Back to Journals » International Journal of General Medicine » Volume 17
Analysis of Laboratory Critical Values During COVID-19 Pandemic at Tertiary Hospital in Saudi Arabia
Authors Jeraiby MA
Received 28 November 2023
Accepted for publication 20 January 2024
Published 2 February 2024 Volume 2024:17 Pages 367—375
DOI https://doi.org/10.2147/IJGM.S449505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Mohammed A Jeraiby
Department of Medical Biochemistry, Faculty of Medicine, Jazan University, Jazan, Saudi Arabia
Correspondence: Mohammed A Jeraiby, Department of Medical Biochemistry, Faculty of Medicine, Jazan University, Jazan, Kingdom of Saudi Arabia, Tel +966-544589494, Email [email protected]; [email protected]
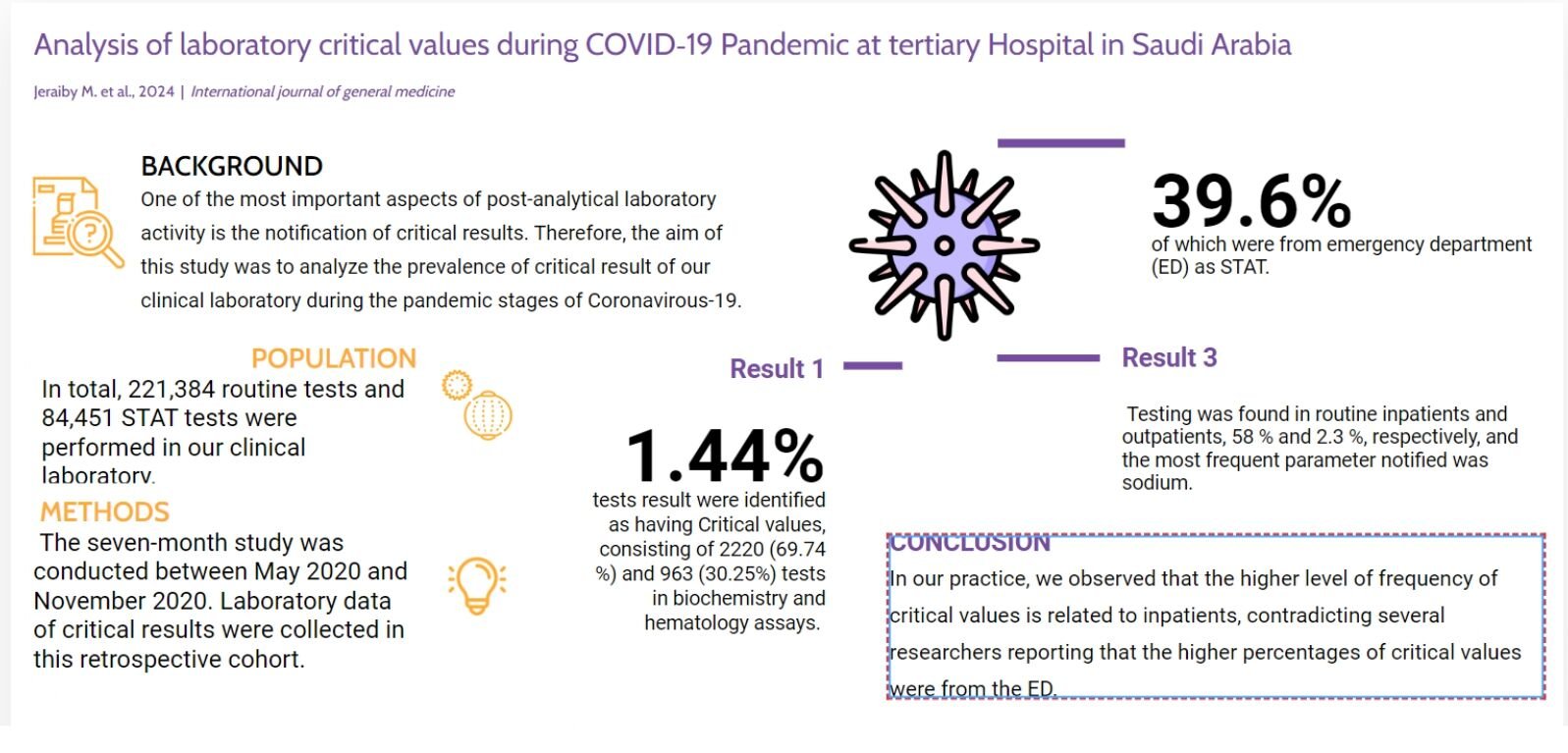
Purpose: One of the most important aspects of post-analytical laboratory activity is the notification of critical results. Therefore, the aim of this study was to illustrate and analyze the prevalence of critical result values of our clinical laboratory investigations during the pandemic stages of coronavirus-19 (COVID-19) and other research pre-pandemic stages.
Methods: The seven-month study was conducted between May 2020 and November 2020. Laboratory data of critical results were collected in this retrospective cohort.
Results: In total, 221,384 routine tests and 84,451 STAT tests were performed in our clinical laboratory. Of the 3183 (1.44%) tests result was identified as having Critical values, consisting of 2220 (69.74%) and 963 (30.25%) tests in biochemistry and hematology assays. Among the tests with critical values, 39.6% of which were from emergency department (ED) as STAT testing (1262) and 60.3% (1921) as TAT testing. Testing was found in routine inpatients and outpatients, 58% and 2.3%, respectively, and the most frequent parameter notified was sodium.
Conclusion: In our practice, we observed that the higher level of frequency of critical values results is related to inpatients, contradicting several researchers reporting that the higher percentages of critical values were from ED.
Keywords: critical values limits, critical values notification, STAT tests, TAT tests, coronavirus-19
Graphical Abstract:
Introduction
A critical value is in accordance with the definition given, “laboratory test result indicating a pathophysiologic state that deviates from normal so much that it could be fatal if medical intervention is not done quickly, and for which an effective action could be implemented.” This concept was first defined in 1972, and it is now widely used all across the world.1 Critical value reporting is now one of the prerequisites for accreditation by lab accrediting organizations. Additionally, through the International Organization for Standardization (ISO), which has been approved as a standard of Good Laboratory Practice, the rapid communication of a critical value as a particular need has been acknowledged and applied globally.2–5 Guidelines for reporting critical results have been released by several organizations, including the British Royal College of Pathologists.6 These organizations have specific standards for laboratory critical values. It provides policies and procedures focused on the identification of critical values with rapid communication of a critical value, including the use of telephone calls and call centers in reporting as well as a list of key values that can help practitioners comprehend tests that could need quick attention.2–5 According to the literature, there is no agreement on the critical value thresholds or the biomarkers that are most likely to produce them. The list of biomarkers and their critical value limits, which each laboratory should develop, must be agreed upon by doctors.7–10 Additionally, separate lists are required for various study groups because key values for neonatal, pediatric, and adult care patients would vary.11 The first step in communicating critical values is the identification of abnormal results by a laboratory staff. For automated lab, middleware instrument or Laboratory information system (LIS) will alert laboratory staff about critical values. In most cases, the staff is the performing technologist. For inpatients and outpatients, the mean time delay to report critical levels is 6 h and 14 min, respectively, 15 to 30 min is the reasonable and acceptable time.12,13
Globally, clinical laboratories have faced numerous challenges as a result of the COVID-19 pandemic. Several laboratory tests may aid clinicians in predicting results and preventing or diagnosing COVID-19 complications, such as IL6, CRP, and white blood cell subset counts, may help predict the severity of COVID-19. D-dimer, a blood coagulation test, is also linked to COVID-19 severity. Cardiac troponin and proBNP (cardiac markers) may aid in the diagnosis and management of COVID-19-related heart problems. Other laboratory tests related with severe COVID-19 include ferritin, LDH, transaminases, and serum albumin.14 That is, we assumed that the rates and characteristics, as well as the impact of COVID-19 in most laboratory tests probably varied from before the pandemic. Thus, this study was conducted to illustrate and analyze the prevalence of Critical result values of our clinical laboratory investigations during pandemic stages of (COVID-19) and compare with literature research pre-pandemic stages.
Materials and Methods
We performed a retrospective study in May 2020. We retrieved laboratory data of critical results during COVID-19 pandemic [from 1st May to 28nd November 2020], of Prince Muhammad Bin Nasser Hospital, Jazan province, Saudi Arabia, following approval from the Ethics Committee of Jazan University, in Jazan, Saudi Arabia (Approval Number. REC-43/07/146), with waived informed consent. The confidentiality of the collected data was strictly maintained. The research adhered to the ethical principles outlined in the Helsinki Declaration and the specific guidelines set by the National Committee of Bioethics in Saudi Arabia. The data were obtained from patient charts and/or laboratory databases as part of routine clinical procedures. Personal information and identifiable details of participants were excluded from the study. Our laboratory is a national clinical laboratory, with sub-specialties such as clinical hematology, biochemistry, Microbiology, Immunology, and Blood bank. The laboratory attained The Saudi Central Board for Accreditation of Healthcare Institutions CEBHI certification in October 2019. All data were obtained from laboratory reports generated from the LIS (AMS Abbott Medical System, Germany). Data were exported from the LIS to Microsoft Excel (Microsoft Office 2003®). Biochemistry and hematology tests were done during their stay in the hospital using (Abbott Architect plus C8000 and Sysmex XN1000) for clinical and immunochemistry tests and for hematology tests, respectively. The following parameters were included in the study: Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Amylase, Blood urea nitrogen (BUN), calcium, Creatine Kinase (CK), CKMB, Creatinine, D-dimer, glucose, hemoglobin, Lactate, Lactate dehydrogenase (LDH), potassium, sodium, Lipase, Magnesium, Phosphate, Red blood cell (RBC), Platelet, White blood cell (WBC), and Bilirubin (Total). Laboratory staff (Technician & Lab. Specialist) are notified of the presence of a critical value by the LIS, which is configured in such a way that a red warning flag automatically shows up when a critical value appears. We have two types of tests, STAT test, request from ED, results will be released within 1 hr from receiving the specimen. In the section of Routine test, results will be released within 2–4 hr for inpatient department or one working day for outpatient department (OPD).
The result is then checked for analytical reliability and errors, once the potential margin for error has been eliminated and the result technically validated by lab staff, the person responsible for the patient’s healthcare is notified by the laboratory staff either by Lab specialist or who is validated the results to get more information from clinician about the status of the patient to make sure there is no technical error. With regard to critical value, our laboratory has established two separate processes for inpatients and outpatients. For inpatients, either the nurse or the medical department is notified of critical value by phone. For outpatients, however, the laboratory staff gets in touch via the hospital’s call center, who call the attending physician as well as send them an alarm notification by the system. The call center then sends confirmation to the laboratory that the above has taken place and will contact patients to come back to clinic as soon as possible. All critical values reported for both inpatients and outpatients must be collected and registered in the laboratory’s internal archive by the laboratory technician or lab specialist who advised the value. Laboratory staff register information about the communication (the critical value results, the laboratory staff who communicated the information and the person who received the information). The lab staff also adds a comment to the critical result in the LIS, such as “critical value notified by phone” with names of laboratory staff who communicated and the nurse who received it, so that this information is recorded in the patient’s record. In our laboratory, these limits were established based on literature sources, and were subsequently revised and approved by lab staff and clinicians.11 In some cases, special critical value Calcium (total) is not considered a critical value unless the serum albumin concentration is higher than 25 g/L and the lab staff first corrected calcium manually if the albumin is low and notify the corrected results. For Cardiology Care Unit (CCU) the troponin is not considered a critical value. Additionally, if the samples belong to nephrology dialysis center predialysis, potassium is not consider critical value unless for post dialysis and is not necessary to report under an agreement between the Nephrology Service and our laboratory. Re-evaluation of these critical values is performed together with clinicians when the need to modify a critical value arises, such as following the publication of new data, new accords with clinical services, or technological improvements.
Statistical Package for the Social Sciences (SPSS version 24) was used for data analysis. Data analysis techniques involve descriptive and inferential statistics. Descriptive statistics were calculated for study variables, ie, frequency and percentage, with their 95% confidence intervals (CIs). p-values <0.05 were considered.
Results
During the 7-month study period coinciding with the COVID-19 pandemic, our clinical laboratory performed more than 221,384 routine tests and 84,451 STAT tests in biochemistry and hematology assays. The frequency of various critical values for each parameter, with their numbers, percentages, reference range, and minimum and maximum, is depicted in Table 1. Nearly 26% and 25% for sodium and potassium were the parameters with the highest percentages among all the critical values, followed by 18% and 12% for hemoglobin and glucose, respectively. The parameters with the lowest percentages were 8%, 4%, 3%, 2%, and 2% for platelets, BUN, WBC, D-dimer, and magnesium, respectively. In total, 3183 (1.44%) critical values were detected, approximately 39.6% of which were from ED STAT testing (1262) and 60.3% from routine inpatient and outpatient TAT testing (1921). Of all the critical results from routine laboratory tests, 58% were found in inpatients (1848). The parameter with the highest frequency in inpatients was sodium 493 cases (26.7%). However, only 2.3% (73 cases) of the critical values were observed in outpatients (Table 2). To recognize an association between the frequency of laboratory parameters and their units (ED, inpatients, and OPD), 95% confidence intervals (CI) was applied as shown in Table 2. All critical value tests were statistically significant (P < 0.001). However, they were insignificant for departments (ED, inpatient, or outpatient) and units (P = 0.719, P = 0.912), respectively, Table 3. Most parameters resulting in critical values from routine laboratory tests belonged to inpatients, followed by ED. The outpatients had the lowest percentage of critical values for routine laboratory tests. The frequency of critical values among outpatients reached 11 analytes (4 in hematology and 7 in biochemistry). Additionally, the greatest frequencies occurred for sodium and potassium (16 and 15), respectively.
 |
Table 1 The Critical Value List and Results for Biochemical and Hematological Parameters (with Minimum and Maximum Single Parameter Results) |
 |
Table 3 Comparison of Critical Results Above or Below Normal Range According to Type of Tests, Department, and Unit |
Discussion
In this study, there was a total of 221.384 tests, among which 3183 cases (1.44%) had critical values consisting of 2220 (69.74%) and 963 (30.25%) tests in biochemistry and hematology assays. The rate is more frequent in inpatients and ED at 58.1% and 39.6%, respectively, ICU had the highest ward in inpatient (19.5%), which were factors affecting critical values. Comparably, we came across similar findings for the ICU and ED as departments with the highest rate of critical values, 45% and 44%, respectively.15 Similarly, Jafari E et al had observed the ICU had the highest critical values 23.2% and ED 12.8%. Similar to our study, the rates were higher in inpatients compared to OPD.16 Kuperman GJ et al separated the tests into high and low values. The PO2 test in their study showed the highest percentage of critical values.17 Similarly, the authors, Jafari E et al, divide the test to low and high and 31.5% of urea tests had the most frequent critical values.16 Based on the critical values reference list, we separated the test results in our study into minimum and maximum critical results. For the sodium level, our findings showed that 26% (823 cases) critical sodium ion with 26.7%, 24.8%, and 1.94% from inpatient department, ED, and OPD, respectively, and the minimum result was 86 mmol/L and maximum result was 197 mmol/L, consisting of 506 cases with low and 317 cases with high. Jafari E et al discovered that there were 94 cases with a critical sodium value rate of 0.09%, 48 of which had low serum sodium levels and 46 of which had high serum sodium levels. ICU admissions made up the majority of cases.16 A rate of 0.55% for critical sodium levels was found in a different study by Howanitz JH and Howanitz PJ, which included 447 and 166 cases of high and low sodium levels, respectively. That study, however, focused on mortality rates.18 For potassium levels, the second greatest percentage of critical values was 25%, consisting of 308, 463 and 15 cases from ED, Inpatient, and OPD, respectively, finding concerning hypokalemia in 60% of cases. According to a study by Jafari E et al, 61 and 69 cases with low and high potassium levels, respectively, made up 0.13% of the cases with critical values; the majority of these patients were admitted to the ICU.16 Contrary to our findings, Zhou F, Zhao B, and Gu D discovered greater admission in internal departments such hepatology and nephrology.19
Moreover, Shubha demonstrated that the results related to biochemistry had the highest number of Critical values (86.3%), and the most often reported analyte was creatinine 52.3%.20 Another study by Piva et al reported that the vast majority of critical results were related to patients in the ICU, and the most frequent parameter notified was potassium (49%).21
Platelet count had the highest rate of critical values, according to a prior report by Sun SP, Garcia J, and Hayden JA, whereas leukocytes had the lowest rate. In their study, it was shown that the critical values were confirmed by repeating more than 95% of tests.22
In terms of calcium levels, according to a study by Jafari E et al, calcium, which was primarily found in the ICU, oncology, and internal wards and consisted of six low and seven high results, had critical values in 13 cases (0.03%).16 In a study conducted by Howanitz JH and Howanitz PJ, critical calcium values were reported in 1.4% of the sample accompanied by a 25% mortality rate.23 In present study, the calcium-critical results from pandemic state showed that no one suffered hypercalcaemia; instead, six cases with critical results for calcium indicated hypocalcaemia and all from inpatient ward (ICU isolation) and confirmed to have a positive diagnosis for COVID-19 infection by real-time reverse transcriptase-polymerase chain reaction (RT-PCR). However, hypocalcaemia is common in critically ill patients and is linked to organ failure and poor prognosis.24–26 The SARS-CoV 2 E gene produces a short transmembrane protein with an ion channel function that is significantly produced during infection of COVID-19, according to experiments conducted in vitro and in vivo. Since calcium ions may pass via these ion channels, a disturbance in calcium homeostasis may encourage the activation of inflammatory pathways.27 These observations suggest that SARS-CoV-2 endocytosis and infection might be influenced by hypocalcemia.28
Although we have provided sufficient evidence to support our conclusion, several limitations exist. Firstly, due to the nature of the study, specifically its retrospective nature conducted at a single tertiary hospital, which may limit the generalizability of the findings to the entire laboratories in Jazan province of Saudi Arabia. Future studies should consider a larger sample size and a multicenter approach to provide a more comprehensive understanding of the critical values rate and evaluate the improvement of the process. Secondly, the study focused on pandemic state, and further research is needed to evaluate the pre-pandemic state.
Conclusion
Our study retrospectively highlights the prevalence and distribution of critical values of our clinical laboratory (biochemistry and hematology) at a tertiary hospital in Saudi Arabia during pandemic stages of COVID-19. Inpatients can have number and frequencies of such critical values for different test values that are closely related to patient outcomes. Emphasizing the need for targeted interventions to address departmental and service setting challenges. Addressing these challenges is critical for improving patient care, properly notifying and recording the results, and promoting critical values of laboratory tests to health care staff while minimizing diagnostic errors and optimizing laboratory processes. It appears that in order to achieve the desired conditions and infrastructures, it will be necessary for each laboratory to have a policy on how to handle reporting critical values based on a number of factors such as nature of the test item, combined with install alarm systems in the patient information system, update laboratory equipment, and train medical staff – particularly physicians and nurses – on how to handle critical values. This will clarify laboratory technician responsibilities and establish consistency in performance.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Lundberg GD. Critical (panic) value notification: an established laboratory practice policy (parameter). JAMA. 1990;263(5):709. doi:10.1001/jama.1990.03440050103044
2. College of American Pathologists. Laboratory General Checklist[components; GEN.41320, GEN.41330, and GEN.41340].; 2011–02; 2011. Available from: http://www.cap.org..
3. The Joint Commission on Accreditation of Healthcare Organizations Laboratory, national patient safety goals (NPSG.02.03.01).; 2012–5; 2012. Available from: http://www.jointcommission.org/.PatientSafety/NationalPatientSafetyGoals.
4. International Organization for Standardization (ISO). ISO 15189:2012. Medical Laboratories – Particular Requirements for Quality and Competence. Geneva, Switzerland: International: Organization for Standardization; 2012.
5. Clinical and Laboratory Standards Institute (CLSI). Management of Critical- and Significant-Risk Result. CLSI Guideline GP47. Wayne, PA: Clinical and Laboratory Standards Institute; 2015.
6. Croal B The communication of critical and unexpected pathology results. The royal college of pathologists; 2024. Available from: https://www.rcpath.org/resourceLibrary/the-communication-of-critical-and-unexpected-pathology-results-pdf.html.
7. Kost GJ. Critical limits for emergency clinician notification at United States children’s hospitals. Pediatrics. 1991;88(597):603. doi:10.1542/peds.88.3.597
8. Kost GJ. Critical limits for urgent clinician notification at US medical centers. JAMA. 1990;263(5):704–707. doi:10.1001/jama.1990.03440050098042
9. Fine RH. Laboratory critical limits. JAMA. 1990;264(3):334. doi:10.1001/jama.1990.03450030050019
10. Lundberg GD. Now read this: the SI units are here. JAMA. 1986;255(17):2329–2339. doi:10.1001/jama.1986.03370170093043
11. Lippi G, Mattiuzzi C. Critical laboratory values communication: summary recommendations from available guidelines. Ann Transl Med. 2016;4(20):400. doi:10.21037/atm.2016.09.36
12. Rocha BCB, Alves JAR, Pinto FPD, Mendes ME, Sumita NM. The critical value concept in clinical laboratory. J Bras Patol Med Lab. 2016;52(1):17–20. doi:10.5935/1676-2444.20160008
13. Howanitz PJ, Steindel SJ, Heard NV.Laboratory critical values policies and procedures: a college of American pathologists q-probes Study in 623 institutions -; 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/12033953/.
14. Cihakova D, Streiff MB, Menez SP, et al. High-value laboratory testing for hospitalized COVID-19 patients: a review. Future Virol. 2021;16(10):691–705. doi:10.2217/fvl-2020-0316
15. Dighe AS, Rao A, Coakley AB, Lewandrowski KB. Analysis of laboratory critical value reporting at a large academic medical center. Am J Clin Pathol. 2006;125(5):758–764. doi:10.1309/R53XVC2U5CH6TNG8
16. Jafari E, Zarnegar F, Kalantari M, Dabiri S, Naghibzadeh-Tahami A. Critical values in laboratory tests of Iranian patients referring to laboratories: a cross-sectional study in Kerman. Arch Iran Med. 2021;24(6):473–477. doi:10.34172/aim.2021.68
17. Kuperman GJ, Boyle D, Jha A, et al. How promptly are inpatients treated for critical laboratory results? J Am Med Inform Assoc. 1998;5(1):112–119. doi:10.1136/jamia.1998.0050112
18. Howanitz JH, Howanitz PJ. Evaluation of serum and whole blood sodium critical values. Am J Clin Pathol. 2007;127(1):56–59. doi:10.1309/Q3Y27QQLEL19340A
19. Zhou F, Zhao B, Gu D. Evaluation of laboratory critical serum potassium values and their association with clinical symptoms in Chinese han patients. J Int Med Res. 2015;43(6):851–861. doi:10.1177/0300060515576011
20. H.v S; Shubha. A study of critical value analysis at hematology and biochemistry sections of laboratory in a multispeciality hospital. Panacea j med sci. 2022; 12(2):430–435. doi:10.18231/j.pjms.2022.081
21. Piva E, Pelloso M, Pennello L, Plebani M. Laboratory critical values: automated notification supports effective clinical decision making. Clin Biochem. 2014;47(13–14):1163–1168. doi:10.1016/j.clinbiochem.2014.05.056
22. Sun SP, Garcia J, Hayden JA. Repeating critical hematology and coagulation values wastes resources, lengthens turnaround time, and delays clinical action. Am J Clin Pathol. 2018;149(3):247–252. doi:10.1093/ajcp/aqx156
23. Howanitz JH, Howanitz PJ. Evaluation of total serum calcium critical values. Arch Pathol Lab Med. 2006;130(6):828–830. doi:10.5858/2006-130-828-EOTSCC
24. Kelly A, Levine MA. Hypocalcemia in the critically ill patient. J Intensive Care Med. 2013;28(3):
25. Ranson JH. Etiological and prognostic factors in human acute pancreatitis: a review. Am J Gastroenterol. 1982;77(9):
26. Imrie CW, Allam BF, Ferguson JC. Hypocalcaemia of acute pancreatitis: the effect of hypoalbuminaemia. Curr Med Res Opin. 1976;4(2):
27. Nieto-Torres JL, DeDiego ML, Verdiá-Báguena C, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10(5):e1004077. doi:10.1371/journal.ppat.1004077
28. Yang C, Ma X, Wu J, et al. Low serum calcium and phosphorus and their clinical performance in detecting COVID-19 patients. J Med Virol. 2021;93(3):1639–1651. doi:10.1002/jmv.26515
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

