Back to Journals » International Journal of General Medicine » Volume 17
A Cross-Sectional Evaluation of Knowledge of Medicine Safety and Frequency of Reading Medication Leaflets and Its Predictors – Insights from Saudi Adults in Riyadh, Saudi Arabia
Authors Alghamdi A, Qadhi OA, Syed W, Samarkandi OA, Basil A Al-Rawi M
Received 6 November 2023
Accepted for publication 10 January 2024
Published 19 January 2024 Volume 2024:17 Pages 175—186
DOI https://doi.org/10.2147/IJGM.S446041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Mihajlo Jakovljevic
Alya Alghamdi,1 Omaimah A Qadhi,2 Wajid Syed,3 Osama A Samarkandi,4 Mahmood Basil A Al-Rawi5
1Department of Community and Mental Health, College of Nursing, Riyadh, 11451, Saudi Arabia; 2Department of Medical-Surgical College of Nursing, King Saud University, Riyadh, 11451, Saudi Arabia; 3Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia; 4Department of Basic Sciences, Prince Sultan College for Emergency Medical Services, King Saud University, Riyadh, 11466, Saudi Arabia; 5Department of Optometry, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
Correspondence: Wajid Syed, Department of Clinical Pharmacy, College of Pharmacy, King Saud University, Riyadh, 11451, Saudi Arabia, Email [email protected]
Background and Aim: On the other hand, patient safety, is of the utmost importance, in addition to health care providers’ counseling and advice, patient information leaflets (PILs) also have a significant impact on health outcomes. This study aimed to assess the Knowledge of medicine safety and Frequency of reading medication Leaflets and their predictors among the Saudi population in Riyadh Saudi Arabia.
Methods and Materials: This cross-sectional study was conducted over three months in 2023 using online structured self-administered questionnaires, on the adults of Saudi Arabia, admitted to provide informed consent, aged ≥ 18 years, able to complete the questionnaires, being Saudi nationals, and currently living in the country. The data analysis was performed using the Statistical Package for Social Science (SPSS) version 27.
Results: In this study, 23.6% (n=123) of respondents always read the PILs, while 14.6% (n=76) of them never read the PILs. In addition, 22.6% (n=118) of them find it difficult to understand the PILs. In this study, 53.9% of them revealed that ADRs are unexpected reactions after taking the normal dose and 15.4% of them do not know what ADRs are. The respondents gender (B= 0.164; SE=0.075; 95CI = 0.017– 0.310; p = 0.029), Social status (B = 0.251; SE = 0.079; 95CI = 0.096– 0.406; p = 0.002) and monthly income (B= 0.136; SE = 0.021; 95CI = 0.095– 0.178; p < 0.001) was the predictor of reading PILs.
Conclusion: In this study, two-thirds of participants took medication and knew why they were taking it. However, only a few of them took the medicine after seeing a doctor, suggesting self-medication practice. In addition, 22.1% of them find it difficult to understand the PILS, and a small number of them would rather read it. Highlighting the significance of seeking medical advice from healthcare professionals before using a medication.
Keywords: medication leaflets, package inserts, knowledge, medicine safety, adverse drug reactions, attitudes, Saudi Arabia
A Letter to the Editor has been published for this article.
Introduction
Medication use is increasing in everyday life, regardless of gender or other characteristics, and accounts for 20% to 60% of healthcare spending in low- and middle-income countries.1,2 According to data from the World Health Organization (WHO), nearly half of patients misuse the prescription drugs they are given, and over half of all medications provided worldwide are administered or prescribed inappropriately, resulting in increasing morbidity and mortality.2 Drug and medication manufacturers give patients leaflets to inform them about the medications they may use and help them prevent medication-related side effects.3–5
Written information regarding the drug is provided by manufacturers in the form of patient information leaflets (PILs), which are paper documents that are enclosed with every medicine package.3–5 Patient information leaflets sometimes referred to as package information leaflets, consumer medicines information, and package inserts.4,5 PILs are supplied by the manufacturer with a template that is uniform and contains the same kinds of data for each drug.3,6 PILs are pieces of standardized written information about the safety, dosage, adverse reactions, drug interactions, and many more things about the specific drug and its effective use of a medication or over-the-counter drugs dispensed by pharmacists and supplied by manufacturers.3 According to the literature, there are three different formats of the PILs.3,7 The primary and most common one is package inserts, found in the box of the medicine, the second one is loose leaflets, and the last one of the patient leaflet which is found electronically on the website.3,7 These countries, including Saudi Arabia, require Patient Information leaflets about drug therapy to be included in the medication supplied to patients, and the content of these is prescribed by regulatory requirements.7,8
Previous research in Saudi Arabia found that the majority of patients had a positive attitude toward patient leaflets and read PILS before taking prescribed medication.6–8 When patients were unable to read the leaflets, they asked others to read them for them.6,8 Patient safety, on the other hand, is the top priority, and the leaflets can help with that. Although studies have shown that PILs can give patients a variety of benefits such as enhanced quality of life, less anxiety, early detection of harmful side effects, and a better knowledge of the treatment plan.3 The patient’s ability to read and understand pharmaceutical instructions and the accompanying leaflet is a crucial element that determines adherence.3
It is a well-known fact that knowledge about medicine safety and attitudes towards leaflets are very important since they are associated with good health outcomes and improved patient knowledge about diseases.3–5 Several studies revealed that the drug information leaflet was the most frequently used source of ADR information9 and limited medication safety knowledge among the Saudi public.10 In addition, earlier studies revealed that the current patient information leaflets do not meet the needs of all patients, or text is too long in the leaflets and the small font, which is difficult to read, the presence of medical terms, methods of folding to fit it in the medication package.5,11 Moreover, in Saudi Arabia, studies addressing public attitudes towards PILs and medication safety knowledge are limited. Therefore, it is important to assess the knowledge of medication safety and attitude towards patient leaflets. Given this, the current study aimed to assess the Knowledge of medicine safety, adverse drug reactions, and Attitudes towards PILs among Saudi adults in Saudi Arabia.
Materials and Methods
This cross-sectional web-based study aimed to assess the Knowledge of medicine safety, adverse drug reactions, and Attitudes toward PILs among Saudi adults. The study was conducted over three months in 2023 using online structured self-administered questionnaires, on the adults of Saudi Arabia, admitted to provide informed consent, aged ≥18 years, able to complete the questionnaires, being a Saudi national, and currently living in the country. Those who did not match the inclusion criteria and incompletely answered surveys were excluded from the study. After receiving the IRB, approval from the Human and Social Research Committee (IRB 23–1203) at King Saud University, Riyadh, Saudi Arabia. Furthermore, the study was conducted according to the declaration of Helsinki guidelines for human research.
Sample Size Estimation
Using an online calculator, the sample size was determined with a 95% confidence interval (CI) and a 5% margin of error (ME), assuming that the population (n = 20,000) was unknown. For this research, a sample size of 377 was necessary. We disseminated to 550 respondents to ensure accuracy, prevent missing responses, and minimize response bias.
Designing the Study Tool
A questionnaire was developed using the literature12–15 to fulfill the study’s aim. It is composed of 4 sections including, respondents’ characteristics, their knowledge of medication use and adverse drug reactions (ADRS), and attitude toward PILs. Regarding the knowledge of individuals, about medication safety (5 items) and ADRs six questions were used to assess that which were adopted from other studies12–15 after subjecting them to a few modifications. The attitude questions were a total of 3 items. All these questions are open-ended. The detailed methodology design, step by step is presented in Figure 1.
 |
Figure 1 Methodology flowchart. |
When the Cronbach’s Alpha coefficient was computed using responses from the pilot study (which involves thirty randomly chosen adults) to fourteen questions assessing knowledge and attitudes regarding medication, adverse drug reactions, and leaflets, the questionnaire reliability test showed an acceptable degree of internal consistency (Cronbach’s Alpha = 0.794). Adult Saudis were approached to inquire about their interest in taking part in the study. The necessary information was gathered, along with questionnaire-based assessments, after the study’s disclosure. Convincing sampling, a non-probability sampling technique, was used to obtain the data. It involves selecting a sample from the population that is closest to the researcher or that is most accessible to them. Additionally, we employed a snowball method in which an individual sent one or more referrals to the research questions. The data was collected under the supervision of the researcher, who strictly followed up with the data collection process. To get maximum responses, the researcher was sent reminders to complete the questionnaires and refer to their family and friends.
Data Analysis
The statistical program for social science (SPSS) version 27 (SPSS Inc., Armonk, New York, United States) was used to conduct the analysis. Frequencies (n) and percentages (%) were estimated using descriptive analysis, and group differences were evaluated using the Chi-square/Fisher exact test. Results with a p-value of <0.05 were deemed statistically significant. Furthermore, the predictors of PILs were evaluated using multiple-regression analysis.
Results
A total of 521 individuals responded to the questionnaires. Among the respondents, the majority 65.5% (n = 341) of them were found between 18 and 30 years old, 56.2% (n = 293) of them were male and 49.5% (n = 258) were graduates, while 8.6% (n = 45) belonged to secondary school. The detailed frequencies about the demographic and some features of the respondents are given in Table 1.
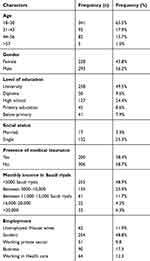 |
Table 1 Demographic Data of Respondents |
Table 2 describes the respondent’s frequency of the use and purpose of utilization of medication. According to findings, 59.9% (n = 312) of the individuals have taken medication, in the past three months, while 37.8% of them have never taken any medications. When we asked who recommended taking the medication third (n = 171; 32.8%) revealed that pharmacist, while 26.1% (n = 136) self-administered without any advice or consultation, and only 15% (n = 78) of them reported that they take medication upon health care practitioners consultation (Figure 2). More than half 59.3% of the respondents knew the purpose of taking medication (n = 309), while 22.3% of them never knew the purpose as shown in Table 2. In addition, the current findings revealed that 46.1% (n = 240) of respondents knew the time and frequency of the medication they were taking, while 34.4% (n = 179) did not know about it. Furthermore, 44.5% (n = 232) of them knew how long it had to take. The detailed responses of the respondents about the frequency and purpose of utilization of medication are given in Table 2.
 |
Table 2 Frequency and Purpose of Utilization of Medication by Respondents |
 |
Figure 2 Recommendation for medication. |
With regard to attitude towards leaflets of medication, 23.6% (n = 123) of them always read the PILs, while 61.8% (n = 322) of them read it sometimes, and 14.6% (n = 76) of them never read the PILs. In addition, 22.6% (n = 118) of them find it difficult to understand the PILs. On the other hand, 57.6% (n = 300) did not feel difficulty understanding the PILs as seen in Table 3.
 |
Table 3 Attitudes Towards Patients Information Leaflet |
Table 4 details the Knowledge of adverse drug reactions among studied respondents. In this view, 16.1% (n = 84) of them reported that adverse drug reactions are any effect of the medication, while 14.6% (n = 76) reported that ADRs are expected reactions after taking the normal dose. However, 53.9% of them revealed that ADRs are unexpected reactions after taking the normal dose and 15.4% of them do not know what ADRs are. Furthermore, 19.2% (n = 100) of them always searched for ADRs of their medication, and 39.7% (n = 207) thought that healthcare providers must report the suspect ADRs. In addition, 32.6% (n = 170) did not experience any ADRs. The detailed responses of the respondents about Knowledge of adverse drug reactions are given in Table 4.
 |
Table 4 Knowledge of Adverse Drug Reactions (ADR) |
The frequency of reading PILs is significantly associated with the age group. For instance, adults aged between 18 and 30 years were found to be always reading PILs compared to respondents with other age groups, indicating a significant association between them (p= 0.001) (Figure 3).
 |
Figure 3 Frequency of reading PILs according to Age. |
Similarly, more women have read PILS compared to men, although more men read PILs sometimes compared to women. The frequency of reading PILs is significantly associated with gender (P = 0.003). The detailed association between the gender and frequency of reading PILs is given in Figure 4. Similarly, the respondents with university degrees always read the PILs, compared to respondents with secondary school and other qualifications, indicating a significant association between them (p = 0.0001) as shown in Figure 5. The frequency of reading PILs was significantly higher among students and health sector employees compared to other professionals indicating significant association (p = 0.0001). The details of the association between the employment and frequency of reading PILs are given in Figure 6.
 |
Figure 4 Frequency of reading PILs according to gender. |
 |
Figure 5 Frequency of reading PILs according to education. |
 |
Figure 6 Frequency of reading PILs according to employment. |
With regard to resources used by the respondents in the search for ADR of their medications, 52.9% (n = 276) of them reported using PILS, followed by pharmacists 38.1% (n = 199) physicians 26.8% (n = 140) and 20.5% (n = 107) reported they do not search for the ADR of the drugs or medications prescribed to them (Figure 7). However, the results of the multiple linear regression model revealed that no significant association was found between the reading PILs, age, education, occupation, and presence of health insurance and were not predictors of the reading PILs (p > 0.05). Except gender (B = 0.164; SE = 0.075; 95CI = 0.017–0.310; p = 0.029), Social status (B = 0.251; SE = 0.079; 95CI = 0. 096–0.406; p = 0.002) and monthly income (B = 0.136; SE = 0.021; 95CI = 0.095–0.178; p < 0.001) was the predictor of reading PILs as shown in Table 5.
 |
Table 5 Multiple Linear Regression Model for Predictors of Reading PILs |
 |
Figure 7 Resources for ADR among respondents. |
Discussion
Appropriate medication use and self-medication are more common and rising day by day in everyone’s life resulting in morbidity and mortality resistance to medicine, medication wastage, and increased cost to health care. In this view, individuals play a crucial role in the management of their medication side effects, as they are responsible for ensuring that they receive appropriate medication for their ailment and that their medications are optimized to effectively manage their condition. As such, individuals must be equipped with a solid foundation of knowledge about the safety, adverse reactions, and pathophysiology of the medicine they use, which can be obtained from the PILs or package inserts. Therefore, this study sought to assess Saudi public’s evaluation of medication safety knowledge and attitudes toward PILs. The results revealed that 59.9% of individuals had taken medicine in the past month more than half (59.3%) of the individuals knew the purpose of taking medication, 46.1% of them knew in what frequency they should take the medication and 44.5% of them knew the duration of taking medication, which revealed acceptable knowledge from the respondents. The findings of this study indicated that most of the respondents had adequate information about the basic aspects of medicine safety, which is very important in obtaining excellent health outcomes and overcoming any adverse effects associated with the products. In Thailand, a previous study by Wongtaweepkij et al, in 2023 reported that the majority (96.2%) of studied individuals knew the procedure of taking medication.16 Furthermore, patients reading the patient information leaflets had slightly higher overall medication safety knowledge scores compared to package inserts.16 Suggesting the significance of patient information leaflets.
In this study, self-medication was found among 26.1% of the individual while 47.8% of them reported that healthcare providers were the source of their medicine. Although previous findings revealed that physicians were the top utilized source for getting medicine information (92.7%), followed by pharmacists (84.7%), PILs (67.4%), searching the Internet (53.6%), and consulting family and friends (31.7%).17 Furthermore, these findings are not surprising, self-medication is relatively high in both Saudi Arabia and other international countries.18,19 For instance self, 79.4% of Korean adults found self-administered their medications.20 Similarly, another recent study estimated 62.7% of the prevalence of self-medication in the Arab region.21 In India, the previous study reported 605 prevalence of self-medication.22 Similarly, 63.5% of the total prevalence of self-medication was reported among Malaysians.23 Self-medication was most frequently reported for fever, common flu, cough, and dental diseases, with myalgia being the most prevalent complaint. To prevent excessive medication use, it is crucial to raise public awareness of medication use, its consequences, and its side effects. We also urged government authorities to enact stringent laws governing the availability of these medications at pharmacies.18–21
With regard to attitudes towards leaflets, 85.4% of the respondents always or sometimes read the patient information leaflet, and only 33% of them read the entire patient information leaflet. However, more than half of them did not feel difficulty in reading and understanding the PILs. Suggesting that most of them do not always prefer to read the PILs and there is need of awareness of the importance of reading PILs among the Saudi public is needed. Although in this study who read the PILs, most of them read indications (39.7%) of the drugs, followed by side effects (10.4%) and 33% read the entire part of the PILs. These findings were similar to earlier findings published in Saudi Arabia, and other countries.16,24–26 For instance, a more recently published study in Saudi Arabia reported that 91.1% of respondents read PILs and the most frequently read PILs were directions on how to use the medication (52.7%) followed by side-effect drugs (30.3%).24 Similar results were reported in the Thailand public where 88% of them received packaging inserts and 26.2% of them received patient information leaflets.16 In Ghana, a previous study reported that 66.7% of respondents read the information on the drug leaflets whilst the remaining 33.3% did not read.3 Furthermore, most of the respondents received PIL-related information online, and high education and family income were found to be significant factors associated with receiving PILS online.16 Similarly, another study among Palestinians revealed that 45% of buyers reported that they always read the PPIs, and 29.3% said that they read the PILs most of the time. High levels of reading the leaflet were found among females.25
In this study, half of the respondents revealed that consumers can directly report suspected adverse drug reactions through the Saudi vigilance program, and over half of the respondents were aware of the existence of the Saudi National Pharmacovigilance Center. Of the respondents, 39.7% agreed that healthcare professionals should be in charge of reporting suspected adverse drug reactions from medications. These findings were comparable to previous studies.26 A similar study found that ADRs can be reported to NCCs or AMCs by patients, consumers, and healthcare professionals alike. The NCC may get individual case safety reports for pharmaceuticals from the pharmaceutical industry.26
This study’s limited sample size and low response rate are among its limitations. The number of responses did not significantly increase despite repeated reminders regarding the questionnaires. Furthermore, the study was limited to the central region only limiting the generalizability of the findings. Despite these drawbacks, our study has several merits. This study first examines the importance of a person’s knowledge in adhering to medicine safety guidelines and avoiding self-medication. Additionally, there is a dearth of this kind of research in this field, particularly among Saudi Arabia’s public. It could have been possible to identify specific problems that needed to be fixed and to promote the safe and appropriate use of medications by delving deeper into some of the responses with a mixed-methods study. For a study such as this one, a workforce planning conference or meeting could act as a spur to encourage a higher response rate from the sample.
Conclusion
In this study, two-thirds of participants took medication and knew why they were taking it. However, only a few of them took the medicine after seeing a doctor, suggesting self-medication practice. In addition, 22.1% of them find it difficult to understand the PILS, and a small number of them would rather read it. Moreover, self-medication is becoming more prevalent on a national and worldwide scale, especially among individuals, which could result in a rise in adverse effects. Before taking any kind of medication to counteract the side effects, it is imperative to identify them and encourage them to read the PILS. Therefore, policymakers, drug manufacturers, medical practitioners, and all other interested parties should invest enough time to create a structure that draws readers in. It is also advised that PILS be simple to comprehend. Therefore, we recommend putting awareness campaigns into place to inform people about their importance for health outcomes in terms of preventing medication errors or adverse drug reactions.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Informed Consent Statement
Informed consent was obtained from a patient, which confirmed that their data would be kept confidential and used exclusively for research purposes.
Institutional Review Board Statement
The study was conducted by the Declaration of Helsinki and approved by the Institutional Review Board of King Saud University College of Medicine, Riyadh, Saudi Arabia.
Acknowledgments
The authors of this study extend their appreciation to Researchers Supporting Project (Project number RSP2024R378), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Research Supporting Project, King Saud University, Riyadh, Saudi Arabia, (RSP2024R378) which provided funding for this work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. World Health Organization. Access to Medicines: Making Market Forces Serve the Poor. Geneva, Switzerland: World Health Organization; 2017.
2. Sánchez-Sánchez E, Fernández-Cerezo FL, Díaz-Jimenez J, et al. Consumption of over-the-counter drugs: prevalence and type of drugs. Int J Environ Res Public Health. 2021;18(11):5530. doi:10.3390/ijerph18115530
3. Owusu FWA, Yeboah GN, Aboagye RA, Amengor CDK, Entsie P. The role of the patient information leaflet in patients’medication therapy: a case study within the Kumasi metropolis of Ghana. Sci World J. 2020;2020:2489137. doi:10.1155/2020/2489137
4. Herber OR, Gies V, Schwappach D, Thürmann P, Wilm S. Patient information leaflets: informing or frightening? A focus group study exploring patients’ emotional reactions and subsequent behavior towards package leaflets of commonly prescribed medications in family practices. BMC Family Practice. 2014;15(1):1–8. doi:10.1186/1471-2296-15-163
5. Hammar T, Nilsson AL, Hovstadius B. Patients’ views on electronic patient information leaflets. Pharm Pract. 2016;14(2):702. doi:10.18549/PharmPract.2016.02.702
6. Decision of the Dusseldorf District Court 26 July 2012 – Case No 4a O 282/10. “Bolar Exemption (Solifenacin) – Germany”. IIC. 2013;44:361–362. doi:10.1007/s40319-013-0034-5
7. Al-Aqeel SA. Evaluation of medication package inserts in Saudi Arabia. Drug Health Patient Saf. 2012;4:33–38. doi:10.2147/DHPS.S29402
8. Bawazir SA, Abou-auda HS, Gubara OA, et al. Public attitude toward drug technical package inserts in Saudi Arabia. J Pharm Technol. 2003;19:209–218. doi:10.1177/875512250301900302
9. El-Dahiyat F, Hammour KA, Farha RA, Manaseer Q, Allan A, Alkhawaldeh R. Jordanians’ knowledge, attitude and practice regarding adverse drug reactions reporting. Saudi Pharm J. 2023;31(7):1197–1201. doi:10.1016/j.jsps.2023.05.016
10. Islam MA, Al-Karasneh AF, Naqvi AA, et al. Public awareness about medicine information, safety, and Adverse Drug Reaction (ADR) reporting in Dammam, Saudi Arabia. Pharmacy. 2020;8(4):222. doi:10.3390/pharmacy8040222
11. Liu F, Abdul-Hussain S, Mahboob S, Rai V, Kostrzewski A. How useful are medication patient information leaflets to older adults? A content, readability and layout analysis. Int J Clin Pharm. 2014;36(4):827–834. doi:10.1007/s11096-014-9973-2
12. Wang N, Ren B, You H, et al. Assessment of medication adherence, medication safety awareness and medication practice among patients with lung cancer: a multicentre cross-sectional study. Health Expect. 2022;25(2):791–801. PMID: 34989054; PMCID: PMC8957719. doi:10.1111/hex.13426
13. Khan G, Haq N, Ahmad N, et al. Public awareness regarding the manufacturer provided information about medicine usage, safety, and adverse drug reactions in Balochistan, Pakistan. Front Pharmacol. 2023;14:1.
14. Alsaad HA, Almahdi JS, Alsalameen NA, Alomar FA, Islam MA. Assessment of self-medication practice and the potential to use a mobile app to ensure safe and effective self-medication among the public in Saudi Arabia. Saudi Pharm J. 2022;30(7):927–933. doi:10.1016/j.jsps.2022.05.010
15. Schwappach DL, Mulders V, Simic D, Wilm S, Thurmann PA. Is less more? Patients’ preferences for drug information leaflets. Pharmacoepidemiol Drug Saf. 2011;20(9):987–995. doi:10.1002/pds.2212
16. Wongtaweepkij K, Sup-Adulchai N, Chanachoat J, Krska J, Jarernsiripornkul N. Evaluation of medicine information leaflets for omeprazole, safety knowledge, and perceptions of taking the medication in Thailand. Patient Preference Adherence. 2023;31:883–893. doi:10.2147/PPA.S397557
17. Almanasef M. Patient information leaflet in the era of digitalisation: a cross-sectional study on patients’ attitudes and practices. Ir J Med Sci. 2023. doi:10.1007/s11845-023-03515-2
18. Alfadly S, Ballaswad MR, Amra AS, et al. Self-medication with antibiotic amongst adults attending community pharmacies in Mukalla district, Yemen. Lat Am J Pharm. 2017;36(2):224–228.
19. Mahmoud MA, Wajid S, Naqvi AA, Samreen S, Althagfan SS, Al-Worafi Y. Self-medication with antibiotics: a cross-sectional community-based study. Latin Am J Pharm. 2020;39(2):348–353.
20. Lee M, Kim K, Rhew K, Choi KH. A knowledge, attitude, and practice survey on medication safety in Korean older adults: an analysis of an ageing society. In: Healthcare. Vol. 9. MDPI; 2021:1365.
21. Abdelwahed AE, Abd-Elkader MM, Mahfouz A, et al. Prevalence and influencing factors of self-medication during the COVID-19 pandemic in the Arab region: a multinational cross-sectional study. BMC Public Health. 2023;23(1):1. doi:10.1186/s12889-023-15025-y
22. Rathod P, Sharma S, Ukey U, et al. Prevalence, pattern, and reasons for self-medication: a community-based cross-sectional study from central India. Cureus. 2023;15(1):e33917. doi:10.7759/cureus.33917
23. Mok CZ, Sellappans R, Ee Loo JS. The prevalence and perception of self-medication among adults in the Klang Valley, Malaysia. Int J Pharm Pract. 2021;29(1):29–36. doi:10.1111/ijpp.12660
24. Al Jeraisy M, Alshammari H, Albassam M, Al Aamer K, Abolfotouh MA. Utility of patient information leaflet and perceived impact of its use on medication adherence. BMC Public Health. 2023;23(1):488. doi:10.1186/s12889-023-15346-y
25. Zaid AN, Sweileh W, Kettana N, Sweileh W, Al-Jabi D. Attitudes of consumers and healthcare professionals towards the patient package inserts-a study in Palestine. Pharmacy Practice. 2012;10(1):57. doi:10.4321/S1886-36552012000100010
26. Kalaiselvan V, Kumar P, Mishra P, Singh GN. System of adverse drug reactions reporting: what, where, how, and whom to report? Indian J Crit Care Med. 2015;19(9):564–566. doi:10.4103/0972-5229.164819
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
