Back to Journals » Clinical and Experimental Gastroenterology » Volume 17
An Overview of the Effects of Tenapanor on Visceral Hypersensitivity in the Treatment of Irritable Bowel Syndrome with Constipation
Authors Singh P, Sayuk GS, Rosenbaum DP, Edelstein S, Kozuka K, Chang L
Received 16 December 2023
Accepted for publication 22 March 2024
Published 10 April 2024 Volume 2024:17 Pages 87—96
DOI https://doi.org/10.2147/CEG.S454526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Santosh Shenoy
Prashant Singh,1 Gregory S Sayuk,2 David P Rosenbaum,3 Susan Edelstein,3 Kenji Kozuka,3 Lin Chang4
1Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA; 2Department of Medicine, Washington University School of Medicine, St Louis, MO, USA; 3Ardelyx, Inc, Waltham, MA, USA; 4Department of Medicine, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA
Correspondence: Prashant Singh, Department of Internal Medicine, University of Michigan, 1500 East Medical Center Drive, Ann Arbor, MI, 48109, USA, Tel +1 734 647 9252, Fax +1 734 936 9849, Email [email protected]
Background: Patients with irritable bowel syndrome with constipation (IBS-C) experience persistent abdominal pain, a common symptom leading to greater healthcare utilization and reports of treatment non-response. Clinically significant improvements in abdominal pain were observed in clinical trials of tenapanor, a first-in-class inhibitor of sodium/hydrogen exchanger isoform 3 (NHE3), for the treatment of IBS-C in adults.
Aim: This narrative review reports the current knowledge about visceral hypersensitivity as a mechanism for abdominal pain in patients with IBS-C and explores the published evidence for hypothesized mechanisms by which tenapanor may reduce visceral hypersensitivity leading to the observed clinical response of decreased abdominal pain.
Findings: Abdominal pain is experienced through activation and signaling of nociceptive dorsal root ganglia that innervate the gut. These sensory afferent neurons may become hypersensitized through signaling of transient receptor potential cation channel subfamily V member 1 (TRPV1), resulting in reduced action potential thresholds. TRPV1 signaling is also a key component of the proinflammatory cascade involving mast cell responses to macromolecule exposure following permeation through the intestinal epithelium. Indirect evidence of this pathway is supported by observations of higher pain in association with increased intestinal permeability in patients with IBS. Tenapanor reduces intestinal sodium absorption, leading to increased water retention in the intestinal lumen, thereby improving gastrointestinal motility. In animal models of visceral hypersensitivity, tenapanor normalized visceromotor responses and normalized TRPV1-mediated nociceptive signaling.
Conclusion: By improving gastrointestinal motility, decreasing intestinal permeability and inflammation, and normalizing nociception through decreased TRPV1 signaling, tenapanor may reduce visceral hypersensitivity, leading to less abdominal pain in patients with IBS-C. Therapies that have demonstrated effects on visceral hypersensitivity may be the future direction for meaningful abdominal pain relief for patients with IBS-C.
Keywords: IBS-C, NHE3, abdominal pain, TRPV1, sodium absorption, intestinal permeability
Introduction
Irritable bowel syndrome (IBS) is a common, chronic disorder of gut–brain interaction, with a multifactorial pathophysiology. Alterations in colonic motor function, changes in gut microbiota, increased intestinal permeability and resulting immune responses, and dysregulation of gut-brain nerve pathways collectively contribute to IBS.1 Patients with IBS experience recurrent abdominal pain associated with altered bowel movements.2,3 According to the Rome IV criteria, IBS diagnosis requires ≥1 weekly episode of abdominal pain for ≥3 months related to ≥2 of the following: change in stool form, change in stool frequency, and/or defecation.2 Based on these criteria, an estimated 4% of the world’s population has IBS.4
IBS is classified into four subtypes based on stool consistency; the most common are IBS with diarrhea and IBS with constipation (IBS-C) and occur in 31.5% and 29.3% of patients with IBS according to the Rome IV criteria, respectively.2,4 In patients with IBS-C, >25% of bowel movements are of Bristol Stool Form Scale types 1 (nut-like lumps) or 2 (sausage-shaped and lumpy), with <25% being of Bristol Stool Form Scale types 6 (mushy) or 7 (pure liquid).2
Although not associated with an increased mortality risk,5 IBS negatively impacts health-related quality of life,6,7 as well as activities of daily living.8,9 Among patients with IBS-C, pain is often regarded as the most troublesome abdominal symptom.10 Indeed, a survey of patients with IBS-C in the United States revealed that abdominal pain was the underlying cause of 91.1% of disease-related emergency department visits or hospitalizations.10 Abdominal pain was an independent predictor of US outpatient and emergency department visits among 434 patients with disorders of gut–brain interactions, including IBS, functional constipation, and functional diarrhea.11 Patients with IBS-C also experience additional abdominal symptoms, including bloating, discomfort, cramping, and fullness.12,13 Strategies to alleviate the symptoms of IBS-C include dietary interventions (eg, fiber supplementation, avoidance of specific foods), over-the-counter laxatives, and prescription medications.3,14,15 Neither dietary fiber nor laxatives have been demonstrated to alleviate abdominal pain.3,15,16 While treatment satisfaction rates vary depending on regimen, overall, 35–64% of patients with IBS-C report being dissatisfied with existing over-the-counter and/or prescription medications.17,18 Lack of improvement in abdominal pain is a common reason for treatment discontinuation, as noted by 29–42% of patients in the CONTOR study.18 Thus, patients with IBS-C remain in need of more effective treatments that target the underlying cause of abdominal pain.
Mechanistic Underpinnings of Abdominal Pain
Abdominal pain in patients with IBS is due in part to perturbations in nociception, a phenomenon referred to as visceral hypersensitivity.19 Increased intestinal permeability and low-grade intestinal inflammation have been hypothesized to act as triggers for visceral hypersensitivity.20,21 Visceral pain scores following colorectal distension were shown to be significantly higher in patients with IBS with increased intestinal permeability (defined as a lactulose-to-mannitol ratio ≥0.07) than in patients with IBS with normal intestinal permeability (lactulose-to-mannitol ratio <0.07).22 Supernatants derived from colonic biopsies of patients with IBS have been demonstrated to increase the paracellular permeability of cultured colonic epithelial cells, and the degree of supernatant-induced permeability has been correlated with the severity of patient-reported abdominal pain.23 A compromised epithelial barrier creates an environment permissive to the movement of macromolecules, mast cells, and mast cell mediators from the intestinal lumen across the epithelium. These macromolecules have the potential to cause inflammation that can trigger visceral hypersensitivity.20,21
The prevalence of visceral hypersensitivity in patients with IBS varies widely from 8% to 90%.24–26 Visceral hypersensitivity can be assessed clinically by colorectal balloon distension.25 Patients with IBS have lower thresholds for the pain and discomfort associated with this procedure compared with healthy volunteers.27–31 While there is evidence that altered central nervous system processing of visceral input may contribute to visceral hypersensitivity, other studies strongly support the importance of peripheral mechanisms of visceral pain in IBS.32,33
Transient receptor potential cation channel subfamily V member 1 (TRPV1) is expressed in nociceptive sensory neurons of the gastrointestinal tract34,35 and plays a role in sensing stimuli such as heat and the presence of capsaicin.36 In mouse models, knockout of the Trpv1 gene significantly reduced the ability of mice to sense (ie, neuronal impairment) and respond to colorectal distension.37 In rat models of colonic hypersensitivity, TRPV1 has been demonstrated to maintain visceral hypersensitivity, and treatment with a TRPV1 antagonist has been shown to blunt visceromotor responses following colorectal distension.38,39 Collectively, these data from animal models implicate TRPV1 as a mediator of visceral hypersensitivity in humans. The number of TRPV1-positive nerve fibers has been shown to be elevated in colonic biopsies of patients with IBS relative to healthy controls.34,35 Both TRPV1 transcription levels and immunoreactivity to TRPV1 protein have been shown to correlate positively with abdominal pain scores in patients with IBS.34,40 Additional clinical evidence in support of the relationship between TRPV1 and visceral hypersensitivity stems from studies in patients with quiescent inflammatory bowel disease whose symptoms do and do not satisfy the Rome criteria for IBS. The number of TRPV1-positive neuronal fibers was 5-fold higher in colorectal biopsies from patients with inflammatory bowel disease who reported ongoing abdominal pain compared with those who did not.41 Furthermore, in multivariate analyses, TRPV1 positivity was the only covariate found to be significantly associated with the abdominal pain score in these patients.41 The association of TRPV1 with visceral hypersensitivity in clinical evidence thus further supports the role of TRPV1 as a mediator of visceral hypersensitivity in patients with IBS.
In other experiments, visceral hypersensitivity was demonstrated to be “transferrable” from patients with IBS to animals, suggesting that soluble factors could also be at play. For example, inoculating germ-free rats with the fecal microbiota from patients with IBS-C with visceral hypersensitivity led to a significant increase in abdominal contractions upon colorectal distension (ie, increased visceral sensitivity) compared with rats inoculated with the fecal microbiota from healthy volunteers.42 Exposure of guinea pig submucosal neurons to colon supernatants from patients with IBS-C induced increased neuronal activation, as measured by spike discharge.43 Additionally, enteric and dorsal root ganglia (DRG) neuronal responses were more pronounced when colon supernatants were derived from patients with IBS with visceral hypersensitivity than from those with normosensitivity.24 IBS-C and IBS-D–derived colonic biopsy supernatants can also stimulate human enteric neuron cultures.44
During intestinal inflammation, the number of activated mast cells, as well as the number of mast cells in close proximity (within 5–10 μm) to colonic neurons, was significantly increased in patients with IBS compared with healthy controls as revealed by examination of the colonic mucosa.45,46 The physiological relevance of mast cell infiltration to increased visceral hypersensitivity in patients with IBS is underscored by the positive correlation between the number of mast cells proximal to colonic nerves and the severity of abdominal pain or discomfort.45 This influx of mast cells is accompanied by significant increases in locally released pro-nociceptive mediators such as histamine, tryptase, prostaglandin E2, prostaglandin D2.45–47 Indeed, each of these mediators has been shown to sensitize TRPV1, and levels of these mediators are elevated in the colonic mucosa of IBS patients compared to healthy controls.47 Furthermore, targeting these mast cell mediators has been shown to suppress the ability of mucosal supernatant derived from patients with IBS to activate TRPV1-expressing DRG isolates from rats.46 In addition to histamine, mast cells also secrete the cytokine interleukin 6 (IL-6).48 Serum and plasma IL-6 levels have been shown to be significantly elevated in patients with IBS relative to healthy controls.49 Further, IL-6 can also activate submucosal and myenteric neurons.50,51 In a rat model of IBS, blockade of IL-6 signaling decreased visceral hypersensitivity, as demonstrated by an increase in the pain threshold for colorectal distension.50
Mechanism of Action of Tenapanor
Tenapanor is a minimally absorbed first-in-class inhibitor of sodium/hydrogen exchanger isoform 3 (NHE3),52–54 an antiporter expressed on the apical membrane of epithelial cells lining the small intestine and proximal colon.55 NHE3 is the predominant NHE isoform involved in the transepithelial absorption of sodium from the gut.56 Tenapanor-mediated inhibition of NHE3 leads to reduced sodium absorption and subsequent retention of water in the intestinal lumen (Figure 1).52–54,57 Water retention, in turn, accelerates intestinal transit, resulting in softer stool consistency and improved gastrointestinal motility.
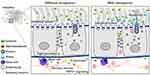 |
Figure 1 Proposed mechanism of action of tenapanor in reducing intestinal sodium absorption and alleviating visceral hypersensitivity in patients with IBS-C.52–54,57–60 (1) Tenapanor-mediated inhibition of NHE3 leads to (2) decreased sodium absorption and proton secretion, which in turn results in (3) a pH-sensitive conformational change in the tight junctions to decrease intestinal permeability to macromolecules. (4) Decreased exposure to luminal macromolecules results in reduced mast cell activation and subsequent (5) reduced TRPV1 signaling. (6) Ultimately, the reduction of TRPV1 signaling leads to normalization of nociceptive signaling and reduction of abdominal pain. Reprinted from King AJ, Chang L, Li Q, et al. NHE3 inhibitor tenapanor maintains intestinal barrier function, decreases visceral hypersensitivity, and attenuates TRPV1 signaling in colonic sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2024. Online ahead of print.57 Abbreviations: IBS-C, irritable bowel syndrome with constipation; NHE3, sodium/hydrogen exchanger isoform 3; TRPV1, transient receptor potential cation channel subfamily V member 1. |
Dose-dependent increases in stool sodium content and luminal fluid retention were reported in rats given single oral doses of tenapanor, and repeated dosing over 4 days resulted in sustained increases in stool sodium that returned to baseline when tenapanor was discontinued.54 In healthy volunteers, twice-daily (BID) doses of tenapanor 15–60 mg increased the amount of stool sodium retention by 20–50 mmol/day;53,54 absorption of 50 mmol sodium is equivalent to 2.8 g of table salt.53 In healthy volunteers, administration of tenapanor led to demonstrable increases in stool frequency and stool weight relative to placebo.53 Importantly, the sodium retention effects of tenapanor are limited to the gut, with no impact on serum/plasma sodium levels.53,58
As discussed above, the presence of abdominal pain is a hallmark of IBS,2 and visceral hypersensitivity may have pathophysiologic relevance to these abdominal pain experiences.19 The mechanistic effects of tenapanor on abdominal pain have been evaluated in a rat model of acetic acid–induced colonic hypersensitivity.59 In this model, oral tenapanor 0.5 mg/kg BID significantly reduced visceromotor responses to colorectal distension relative to vehicle. Interestingly, the visceromotor responses observed in tenapanor-treated, acetic acid–sensitized rats with colonic visceral hypersensitivity were comparable to those recorded in naive rats (ie, those not treated with acetic acid). Additionally, in single-cell patch-clamp experiments in colon-specific DRG obtained from acetic acid–sensitized rats, tenapanor reversed the DRG hyperexcitability following exposure to the TRPV1 agonist capsaicin, which suggests that tenapanor can normalize TRPV1-mediated nociceptive signaling (Figure 1).59 Interestingly, studies with human colonic epithelial monolayers have shown that tenapanor reduces intestinal cell permeability to macromolecules.60 Given the relationship between intestinal permeability and visceral hypersensitivity noted above, it is likely that improvement in barrier function by tenapanor is in part responsible for improvement in visceral hypersensitivity.
Clinical Effects of Tenapanor
The ability of tenapanor to inhibit sodium absorption and normalize enteric sensory neuron excitability and intestinal permeability translates to clinical improvements in global IBS-C symptoms, including amelioration of abdominal pain and increases in bowel movement frequency in patients with IBS-C. In the Phase 3 T3MPO-1 (NCT02621892) and T3MPO-2 (NCT02686138) studies, which were of 12 and 26 weeks’ duration, respectively, the primary endpoint was the proportion of patients with IBS-C who experienced both a ≥30% reduction in mean weekly worst abdominal pain score (abdominal pain response) and an increase of ≥1 complete spontaneous bowel movement relative to baseline in the same week for ≥6 of the first 12 weeks of treatment.61,62 Significantly, more patients randomized to receive tenapanor 50 mg BID met the primary endpoint compared with those who received placebo in T3MPO-1 (27.0% vs 18.7%; P=0.020) and T3MPO-2 (36.5% vs 23.7%; P<0.001).
In secondary analyses, proportionally more patients in the tenapanor group than in the placebo group had a clinically meaningful abdominal pain response for ≥6 of the first 12 weeks of treatment (T3MPO-1, 44.0% vs 33.1%; P=0.008; T3MPO-2, 49.8% vs 38.3%; P=0.004), ≥9 of the first 12 weeks of treatment (T3MPO-1, 30.3% vs 19.4%; P=0.003; T3MPO-2, 35.8% vs 26.7%; P=0.015), and ≥13 of 26 weeks of treatment (T3MPO-2, 50.2% vs 40.0%; P=0.013) (Table 1; Figure 2).61,62 Also, significantly more tenapanor-treated patients experienced durable improvements in abdominal pain, defined as a reduction of ≥30% in mean weekly abdominal pain score for ≥9 of the first 12 weeks of treatment including ≥3 weeks during weeks 8–12 (T3MPO-1, 29.3% vs 19.4%; P=0.006; T3MPO-2, 34.8% vs 26.7%; P=0.028) (Table 1; Figure 2). At each week of the 12-week T3MPO-1 and 26-week T3MPO-2 trials, tenapanor 50 mg BID led to statistically significant reductions in mean abdominal pain score compared with placebo. Additionally, among 186 patients in the T3MPO-2 trial who received tenapanor for the 26-week treatment period and reported weekly average abdominal pain score at baseline and at week 26, the percentage reporting moderate-to-severe abdominal pain (weekly average pain score of 3–10 on a 0–10 scale) decreased from 100% prior to treatment to 38% after 26 weeks of treatment with tenapanor, with 31% reporting none (weekly average pain score of <1) (Unpublished data). Tenapanor also alleviated other abdominal symptoms experienced by patients with IBS-C such as bloating, discomfort, cramping, and fullness.63
 |
Table 1 Summary of Tenapanor Efficacy Demonstrated in the T3MPO-1 and T3MPO-2 Studies |
 |
Figure 2 Abdominal pain responses in patients with IBS-C participating in the (A) T3MPO-1 and (B) T3MPO-2 studies.61,62 The adjusted RR was based on the ratio of response rates with tenapanor 50 mg BID compared with placebo, stratified by pooled investigator sites using the Cochran–Mantel–Haenszel method. The Cochran–Mantel–Haenszel P value was based on a test of 1 degree of freedom for association between treatment, stratified by pooled investigator sites. An “abdominal pain response” was defined as a decrease in mean weekly worst abdominal pain of ≥30.0% from baseline. A “6/12 week response” was defined as a reduction of ≥30% in mean weekly abdominal pain score for ≥6 of the first 12 weeks of treatment. A “9/12 week response” was defined as a reduction of ≥30% in mean weekly abdominal pain score for ≥9 of the first 12 weeks of treatment. A “13/26 week response” was defined as a reduction of ≥30% in mean weekly abdominal pain score for 13 of 26 weeks of treatment. A “Durable response” was defined as a reduction of ≥30% in mean weekly abdominal pain score for ≥9 of the first 12 weeks of treatment as well as for ≥3 weeks during weeks 8 to 12. (A) Adapted from Chey WD, Lembo AJ, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: A 12-week, placebo-controlled phase 3 trial (T3MPO-1). Am J Gastroenterol. 2020;115(2):281–293 (https://doi.org/10.14309/ajg.0000000000000516).61 (B) Adapted from Chey WD, Lembo AJ, Yang Y, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: A 26-week, placebo-controlled phase 3 trial (T3MPO-2). Am J Gastroenterol. 2021;116(6):1294–1303 (https://doi.org/10.14309/ajg.0000000000001056).62 Abbreviations: BID, twice daily; IBS-C, irritable bowel syndrome with constipation; RR, relative risk. |
Consistent with tenapanor’s action to increase water retention in the intestinal lumen and improve gastrointestinal motility, most treatment-emergent adverse events in the phase 3 studies were gastrointestinal in nature. The most common adverse event reported among tenapanor-treated patients was diarrhea (14.6% in T3MPO-1, 16.0% in T3MPO-2, and 11.1% in T3MPO-3) (Table 2). Diarrhea often occurred within the first 3 weeks of treatment. Most episodes were transient, resolving within 1 week and were of mild or moderate severity.61,62,64 In the clinical trial setting, diarrhea was associated with a low rate (<10%) of treatment discontinuation. Based on the efficacy and safety data derived from T3MPO-1 and −2, tenapanor was approved by the US Food and Drug Administration in 2019 for the treatment of adults with IBS-C.65 In 2022, the American Gastroenterological Association endorsed the use of tenapanor for the treatment of IBS-C, based on the certainty of scientific evidence in its most recent clinical practice guideline update at the time of this writing.14
 |
Table 2 Summary of Tenapanor Safety Demonstrated in the T3MPO-1 and T3MPO-2 Studies |
Conclusion
Abdominal pain is perhaps the most troublesome abdominal symptom experienced by IBS-C patients, significantly impacting healthcare utilization and quality of life.10 The pathophysiology of abdominal pain is believed to be multifactorial, resulting from visceral hypersensitivity, which may be exacerbated by increased intestinal permeability and associated inflammatory responses.19–21,66 Patients with IBS-C also experience other abdominal symptoms, such as bloating, discomfort, cramping, and fullness.12,13 Tenapanor alleviates abdominal symptoms and constipation in patients with IBS-C through a unique and innovative mechanism of action, acting locally to inhibit NHE3, while travelling through the gut lumen until it is excreted rather than being absorbed.61–63 The clinical effects of tenapanor on abdominal pain may be mediated through reducing visceral hypersensitivity by normalizing TRPV1 activation as well as restoring intestinal barrier function, based on results from nonclinical studies.59,60 The use of tenapanor in patients with IBS-C was endorsed by the American Gastroenterological Association based on its efficacy in increasing complete spontaneous bowel movement frequency and improving abdominal pain, while being safe and tolerable with transient, mild-to-moderate diarrhea being the most common adverse event. Visceral hypersensitivity contributes to the underlying etiology of abdominal pain in IBS-C, and treatments targeting the underlying visceral hypersensitivity could bring meaningful relief to patients with IBS-C.
Abbreviations
BID, twice daily; DRG, dorsal root ganglia; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IL-6, interleukin 6; NHE, sodium/hydrogen exchanger; NHE3, sodium/hydrogen exchanger isoform 3; RR, relative risk; TRPV1, transient receptor potential cation channel subfamily V member 1.
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Ashfield MedComms (US), an Inizio company, and was funded by Ardelyx, Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Medical writing and editing support for this review article was funded by Ardelyx, Inc.
Disclosure
Prashant Singh reports no conflicts of interest in this work. Gregory S. Sayuk consulted for AbbVie/Ironwood, Ardelyx, Inc., Regeneron/Sanofi, and Salix and served on speakers bureaus for and received honoraria from AbbVie/Ironwood, Ardelyx, Inc., GI Health Foundation, Regeneron/Sanofi, Rome Foundation, and Salix. David P. Rosenbaum, Kenji Kozuka and Susan Edelstein are employees of Ardelyx, Inc. Lin Chang has served on a scientific advisory board for Ardelyx, Inc. Lin Chang also reports personal fees/grants from Atmo, Arena, Bausch Health, Ironwood, and AbbVie; stock options from Trellus Health, Food Marble, and ModifyHealth, outside the submitted work. In addition, Lin Chang has a patent Ref.: 2018-578-2 (Serial number 63/499,017) issued. These authors report no other conflicts of interest in this work.
References
1. Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396(10263):1675–1688. doi:10.1016/S0140-6736(20)31548-8
2. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. doi:10.1053/j.gastro.2016.02.031
3. Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116(1):17–44. doi:10.14309/ajg.0000000000001036
4. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(10):908–917. doi:10.1016/S2468-1253(20)30217-X
5. Staller K, Olén O, Söderling J, et al. Mortality risk in irritable bowel syndrome: results from a nationwide, prospective cohort study. Am J Gastroenterol. 2020;115(5):746–755. doi:10.14309/ajg.0000000000000573
6. Cassar GE, Youssef GJ, Knowles S, Moulding R, Austin DW. Health-related quality of life in irritable bowel syndrome: a systematic review and meta-analysis. Gastroenterol Nurs. 2020;43(3):E102–E122. doi:10.1097/SGA.0000000000000530
7. Trindade IA, Melchior C, Törnblom H, Simrén M. Quality of life in irritable bowel syndrome: exploring mediating factors through structural equation modelling. J Psychosom Res. 2022;159:110809. doi:10.1016/j.jpsychores.2022.110809
8. Ballou S, McMahon C, Lee HN, et al. Effects of irritable bowel syndrome on daily activities vary among subtypes based on results from the IBS in America survey. Clin Gastroenterol Hepatol. 2019;17(12):2471–2478 e2473. doi:10.1016/j.cgh.2019.08.016
9. Singh P, Staller K, Barshop K, et al. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J Gastroenterol. 2015;21(26):8103–8109. doi:10.3748/wjg.v21.i26.8103
10. Lacy BE, Parikh M, Taylor DCA, et al. Prevalence and impact of abdominal symptoms in patients with IBS-C. Am J Gastroenterol. 2021;116(1):S229–S230. doi:10.14309/01.ajg.0000774524.77992.13
11. Yu V, Ballou S, Hassan R, et al. Abdominal pain and depression, not bowel habits, predict health care utilization in patients with functional bowel disorders. Am J Gastroenterol. 2021;116(8):1720–1726. doi:10.14309/ajg.0000000000001306
12. Heidelbaugh JJ, Stelwagon M, Miller SA, Shea EP, Chey WD. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015;110(4):580–587. doi:10.1038/ajg.2015.67
13. Fehnel SE, Ervin CM, Carson RT, et al. Development of the diary for irritable bowel syndrome symptoms to assess treatment benefit in clinical trials: foundational qualitative research. Value Health. 2017;20(4):618–626. doi:10.1016/j.jval.2016.11.001
14. Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA clinical practice guideline on the pharmacological management of irritable bowel syndrome with constipation. Gastroenterology. 2022;163(1):118–136. doi:10.1053/j.gastro.2022.04.016
15. Mearin F, Ciriza C, Minguez M, et al. Clinical practice guideline: irritable bowel syndrome with constipation and functional constipation in the adult. Rev Esp Enferm Dig. 2016;108(6):332–363. doi:10.17235/reed.2016.4389/2016
16. Schoenfeld PS. Advances in IBS 2016: a review of current and emerging data. Gastroenterol Hepatol. 2016;12(8 Suppl 3):1–11.
17. Quigley EMM, Horn J, Kissous-Hunt M, Crozier RA, Harris LA. Better understanding and recognition of the disconnects, experiences, and needs of patients with irritable bowel syndrome with constipation (BURDEN IBS-C) study: results of an online questionnaire. Adv Ther. 2018;35(7):967–980. doi:10.1007/s12325-018-0733-x
18. Taylor DCA, Abel JL, Martin C, et al. Comprehensive assessment of patients with irritable bowel syndrome with constipation and chronic idiopathic constipation using deterministically linked administrative claims and patient-reported data: the Chronic Constipation and IBS-C Treatment and Outcomes Real-World Research Platform (CONTOR). J Med Econ. 2020;23(10):1072–1083. doi:10.1080/13696998.2020.1799816
19. Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1–mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150(4):875–887. doi:10.1053/j.gastro.2015.12.034
20. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G775–G785. doi:10.1152/ajpgi.00155.2012
21. Zhang L, Song J, Hou X. Mast cells and irritable bowel syndrome: from the bench to the bedside. J Neurogastroenterol Motil. 2016;22(2):181–192. doi:10.5056/jnm15137
22. Zhou Q, Zhang B, Verne NG. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146(1):41–46. doi:10.1016/j.pain.2009.06.017
23. Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201. doi:10.1136/gut.2007.140806
24. Buhner S, Braak B, Li Q, et al. Neuronal activation by mucosal biopsy supernatants from irritable bowel syndrome patients is linked to visceral sensitivity. Exp Physiol. 2014;99(10):1299–1311. doi:10.1113/expphysiol.2014.080036
25. Deiteren A, de Wit A, van der Linden L, De Man JG, Pelckmans PA, De Winter BY. Irritable bowel syndrome and visceral hypersensitivity: risk factors and pathophysiological mechanisms. Acta Gastroenterol Belg. 2016;79(1):29–38.
26. Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The role of visceral hypersensitivity in irritable bowel syndrome: pharmacological targets and novel treatments. J Neurogastroenterol Motil. 2016;22(4):558–574. doi:10.5056/jnm16001
27. Bradette M, Delvaux M, Staumont G, Fioramonti J, Bueno L, Frexinos J. Evaluation of colonic sensory thresholds in IBS patients using a barostat: definition of optimal conditions and comparison with healthy subjects. Dig Dis Sci. 1994;39(3):449–457. doi:10.1007/BF02088327
28. Kuiken SD, Lindeboom R, Tytgat GN, Boeckxstaens GE. Relationship between symptoms and hypersensitivity to rectal distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22(2):157–164. doi:10.1111/j.1365-2036.2005.02524.x
29. Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109(1):40–52. doi:10.1016/0016-5085(95)90267-8
30. Roberts C, Albusoda A, Farmer AD, Aziz Q. Factors influencing rectal hypersensitivity in irritable bowel syndrome: a systematic review and meta‐analysis. Neurogastroenterol Motil. 2023;35(4):e14515. doi:10.1111/nmo.14515
31. Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–1192. doi:10.1016/0016-5085(90)90332-U
32. Mayer EA, Labus J, Aziz Q, et al. Role of brain imaging in disorders of brain–gut interaction: a Rome Working Team Report. Gut. 2019;68(9):1701–1715. doi:10.1136/gutjnl-2019-318308
33. Barbara G, Cremon C, De Giorgio R, et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Current Gastroenterol Reports. 2011;13(4):308–315. doi:10.1007/s11894-011-0195-7
34. Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi:10.1136/gut.2007.138982
35. Yiangou Y, Facer P, Dyer NHC, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357(9265):1338–1339. doi:10.1016/S0140-6736(00)04503-7
36. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi:10.1038/39807
37. Jones RCW, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25(47):10981–10989. doi:10.1523/JNEUROSCI.0703-05.2005
38. Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132(2):615–627. doi:10.1053/j.gastro.2006.11.014
39. Wiskur BJ, Tyler K, Campbell-Dittmeyer K, Chaplan SR, Wickenden AD, Greenwood-Van Meerveld B. A novel TRPV1 receptor antagonist JNJ-17203212 attenuates colonic hypersensitivity in rats. Methods Find Exp Clin Pharmacol. 2010;32(8):557–564. doi:10.1358/mf.2010.32.8.1507853
40. Keszthelyi D, Troost FJ, Jonkers DM, et al. Alterations in mucosal neuropeptides in patients with irritable bowel syndrome and ulcerative colitis in remission: a role in pain symptom generation? Eur J Pain. 2013;17(9):1299–1306. doi:10.1002/j.1532-2149.2013.00309.x
41. Akbar A, Yiangou Y, Facer P, et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59(6):767–774. doi:10.1136/gut.2009.194449
42. Crouzet L, Gaultier E, Del’Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25(4):e272–e282. doi:10.1111/nmo.12103
43. Buhner S, Li Q, Berger T, et al. Submucous rather than myenteric neurons are activated by mucosal biopsy supernatants from irritable bowel syndrome patients. Neurogastroenterol Motil. 2012;24(12):1134–e1572. doi:10.1111/nmo.12011
44. Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137(4):1425–1434. doi:10.1053/j.gastro.2009.07.005
45. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi:10.1053/j.gastro.2003.11.055
46. Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. doi:10.1053/j.gastro.2006.11.039
47. Grabauskas G, Wu X, Gao J, Li JY, Turgeon DK, Owyang C. Prostaglandin E(2), produced by mast cells in colon tissues from patients with irritable bowel syndrome, contributes to visceral hypersensitivity in mice. Gastroenterology. 2020;158(8):2195–2207 e2196. doi:10.1053/j.gastro.2020.02.022
48. Lyons DO, Pullen NA. Beyond IgE: alternative mast cell activation across different disease states. Int J Mol Sci. 2020;21(4):1498. doi:10.3390/ijms21041498
49. Bashashati M, Moradi M, Sarosiek I. Interleukin-6 in irritable bowel syndrome: a systematic review and meta-analysis of IL-6 (-G174C) and circulating IL-6 levels. Cytokine. 2017;99:132–138. doi:10.1016/j.cyto.2017.08.017
50. Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin‐6 and corticotropin‐releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592(23):5235–5250. doi:10.1113/jphysiol.2014.279968
51. O’Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G241–G252. doi:10.1152/ajpgi.00385.2010
52. Johansson S, Rosenbaum DP, Knutsson M, Leonsson-Zachrisson M. A Phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol. 2017;21(3):407–416. doi:10.1007/s10157-016-1302-8
53. Rosenbaum DP, Yan A, Jacobs JW. Pharmacodynamics, safety, and tolerability of the NHE3 inhibitor tenapanor: two trials in healthy volunteers. Clin Drug Investig. 2018;38(4):341–351. doi:10.1007/s40261-017-0614-0
54. Spencer AG, Labonte ED, Rosenbaum DP, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6(227):227ra236. doi:10.1126/scitranslmed.3007790
55. Nikolovska K, Seidler UE, Stock C. The role of plasma membrane sodium/hydrogen exchangers in gastrointestinal functions: proliferation and differentiation, fluid/electrolyte transport and barrier integrity. Front Physiol. 2022;13:899286. doi:10.3389/fphys.2022.899286
56. Gawenis LR, Stien X, Shull GE, et al. Intestinal NaCl transport in NHE2 and NHE3 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282(5):G776–G784. doi:10.1152/ajpgi.00297.2001
57. King AJ, Chang L, Li Q et al. (2024). NHE3 inhibitor tenapanor maintains intestinal barrier function, decreases visceral hypersensitivity, and attenuates TRPV1 signaling in colonic sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2024. doi:10.1152/ajpgi.00233.2023
58. King AJ, Siegel M, He Y, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci Transl Med. 2018;10(456):eaam6474. doi:10.1126/scitranslmed.aam6474
59. Li Q, King AJ, Liu L, Zhu Y, Caldwell JS, Pasricha PJ. Tenapanor reduces IBS pain through inhibition of TRPV1-dependent neuronal hyperexcitability in vivo. Am J Gastroenterol. 2017;112:S255. doi:10.14309/00000434-201710001-00484
60. Wang J, Larauche M, Siegel M, et al. Tenapanor attenuates increased macromolecule permeability in human colon monolayer cultures induced by inflammatory cytokines and human fecal supernatants. Gastroenterology. 2018;154(Suppl 1):S326. doi:10.1016/S0016-5085(18)31424-0
61. Chey WD, Lembo AJ, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: a 12-week, placebo-controlled phase 3 trial (T3MPO-1). Am J Gastroenterol. 2020;115(2):281–293. doi:10.14309/ajg.0000000000000516
62. Chey WD, Lembo AJ, Yang Y, Rosenbaum DP. Efficacy of tenapanor in treating patients with irritable bowel syndrome with constipation: a 26-week, placebo-controlled phase 3 trial (T3MPO-2). Am J Gastroenterol. 2021;116(6):1294–1303. doi:10.14309/ajg.0000000000001056
63. Lembo AJ, Chey WD, Harris LA, et al. Abdominal symptom improvement during clinical trials of tenapanor in patients with irritable bowel syndrome with constipation: a post hoc analysis. Am J Gastroenterol. 2024. doi:10.14309/ajg.0000000000002685
64. Lembo AJ, Friedenberg KA, Fogel RP, et al. Long-term safety of tenapanor in patients with irritable bowel syndrome with constipation in the T3MPO −3 study. Neurogastroenterol Motil. 2023;35(11):e14658. doi:10.1111/nmo.14658
65. IBSRELA (Tenapanor) Tablets [Prescribing Information]. Waltham, MA: Ardelyx, Inc.; 2022.
66. Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2(1):16014. doi:10.1038/nrdp.2016.14
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
