Back to Journals » Pathology and Laboratory Medicine International » Volume 15
A Unique Pleomorphic Adenoma in the Minor Salivary Glands of the Upper Lip: A Case Report
Authors Alrehaili J
Received 7 May 2023
Accepted for publication 19 July 2023
Published 21 July 2023 Volume 2023:15 Pages 37—41
DOI https://doi.org/10.2147/PLMI.S415283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Paul Zhang
Jihad Alrehaili
Department of Pathology, College of Medicine, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, Saudi Arabia
Correspondence: Jihad Alrehaili, Tel +966-112037100, Email [email protected]
Abstract: Pleomorphic adenoma (PA) is a common benign tumor of the salivary glands, but its occurrence in the minor salivary glands of the upper lip is rare. In the a case report of a 24-year-old female with PA of the minor salivary glands in the upper lip, exhibiting unique histological characteristics such as features resembling schwannoma, keratinization, myxolipomatous, oncocytic, and mucinous traits resembling mucoepidermoid carcinoma. The main symptom of this condition is a gradual painless enlargement of the affected area. Histopathological analysis confirmed the diagnosis of PA. This case highlights the importance of public education in recognizing and treating painless masses. Further research is needed to explore PA’s histological and molecular characteristics to establish prognostic factors such as the risk of recurrence.
Keywords: pleomorphic adenoma, salivary glands, upper lip, painless mass
Introduction
Pleomorphic adenoma (PA) is a common benign neoplasm of the salivary glands that arises from the proliferation of parenchymatous glandular cells with myoepithelial components in response to the neoplastic process in the glands.1 Although PA exhibits distinctive histological features, it can sometimes be mistaken for malignancy due to its ability to mimic invasion, display atypical or metaplastic cytomorphology, and present morphological features that resemble those of established salivary gland carcinomas.2 The detection of PA poses several challenges due to these factors. PA typically presents as a slow-growing, painless, nodular mass commonly affecting the parotid or submandibular, sublingual, and minor salivary glands.3,4 In many cases, PA goes unnoticed by patients because it is painless. Delay in medical treatment can lead to enlargement of the tumor, making surgical removal more difficult and increasing the risk of recurrence.
PA accounts for 60–65% of all major and minor salivary gland tumors and has a higher incidence in women, with a ratio ranging from 2:1 to 3.2:1, and prevalence in the fifth to seventh decades of life.5,6 PA most frequently affects the palate (60%) and the upper lip (10–20%) but can also occur at other sites, including the buccal mucosa, floor of the mouth, retromolar area, tongue, upper gingival sulcus, nasal cavity, and paranasal sinuses.7–9 Histologically, PA is characterized by cells similar to pleomorphic epithelial and mesenchymal, including ductal and non-ductal epithelial cells and chondroid, myxoid, and osseous mesenchyme-like tissues.10 The etiopathogenesis of PA remains unclear, but molecular studies suggest chromosomal abnormalities at 8q12 and 12q15 in the epithelial tumor.11
Upon histological examination, PA is characterized by a diverse array of epithelial cells arranged in a cord-like pattern and may exhibit features such as squamous differentiation, plasmacytoid appearance, and myoepithelial cells, which contribute to the abundant extra-cellular matrix composed of cartilage, collagen, mucoid, and osseous stroma.12 Histological diagnosis of PA (benign mixed tumors) can be challenging. These lesions may exhibit features suggestive of malignancy, such as capsular infiltration, hypercellularity, cell atypia, necrosis, and vascular invasion.13 In order to prevent complications and ensure the best possible outcome for a patient suffering from PA, early detection and prompt treatment are crucial. This report presents a case of PA on the upper lip, a common location for this salivary neoplasm.
Case Report
A 24-year-old female patient presented to the Outpatient Department (OPD) with a painless lump and progressive swelling of the right upper lip, which had been present for the last 18 months. The patient’s medical history was unremarkable, no history of trauma, genetic disorders, or other abnormalities was found during clinical examination, and no medication was taken. A firm and well-defined swelling were found on the right side of the upper lip, which was freely moving, non-cystic, and rubbery in consistency but not tender, and no signs of inflammation were observed. The lesion was surgically excised without compromising the surrounding tissues, and the sample was sent to the pathology laboratory for histopathological analysis. The excised specimen was received in formalin, labeled “Right upper lip swelling”, and comprised a piece of encapsulated tan-pinkish tissue measuring 0.5×0.4 x 0.3 cm. The specimen was submitted entirely in one cassette without compromising the surrounding tissues. Subsequently, the patient was discharged and attended monthly follow-ups for 12 months to monitor for signs of recurrence or other complications. The present case did not show evidence of local recurrence 12 months after surgery.
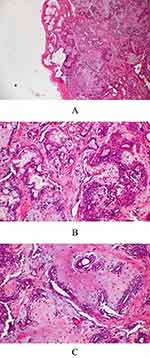
The excised specimen was subjected to histopathological examination, which revealed a well-encapsulated epithelial salivary gland tumor. The tumor comprised an island and sheets of plasmacytoid cells arranged in duct-like structures, with irregular tubules and patterned strands of benign neoplastic cells surrounded by chondromyxoid stroma. Some cystic areas are identified, with variable stroma exhibiting fibrous, chondroid, and myxoid appearance (Figure 1A-C). Based on these histological features, a diagnosis of PA was established.
Discussion
Despite advancements in the field, the progression rate of PA still needs to be better understood. PA is typically a slow-growing, painless tumor; however, a few cases have been reported in which rapid growth has occurred, especially in the palate. Due to its rarity, caution is necessary when diagnosing a PA on the lip.14 Similar clinical manifestations were observed in the current case, which presented with an upper lip lesion. Recent studies have shown a remarkably high prevalence of PA in the upper lip compared to the lower lip, with a ratio of 6:1.15 In a study of 75 patients with small intraoral salivary gland tumors, 15 of the 29 benign tumors were in the palatal region, with only four on the upper lip and one on the lower lip.11 Benign tumors occur on the upper lip, while malignant lesions usually occur on the lower lip.11 Differences in the development of lesions in the upper and lower lips may be attributed to differences in embryonic development stages. The most effective treatment for minor salivary gland tumors is surgical removal, with a margin of normal tissue surrounding cancer excised by at least 1 to 2 cm if malignant.16 In the current study, our female patient from Saudi possessed a tumor on the upper lip with a rare neoplasm. A similar condition was reported in a male patient (33 yrs) from Nigeria, with painless swelling on the right side of the upper lip.17
However, long-term patient follow-up is crucial to reduce the risk of late recurrence since salivary gland tumors can recur after a considerable period. It is recommended that patients are monitored for at least five years after the initial surgery. A retrospective study by McGregor et al revealed that of 31 patients under 30 years of age treated for PA, 42% (n = 16) experienced tumor recurrence after initial treatment, all of which occurred in the parotid gland.18 In another retrospective study of 392 patients, a ratio of 2.19: 1 of malignancies was observed, where 68.2% were benign.5 Other studies suggest a higher risk of recurrence in patients diagnosed with PA before age 30.19 Wittekindt et al analyzed 108 consecutive patients and found that the risk of clinically evident recurrence of pleomorphic adenoma after 1, 5, and 15 years of follow-up was 16%, 42%, and 75% of the participants, respectively.20 The most common explanation for PA recurrence is thought to be related to pathologies, such as capsule thickness or absence of a capsule.21,22 If the capsule ruptures or an incomplete removal is performed during surgery, residual tumor cells will remain, leading to recurrence. This case report adds to the literature and contributes to developing clinical guidelines for diagnosing and treating PA. The study’s main limitation is the incongruity observed in the imaging examinations of the patients. Magnetic resonance imaging (MRI), ultrasound (US), and computed tomography (CT) are commonly recommended diagnostic tools to accurately determine the size and location of a tumor in patients with PA.
Conclusion
As PA is usually asymptomatic, patients may not be aware of its presence, making diagnosis challenging, especially when distinguishing it from inflammatory jaw lesions and oral tumors that exhibit overlapping clinical signs and radiographic characteristics. However, a definitive diagnosis can only be achieved through histopathological examination. Total surgical excision is recommended as the definitive treatment for PA, with an excellent prognosis. However, patients should be closely monitored over an extended period due to the risk of recurrence and the possibility of malignant transformation.
Informed Consent Statement
The author certify that he has obtained appropriate patient consent form. In the form the patient has given her consent for images and other clinical information to be reported in the journal. The patients understand that her name and initial will not be published, and due efforts will be made to conceal her identity. And author declare there is no ethical issue.
Author Contributions
Author contributed significantly to the work that was published, whether it be in the ideation, study design, execution, data collection, analysis, and interpretation, or in each of these areas. He also participated in the writing, editing, and critical review of the article, gave their final approval for the version that would be published, decided on the journal to which the article would be submitted, and agreed to be held accountable for all aspects of the work.
Funding
This study has not received any external funding.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Matsumiya-Matsumoto Y, Morita Y, Uzawa N. Pleomorphic adenoma of the salivary glands and epithelial-mesenchymal transition. J Clin Med. 2022;11(14):4210.
2. Hernandez‐Prera JC, Skálová A, Franchi A, et al. Pleomorphic adenoma: the great mimicker of malignancy. Histopathology. 2021;79(3):279–290.
3. Buchner A, Merrell PW, Carpenter WM. Relative frequency of intra-oral minor salivary gland tumors: a study of 380 cases from northern California and comparison to reports from other parts of the world. J Oral Pathol & Med. 2007;36(4):207–214.
4. Bhatia JSS. Pleomorphic adenoma of upper lip: a rare case presentation. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 1):755–758.
5. Shishegar M, Ashraf MJ, Azarpira N, Khademi B, Hashemi B, Ashrafi A. Salivary gland tumors in maxillofacial region: a retrospective study of 130 cases in a southern Iranian population. Patholog Res Int. 2011;2011:934350.
6. Ali I, Gupta AK, Singh S. Pleomorphic adenoma of the upper lip. Natl J Maxillofac Surg. 2011;2(2):219–221.
7. Kataria SP, Tanwar P, Sethi D, Garg M. Pleomorphic adenoma of the upper lip. J Cutan Aesthet Surg. 2011;4(3):217–219.
8. Sharma Y, Maria A, Chhabria A. Pleomorphic adenoma of the palate. Natl J Maxillofac Surg. 2011;2(2):169–171.
9. Sireesha K, Anuradha A, Srinivas GV, et al. Minor salivary gland pleomorphic adenoma: an inconceivable diagnosis in a 62 year old female. Int J Res Rev. 2020;7(6):356–359.
10. Singh A, Phulware RH, Ahuja A, Gupta A, Kaushal M. Pleomorphic Adenoma with Extensive Squamous and Adipocytic Metaplasia Mimicking as Low Grade Mucoepidermoid Carcinoma on FNAC. Indian J Otolaryngol Head Neck Surg. 2022;74(Suppl 2):2132–2135.
11. Taiwo AO, Akinshipo A, Braimah RO, Ibikunle AA. Pleomorphic Adenoma of the Upper Lip: a Case Report. Saudi J Med Med Sci. 2018;6(1):32–35. doi:10.4103/sjmms.sjmms_109_16
12. Seifert G, Brocheriou C, Cardesa A, Eveson JW. WHO international histological classification of tumours tentative histological classification of salivary gland tumours. Pathol - Res Pract. 1990;186(5):555–581.
13. Skalova A, Altemani A, Di Palma S, et al. Pleomorphic adenoma of the salivary glands with intravascular tumor deposits. Am J Surg Pathol. 2012;36(11):1674–1682.
14. Jaber MA. Intraoral minor salivary gland tumors: a review of 75 cases in a Libyan population. Int J Oral Maxillofac Surg. 2006;35(2):150–154.
15. Shrestha A, Reddy NS, Ganguly SN. Pleomorphic adenoma of the upper lip: a case report. J Coll Med Sci. 1970;6(1):51–53.
16. Thompson LDR, Bauer JL, Chiosea S, et al. Canalicular adenoma: a clinicopathologic and immunohistochemical analysis of 67 cases with a review of the literature. Head Neck Pathol. 2015;9(2):181–195.
17. Owens OT, Calcaterra TC. Salivary gland tumors of the lip. Arch Otolaryngol. 1982;108(1):45–47.
18. McGregor AD, Burgoyne M, Tan KC. Recurrent pleomorphic salivary adenoma—the relevance of age at first presentation. Br J Plast Surg. 1988;41(2):177–181.
19. Moonis G, Patel P, Koshkareva Y, Newman J, Loevner LA. Imaging characteristics of recurrent pleomorphic adenoma of the parotid gland. AJNR Am J Neuroradiol. 2007;28(8):1532–1536.
20. Wittekindt C, Streubel K, Arnold G, Stennert E, Guntinas-Lichius O. Recurrent pleomorphic adenoma of the parotid gland: analysis of 108 consecutive patients. Head Neck. 2007;29(9):822–828.
21. Stennert E, Guntinas-Lichius O, Klussmann JP, Arnold G. Histopathology of pleomorphic adenoma in the parotid gland: a prospective unselected series of 100 cases. Laryngoscope. 2001;111(12):2195–2200.
22. Dulguerov P, Todic J, Pusztaszeri M, Alotaibi NH. Why do parotid pleomorphic adenomas recur? A systematic review of pathological and surgical variables. Front Surg. 2017;4:26.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.