevalence of anaemia and the associated factors among hospitalised people living with HIV receiving antiretroviral therapy in Southwest China: a cross-sectional study. BMJ open. 2022;12(7):e059316. doi:10.1136/bmjopen-2021-059316
34. Gunda DW, Nkandala I, Kilonzo SB, Kilangi BB, Mpondo BC. Prevalence and risk factors of mortality among adult HIV patients initiating ART in rural setting of HIV care and treatment services in North Western Tanzania: a retrospective cohort study. J Sex Transm Dis. 2017;2017:1–8. doi:10.1155/2017/7075601
35. Kposowa AJ. Marital status and HIV/AIDS mortality: evidence from the US national longitudinal mortality study. Int J Infect Dis. 2013;17(10):e868–e74. doi:10.1016/j.ijid.2013.02.018
36. Araújo-Pereira M, Krishnan S, Salgame P, et al. Effect of the relationship between anaemia and systemic inflammation on the risk of incident tuberculosis and death in people with advanced HIV: a sub-analysis of the REMEMBER trial. EClinicalMedicine. 2023;2023:60.
37. Cobelens F, Kerkhoff AD. Tuberculosis and anemia—cause or effect? Environ Health Prev Med. 2021;26(1):93. doi:10.1186/s12199-021-01013-4
38. Harding B, Whitney B, Nance R, et al. Anemia risk factors among people living with HIV across the United States in the current treatment era: a clinical cohort study. BMC Infect Dis. 2020;20:1–8. doi:10.1186/s12879-020-04958-z
39. Manyanga VP, Minzi O, Ngasala BJ. Prevalence of malaria and anaemia among HIV infected pregnant women receiving co-trimoxazole prophylaxis in Tanzania: a cross sectional study in Kinondoni Municipality. BMC Pharmacol Toxicol. 2014;15(1):1–9. doi:10.1186/2050-6511-15-24
40. Menon MP, Yoon SS. Prevalence and factors associated with anemia among children under 5 years of age—Uganda, 2009. Am J Trop Med Hyg. 2015;93(3):521. doi:10.4269/ajtmh.15-0102
41. Alswailem AM, Alahmad SM, Alshehri MA. The prevalence of iron deficiency anemia and its associated risk factors among a sample of females in Riyadh, Saudi Arabia. J Hosp Med. 2018;72(6):4625–4629. doi:10.21608/ejhm.2018.9791
42. Alreshidi MA, Haridi HK. Prevalence of anemia and associated risk factors among pregnant women in an urban community at the North of Saudi Arabia. J Prev Med Hyg. 2021;62(3):E653. doi:10.15167/2421-4248/jpmh2021.62.3.1880
43. Geletaw T, Tadesse MZ, Demisse AG. Hematologic abnormalities and associated factors among HIV infected children pre-and post-antiretroviral treatment, North West Ethiopia. J Blood Med. 2017;Volume 8:99–105. doi:10.2147/JBM.S137067
44. Tripathi A, Misra R, Kalra P, Gupta N, Ahmad RJJ. Bone marrow abnormalities in HIV disease. JAPI. 2005;53:705–710.
45. Belay AS, Genie YD, Kebede BF, Kassie A, Molla A. Time to detection of anaemia and its predictors among women of reproductive-age living with HIV/AIDS initiating ART at public hospitals, Southwest Ethiopia: a multicentre retrospective follow-up study. BMJ open. 2022;12(4):e059934. doi:10.1136/bmjopen-2021-059934
46. Ferede G, Wondimeneh Y. Prevalence and related factors of anemia in HAART-naive HIV positive patients at Gondar University Hospital, Northwest Ethiopia. BMC Blood Disord. 2013;13(1):1–5. doi:10.1186/2052-1839-13-8
47. Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17(2):138.
48. Gunda DW, Kilonzo SB, Mpondo BC. Magnitude and correlates of moderate to severe anemia among adult HIV patients receiving first line HAART in Northwestern Tanzania: a cross sectional clinic based study. Pan Afr Med J. 2016;23(1). doi:10.11604/pamj.2016.23.26.8268
49. Melese H, Wassie MM, Woldie H, Tadesse A, Mesfin N. Anemia among adult HIV patients in Ethiopia: a hospital-based cross-sectional study. HIV/AIDS Res Palliat Care. 2017;Volume 9:25–30. doi:10.2147/HIV.S121021
50. Obirikorang C, Issahaku RG, Osakunor DNM, Osei-Yeboah J. Anaemia and iron homeostasis in a cohort of HIV-infected patients: a cross-sectional study in Ghana. AIDS Res Treat. 2016;2016:1–8. doi:10.1155/2016/1623094
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.