Back to Journals » International Medical Case Reports Journal » Volume 17
A Rare Manifestation of Secondary Hyperparathyroidism Due to Brown Tumors: A Case Report
Authors Boudina M , Zisimopoulou E, Rakitzi P, Barbanis S, Syndouka E, Zouli C, Fotiadou A, Stamati MS, Balodimou C, Christantoniou G, Chrisoulidou A
Received 25 August 2023
Accepted for publication 16 January 2024
Published 26 February 2024 Volume 2024:17 Pages 143—147
DOI https://doi.org/10.2147/IMCRJ.S437191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Maria Boudina,1 Eleana Zisimopoulou,1 Pantelitsa Rakitzi,1 Sotirios Barbanis,2 Eleni Syndouka,3 Chrysanthi Zouli,1 Aimilia Fotiadou,1 Mariana Stamati Stamati,1 Chrysanthi Balodimou,1 George Christantoniou,1 Alexandra Chrisoulidou1
1Department of Endocrinology, Theagenio Cancer Hospital, Thessaloniki, Greece; 2Department of Pathology, Theagenio Cancer Hospital, Thessaloniki, Greece; 3Department of Radiology, Theagenio Cancer Hospital, Thessaloniki, Greece
Correspondence: Maria Boudina, Department of Endocrinology, Theagenio Cancer Hospital, Thessaloniki, Greece, Email [email protected]
Purpose: Brown tumors, also known as cystic fibrosa, are rare, benign, osteolytic, fibrotic lesions of the bones that occur secondary to hyperparathyroidism. They are caused by increased osteoclastic activity leading to an abnormal bone metabolism.
Case Description: Here, we present the case of a 58-year-old male, who presented with painful bony lesions, initially attributed to metastatic disease. After biochemical workout, imaging and biopsy, the nature of the lesions was revealed. We discuss the differential diagnosis and clinical management of the disease.
Conclusion: Patients with brown tumors should be assessed in the differential diagnosis of bony lesions and should always be tested for hyperparathyroidism. An early diagnosis is crucial for the successful treatment of such patients.
Keywords: brown tumor, hyperparathyroidism, chronic kidney disease, case report
Introduction
Hyperparathyroidism (HP) is a disorder in which there is an excess of parathyroid hormone (PTH) secretion. It can be classified, according to the origin of defect, into primary, secondary and tertiary.1 Chronic renal failure is a common cause of secondary HP, triggered by hypocalcaemia, hyperphosphatemia or decreased active vitamin D. The prolonged stimulatory effect of parathyroid hormone on the skeleton causes increased bone turnover, resulting in resorption and replacement of the bone with fibrotic tissue, an entity called brown tumors.2 The underlying pathophysiology has become more complex recently, due to the progressive awareness of the important role of fibroblast growth factor 23 (FGF23), α—klotho, as well as the Wnt-b-catenin signaling pathway. Firstly, during the early stages of chronic kidney disease, there is low bone turnover, due to resistance to the action of PTH, reduced calcitriol levels, malnutrition and uremia. High bone turnover disease (osteitis fibrosa) occurs only later on, when increased PTH levels are able to overcome skeletal PTH resistance, leading to the formation of brown tumors.3 Thus, big lesions appear, anywhere in the skeleton. The commonest locations of the lesions are mandible, clavicle, ribs and pelvis. They are rarely seen in clinical practice nowadays, due to the early detection of parathyroid abnormalities in biochemical laboratory tests. Brown tumors have been reported to occur in 1.5% to 4.5% of patients with primary or secondary HP.4,5 Patients with brown tumors should be assessed in the differential diagnosis of bony lesions and should always be tested for HP. An early diagnosis is crucial for the successful treatment of such patients.
Case Report
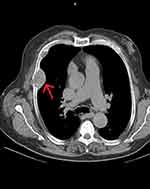
A 58-year-old male was referred to the primary care because of pleurodynia. He was an obese smoker with a heavy medical history of hypertension, chronic kidney disease stage III, obstructive pulmonary disease, heart failure and atrial fibrillation. He reported a two-year history of hyperthyroidism treated with methimazole. The patient underwent a thorax CT, which revealed an extended goiter and extra pulmonary masses infiltrating the thoracic cage and the ribs (Figure 1). The masses were considered metastatic in origin, and the patient was referred to the oncology department of the hospital for additional imaging and staging. Bone scintigraphy indicated increased uptake in left scapula, ribs, left ileum and tibia. However, no primary tumor was identified from thorax, abdomen and brain CT. Biochemistry at the time revealed a correction for albumin calcium of 9.2 mg/dl (8.4–10.1), phosphorus at 2.38 mg/dl (2.5–4.5) and alkaline phosphatase 580 IU/L (30–120). The patient was then referred for an endocrine assessment. He was found to be clinically and biochemically euthyroid. Additional blood tests showed high PTH levels at 1184 pg/mL (15–65) and a mild vitamin D3 deficiency (17.3 ng/mL). The renal function was impaired (eGFR 50 – Stage 3A). Patient’s characteristics are presented in Table 1.
 |
Table 1 Patient’s Characteristics |
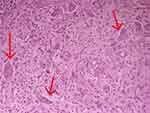
Taking into consideration the laboratory results, the diagnosis of secondary HP was made. Since imaging was inconclusive about the nature of bony lesions, it was decided to undergo a bone biopsy. The examined specimen revealed a morphologically bland fibroblastic spindle cell proliferation admixed with numerous osteoclastic giant cells. According to pathology, despite the absence of evident hemorrhage, in context, the appearance of the lesion was suggestive of bone changes due to HP. Therefore, the diagnosis of brown tumors was confirmed by histological examination (Figure 2).
The patient received supplementation with calcium, vitamin D, vitamin D analogues and cinacalcet. There was a significant improvement in the laboratory tests with a striking reduction of PTH levels at 103 pg/mL. Moreover, 18 months after the initiation of the treatment, a thorax CT was repeated and revealed a regression of the tumors in the thoracic cage (4.7x3.9 to 4.3 × 2.8 cm) and the ribs. The lesions appeared well defined and ossification had started (Figure 3).
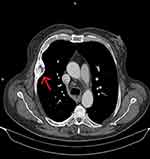 |
Figure 3 Thorax CT after i.v contrast, 18 months post treatment showed regression of the tumor in the thoracic cage (from 4.7×3.9 to 4.3×2.8 cm) and the ribs, as shown with the red arrow. |
Discussion
The prevalence of HP has increased over the latest years because of the early detection of biochemical abnormalities on routine tests. However, in some cases, it remains under-diagnosed and physicians have to recognize and treat the underlying complications, before addressing the primary pathology.6
Brown tumors represent a late event in untreated hyperparathyroidism. These tumors appear more often in primary HP, although they are also seen in secondary and tertiary HP.7,8 Clinically, a brown tumor presents as a slowly growing palpable mass and may cause bone pain or pathological fracture. Nonspecific general clinical features, such as weakness, polyuria, weight loss and recurrent nephrolithiasis, may also be associated with the accompanying HP. However, the suspicion of a brown tumor is based on radiological features either on plain film, computed tomography or MRI. If radiology is doubtful, as was in our case, biopsy of the bone tumor is recommended for the final diagnosis.9
The formation of brown tumors is the result of intense osteoclastic activity, fibroblast proliferation and bone resorption.10,11 The term is used because of reddish brown color caused by its vascularity, hemorrhage and deposits of hemosiderin.12 Once brown tumors are diagnosed, the correction of the underlying endocrine disorder is the treatment of choice, leading to their regression.13 In cases of primary HP, radiological and scintigraphy investigation is suggested, and surgical removal of the parathyroid adenoma will lead to regression of symptoms and signs of the disease. In cases of secondary HP, identification of the underlying disorder, most commonly vitamin D deficiency or chronic renal failure, will be necessary. In any case, the optimal treatment aims at the reduction of PTH levels and improvement of phosphorus levels.
The differential diagnosis of a lytic bone tumor, such as a brown tumor, includes bone metastasis or myeloma, simple bone cyst, infection (osteomyelitis) or infarction (bone infarction), eosinophilic granuloma, osteoblastoma, chondroblastoma or non-ossifying fibroma14,15 (Box 1). In our case, the patient presented with painful bone lesions that initially were considered bone metastases, which represents the main differential problem in these cases. Thorough medical history and clinical examination are mandatory to reveal signs and symptoms, in addition to biochemical, hormonal, radiologic and scintigraphic evaluation in these patients. Differential diagnosis is important, as it will define the treatment of choice and the overall prognosis of the patient.
 |
Box 1 Differential Diagnosis of Bone Lesions |
Despite the rarity of the disease, clinicians should include brown tumors in the differential diagnosis of bony lesions and should always test for HP. Nowadays, due to high accessibility of diagnostic tools and especially biochemical tests, high PTH levels should be further assessed, in order to classify the hyperparathyroidism and be alert to its complications. A high index of suspicion will lead to an early diagnosis. More case studies are needed in order to differentiate bone lesions.
Ethical Approval and Consent for Publication
Institutional Review board (IRB) approval for this study was not required. The patient gave his consent for the publication of information related to his medical condition.
Funding
There is no funding to report.
Disclosure
The authors declare they have no competing interest for this study.
References
1. Dawale K, Agrawal A. Parathyroid hormone secretion and related syndromes. Cureus. 2022;14(10). doi:10.7759/cureus.30251
2. Muppidi V, Meegada SR, Rehman A. Secondary Hyperparathyroidism. StatPearls; 2023.
3. Drüeke TB. Hyperparathyroidism in chronic kidney disease. Endotext. 2021;2021:1.
4. Lessa MM, Sakae FA, Tsuji RK, et al. Brown tumor of the facial bones: case report and literature review. Ear Nose Throat J. 2005;84:432–434. doi:10.1177/014556130508400714
5. Pappu R, Jabbour SA, Reginato AM, Reginato AJ. Musculoskeletal manifestations of primary hyperparathyroidism. Clin Rheumatol. 2016;35(12):3081–3087. doi:10.1007/s10067-016-3450-3
6. Saliba W, El-Haddad B. Secondary hyperparathyroidism: pathophysiology and treatment. J Am Board Fam Med. 2009;22(5):574–581. doi:10.3122/jabfm.2009.05.090026
7. Flores R, Lopes J, Caridade S. Secondary hyperparathyroidism presenting as a brown tumor: a case report and review of the literature. Cureus. 2023;15:1.
8. Engur CO, Ones T, Filizoglu N, Kesim S, Ozguven S. Brown tumors secondary to tertiary hyperparathyroidism masquerading as lytic or sclerotic skeletal metastases on preoperative/postoperative 18f-fluorodeoxyglucose positron emission tomography/computed tomography: a case report. Indian J Nucl Med. 2022;37(3):288–289. doi:10.4103/ijnm.ijnm_195_21
9. Xie C, Tsakok M, Taylor N, Partington K. Imaging of brown tumours: a pictorial review. Insights Imaging. 2019;10(1):75. doi:10.1186/s13244-019-0757-z
10. Meydan N, Barutca S, Guney E, et al. Brown tumors mimicking bone metastases. Natl Med Assoc. 2006;98(6):9502.
11. Kalambokis G, Economou G, Kamina S, Papachristou DJ, Bai M, Tsianos EV. Multiple brown tumors of the ribs simulating malignancy. J Endocrinol Invest. 2005;28:738–740. doi:10.1007/BF03347558
12. Ashrafi SKA, Suhail Z, Khambaty Y. Brown tumor of maxilla – a rare occurrence. Pak J Otolaryngol. 2010;26:61–62.
13. Salamone D, Muresan S, Muresan M, Neagoe R. Multilevel brown tumors of the spine in a patient with severe secondary hyperparathyroidism. A case report and review of the literature. Ann Ital Chir. 2016;87:1.
14. Silva E, Ferreira R, Lourenço MH, Marques B, Duarte S. Multiple brown tumors secondary to parathyroid carcinoma: a challenging diagnosis. Cureus. 2022;14(11).
15. Rossi B, Ferraresi V, Appetecchia MA, Novello M, Zoccali C. Giant cell tumor of bone in a patient with diagnosis of primary hyperparathyroidism: a challenge in differential diagnosis with brown tumor. Skeletal Radiol. 2014;43:693–697. doi:10.1007/s00256-013-1770-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.