Back to Journals » Journal of Inflammation Research » Volume 17
A Correlation Evaluation Between the Peripheral Blood Index and the Prognosis of Advanced Esophageal Squamous Cell Carcinoma Patients Treated with Camrelizumab
Authors Shang H, Chen Y, Wang Q, Yang Y, Zhang J
Received 19 November 2023
Accepted for publication 20 March 2024
Published 29 March 2024 Volume 2024:17 Pages 2009—2021
DOI https://doi.org/10.2147/JIR.S450669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Haotian Shang, Yanan Chen, Qiulu Wang, Yongliang Yang, Jingyu Zhang
Department of Oncology, Clinical College of Second People’s Hospital of Lianyungang, Bengbu Medical College, Lianyungang, People’s Republic of China
Correspondence: Jingyu Zhang, Department of Oncology, Clinical College of Second People’s Hospital of Lianyungang, Bengbu Medical College, No. 41, Hailian East Road, Haizhou District, Lianyungang City, Jiangsu Province, People’s Republic of China, Email [email protected]
Purpose: This study aimed to investigate the relationship between peripheral blood indices and the efficacy and prognosis of advanced esophageal squamous cell carcinoma (ESCC) patients treated with camrelizumab.
Patients and Methods: We retrospectively analyzed 64 patients who received camrelizumab for advanced ESCC at the Second People’s Hospital of Lianyungang City between July 2020 and June 2022. The study included examination of the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), the systemic inflammation index (SII), the lymph-to-monocytes ratio (LMR), the absolute lymphocyte count (ALC), and lactate dehydrogenase (LDH). We used multivariate logistic regression analysis to explore the link existing between peripheral blood and the efficacy of treatment. Determination of potential prognostic factors for Progression-free survival (PFS) and Overall survival (OS) using Cox regression analysis. The nomogram model was developed based on the results of the Cox multivariate analysis. Patients were divided into three groups according to the reduction in LDH and LDL levels before treatment, and the Kaplan-Meier survival curves for the three groups were compared and ROC curves for LDH combined with PLR were plotted.
Results: Lower LDH (OR=6.237, 95% CI: 1.625– 23.944) were independently associated with disease control rates(DCR). LDH was independently correlated with PFS (HR: 0.227 95% CI: 0.099– 0.517). LDH and PLR were independently linked to OS. The C index of the nomogram model is 0.819, indicating good predictive performance. Kaplan-Meier Survival Curve suggested better OS in patients with reduced pretreatment LDH and PLR. The area under the ROC curve showed that the LDH index combined with the PLR index predicts patient survival better than the index alone.
Conclusion: LDH combined with PLR predicted prognosis in patients with ESCC treated with camrelizumab.
Keywords: esophageal squamous cell carcinoma, camrelizumab, immunotherapy, peripheral blood, prognostic index
Introduction
Esophageal cancer (EC) ranks eighth in terms of incidence and sixth in terms of mortality rate worldwide.1 In contrast to esophageal adenocarcinoma (EAC), esophageal squamous cell carcinoma (ESCC) presents the most common histologic type worldwide.2 The standard treatment for ESCC is radiation, chemotherapy, or surgery.3 For patients with advanced EC, combining chemotherapy and immunotherapy provides a longer survival benefit compared to chemotherapy alone.4 A growing body of data suggests that histologic type is related to the prognosis of esophageal cancer. Programmed death 1 (PD-1) inhibitors can inhibit the programmed death of immune checkpoint proteins, and ESCC was more sensitive to PD-1 inhibitors than EAC.5 PD-1 inhibitors activate T lymphocytes, which inhibit cancer cell growth and improve overall patient survival.6 PD-1 inhibitors are safer than chemotherapy and extend patients’ overall survival(OS).7 Camrelizumab is a humanized anti-PD-1 monoclonal antibody. Combining chemotherapy with camrelizumab significantly improved overall and progression-free survival in patients with advanced ESCC compared with chemotherapy and placebo.8 However, not all patients with ESCC have better efficacy after immunotherapy. Programmed cell death ligand 1 (PD-L1) is currently an effective marker for predicting the efficacy of immunotherapy for EC. PD-L1 combined with Tumor Mutational Burden (TMB) and Tumor Infiltrating Lymphocyte (TIL) could improve the predictive accuracy. However, due to the specific response mechanism of tumor tissues, PD-L1 combined with TMB can predict the efficacy of immunotherapy in most tumor patients, but some patients may still have false negatives.9 The clinical method of detecting PD-L1 is complex, involving thresholds and antibody selection. PD-L1 measurement involves not only a lack of harmonized standards but also requires complex and expensive laboratory techniques.10,11 Therefore, we need to find simpler and more convenient markers to predict the efficacy of immunotherapy, so that more patients will benefit.
Inflammation has been considered an important factor in tumor development and prognosis.12 It is well-documented that chronic inflammatory stimulation raises the risk of cancer, promotes tumor progression and contributes to metastatic spread. Hence, chronic inflammatory processes of inflammatory cells and cytokines can serve as tumor-promoting factors, affecting cell survival, proliferation, invasion, and angiogenesis.13 A great deal of research has demonstrated that several potential inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic inflammation index (SII) have a predictive value for the prognosis of the lung,14 gastric,15,16 esophageal,17 and colorectal18 cancers in immunotherapy. For ESCC patients receiving immunotherapy, their NLR metrics at 6 weeks after treatment were predictive of response to PD-1 inhibitor therapy.17 However, the outcomes and prognosis of peripheral blood inflammatory markers and PD-1 inhibitors in ESCC patients prior to immunotherapy still deserve further exploration.
Lactate dehydrogenase (LDH) is a key marker of aerobic glycolysis in cancer cells, which promotes the survival of cancer cells. In a variety of malignancies such as small cell lung cancer, lymphoma, and multiple myeloma, elevated baseline LDH has been shown to relate to poor prognosis.19 However, the impact of LDH prior to immunotherapy and ESCC prognosis is uncertain. This study retrospectively analyzed 64 patients with ESCC treated with camrelizumab to assess the relationship between peripheral blood indices and LDH and the efficacy and prognosis of immunotherapy. We built a predictive score for immunotherapy in ESCC to select a more accurate population of immunotherapy beneficiaries.
Materials and Method
Patient Selection
This study included 64 patients with advanced or metastatic ESCC patients who received camrelizumab in the Second People’s Hospital of Lianyungang between July 2020 and June 2022. We collected the patient’s sex, age, smoking history, alcohol history, clinical stage, treatment modality, Eastern Cancer Cooperative Performance Status (ECOG PS) score, presence or absence of radiotherapy, and peripheral blood indicators 1 week before immunotherapy. The inclusion criteria were as follows: (1) All ESCC patients were pathologically diagnosed (2) Patients with advanced ESCC treated with PD-1 inhibitors (3) patients with an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score at 0–2 (4) No other hematologic disorder (5) Patients having intact peripheral hematological parameters (6) Patients sign immunotherapy consent forms before treatment. The exclusion criteria were as follows: (1) Patients suffering from other malignant tumors (2) Patients whose clinical data are incomplete and whose pathologic diagnosis is unclear (3) Patients with immune system disease (4) Patients with connective tissue disease (5) The clinical stage was unclear.
Efficacy Evaluation
Evaluation of the patient’s treatment efficacy with computed tomography or esophagography at 6–8 weeks of treatment. Evaluated response to treatment using the standard response assessment criteria for solid tumors (RECIST version 1.1). Complete remission (CR) was confirmed to the complete disappearance of all lesions and the maintenance of at least 4 weeks after complete disappearance. Partial remission (PR) means a percentage reduction in the largest diameter of the target lesion ≥ 30% and maintained for at least 4 weeks. Stable disease (SD) means that the target lesion is in partial remission, remains in its original state, or is partially progressive and remains lasting at least 4 weeks Disease progression (PD) has been defined as the proportional increase in the sum of the largest diameter of the target lesion ≥ 20% or the appearance of a new lesion. The objective remission rate (ORR) was calculated as the sum of CR and PR. The disease control rate (DCR) was calculated as the sum of CR, PR, and SD. Progression-free survival (PFS) was measured as the interval of time between the start of treatment and disease progression or death. Overall survival (OS) measured how long it took from the start of initial treatment to death or last follow-up visit.
Statistical Analysis
Categorical variables were presented in a distributional manner using numbers and percentages. With data that is not normally distributed, it is expressed as the median (interquartile range). Youden indexes for NLR, PLR, SII and LDH were calculated from the ROC curves (Youden’s index=sensitivity+specificity-1). Patients were categorized into high and low groups based on cutoff values. Evaluation of clinical characteristics and correlation between inflammatory indicators and treatment response using logistic regression analysis. Kaplan-Meier curves were applied to plot the survival curves of PFS and OS, and Log rank test was used for between-group comparisons. Cox proportional risk models were utilized to verify the relationship between variables and prognosis. Nomogram was drawn according to the results of the multivariate Cox analysis (using R version 4.2.3 of the rms software package). Used C-index, AUC values, and calibration curves to assess the performance of the nomogram. LDH-PLR scores were developed based on independent prognostic factors for OS, and ROC curves predicting LDH-PLR scores were plotted. P < 0.05 was considered statistically significant. All analyses were statistically analyzed using SPSS26.0 and R 4.2.3.
Result
Patient Characteristics
A total of 64 patients were involved in this study. The baseline characteristics of patients are listed in Table 1. The average age of the patients was 63 years (IQR: 52–85), of which 81.2% were male and 18.8% female. With approximately 80% having no history of smoking or alcohol consumption. The number of patients clinically diagnosed with stage III and IV was almost equal, with approximately 93.8% of patients having an ECOG PS score of 0–1. Of all the patients, 67.2% had received previous radiation therapy. 82.9% of the patients opted for chemotherapy in combination with immunotherapy, and only 11 patients received immunotherapy alone. After 6–8 weeks of treatment, 6 patients reached CR, 12 achieved PR, 27 reached SD and 19 reached PD. The ORR and DCR were 28.2% and 70.4%, respectively.
 |
Table 1 Patients’ Characteristics at Baseline |
Cut-off Values for Peripheral Blood Indexes
As revealed in Figure 1, ROC curves for peripheral blood indices were plotted, with 1-year OS as the endpoint for patients. The optimum cut-off value was determined based on Youden’s index. The best critical value for NLR was 4.6 with an AUC of 0.841. The best critical value for PLR was 194.5 with an AUC of 0.842. The best critical value for SII was 725.4 with an AUC of 0.792. The best critical value for LDH was 277 with an AUC of 0.731. Based on the optimal critical value the patients were categorized into two groups, high and low.
 |
Figure 1 Curves for NLR, PLR, SII, and LDH. |
Relationship Between Peripheral Blood Index and Clinical Characteristics and Therapeutic Effect
As shown in Tables 2 and 3, we found that LDH ≤ 277 (OR=4.524, 95% CI: 1.309–15.632), NLR ≤ 4.6 (OR=3.962, 95% CI: 1.329–11.809), and PLR ≤ 194.5 (OR=3.250, 95% CI: 1.117–9.454) were associated with ORR.LDH ≤277 (OR=11.333, 95% CI: 3.366–38.154), NLR ≤4.6 (OR=12.667, 95% CI: 3.198–50.164), and SII ≤725.4 (OR=7.667, 95% CI. 1.966–29.896) were associated with DCR. Multivariable logistic regressions were included for variables with p<0.05 and found that LDH≤277 was independently related to DCR, and no variables significantly related to ORR were found.
 |
Table 2 Univariate and Multivariate Analysis of ORR |
 |
Table 3 Univariate and Multivariate Analysis of DCR |
Relationship Between Peripheral Blood Index and Clinical Characteristics and Survival Outcomes
As shown in Tables 4 and 5, we did univariate and multifactorial analyses of PFS and OS using the Cox regression analysis. LDH, NLR, and SII were related to PFS in the univariate analysis. Tumor stage, LDH, NLR, PLR, and SII were correlated with OS. We included variables with P<0.05 in the multivariate analysis. The pretreatment LDH ≤277 group (HR 0.227, 95% CI 0.099–0.517) was independently associated with PFS, however, NLR and SII had no significant effect on the prediction of PFS. The Kaplan-Meier curve indicated that low group LDH was associated with longer PFS. Tumor stage, pretreatment LDH ≤ 277 group (HR 0.269, 95% CI (0.135–0.539)) and PLR ≤ 194.5 group (HR 0.356, 95% CI 0.135–0.942) were independently related to OS. We did not observe that NLR and SII were independently related to OS. Low group LDH and low group PLR were related to better OS (Figure 2).
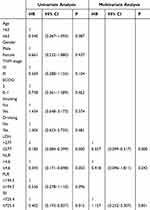 |
Table 4 Univariate and Multivariate Analysis of PFS |
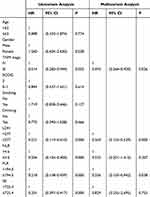 |
Table 5 Univariate and Multivariate Analysis of OS |
Construction and Assessment of the Nomogram Model
We constructed a nomogram model containing PLR, LDH, and tumor stage based on the results of Cox multifactorial analysis to predict esophageal cancer patients’ survival at 6, 12, and 18 months (Figure 3). The C-index value was 0.819 (95% CI 0.764–0.874). The AUC for 6-month, 12-month, and 18-month OS were 0.935, 0.867, and 0.921, respectively. We plotted the internal calibration curves using the rms software package in R language and found optimal concordance between forecast and observed survival probabilities after 12 months of treatment (Figure 4).
 |
Figure 3 A nomogram for 6-, 12- and 18-month OS for patients with esophageal cancer. |
Combined LDH with PLR Predicted Survival in Patients with Advanced ESCC
We constructed an LDH-PLR (LP) score based on independent predictors of OS, stratifying patients using a scoring system. The LP score was calculated as follows: LDH>277 and PLR>194.5 (yes=0), LDH≤277 or PLR≤194.5 (yes=1), LDH≤277 and PLR≤194.5 (yes=2). We divided the patients into 3 groups, 24 patients with a score of 1 and 23 patients with a score of 2. As shown in Figure 5a, we plotted the Kaplan-Meier curves for each group and found that patients with low LDH and low PLR (LP score =2) were associated with higher OS. We used ROC curves to compare the predictive value of LDH combined with PLR with a single indicator and demonstrated that LDH combined with PLR had better predictive value. (AUC=0.876, 95% CI:0.783–0.969) (Figure 5)
Discussion
Esophageal cancer shows a large incidence and poor prognosis. For patients with advanced esophageal cancer, the efficacy of traditional treatments remains unsatisfactory.20 Immune checkpoint inhibitors (ICIs) are becoming the major means of treatment. PD-1 inhibitors exert anti-tumor effects by blocking receptor-ligand specific binding, a safe and effective treatment modality.21 Camrelizumab was a PD-1 inhibitor that was developed uniquely in China. Clinical Phase I trials showed good treatment outcomes for camrelizumab for patients with a number of advanced tumors.22 A clinical trial of 596 Patients suffering from advanced or metastatic squamous cell carcinoma of the esophagus found that camrelizumab combination chemotherapy significantly improves overall survival and progression-free survival than placebo and chemotherapy.8 In advanced esophageal cancer, chemotherapy combined with immunotherapy significantly improves survival compared with chemotherapy alone in patients with squamous carcinoma with high PD-L1 expression.23 However, there was a lack of clarity regarding effective markers for predicting the efficacy of PD-1 inhibitor therapy in patients with ESCC. Our study investigated the correlation between peripheral blood inflammation index and LDH indexes before camrelizumab treatment and the prognosis of ESCC patients. OS and PFS were used as the primary study outcomes in our study. The LDH index was independently linked to PFS. We found a modest correlation between LDH, NLR, PLR, and SII and OS. However, according to the COX multifactorial results, LDH and PLR before receiving PD-1 inhibitor treatment were further found to be effective indicator for independent prognostic markers of OS in ESCC patients. We also analyzed DCR and ORR as study outcomes and showed that the inflammatory index did not correlate with either, but LDH correlated with DCR.
At present, the medical community has established that inflammation is linked closely to the occurrence and progression of cancer.13 PLR was an indicator of inflammation that combines platelets and lymphocytes. Thrombocytosis in patients with solid tumors can be very common, as inflammation associated with cancer can cause tissue damage that leads to platelet activation and aggregation.24 The results of a meta-analysis showed that PLR has some predictive ability but average sensitivity for PFS and OS in esophageal cancer.25 Liu Jiang et al analyzed retrospectively 90 patients with advanced metastatic ESCC receiving camrelizumab and determined therapy PLR be an effective independent prognostic factor for patients’ PFS and OS.26 The finding of our study was further proof of this idea. In our study, we only discovered that lower PLR before immunotherapy was related to longer OS in patients. We concluded that this may be linked to the mechanism by which the inflammatory response affects cancer growth, invasion, and metastasis. Cytokines and chemokines produced in the inflammatory response can promote angiogenesis and extracellular matrix remodeling to create a favorable microenvironment for cancer growth.27 However, we did not find a significant relationship between PLR and DCR with ORR. Several studies have found that PLR combined with SII is a better predictor of treatment efficacy against anti-PD1 in ESCC patients than PLR metrics alone.28 Rulan Ma et al did not find that PLR metrics had predictive value for ORR in patients with locally advanced esophageal cancer treated with PD-1 inhibitors.29 In our study, we found that PLR was probably an influencing factor for ORR and found no link between the two by further multifactorial analysis. Therefore, we believe that PLR is an important predictor of OS in ESCC patients.
High tumor load and metabolism leads to elevated circulating levels of lactate dehydrogenase (LDH) in the body, indicating a negative tumor prognosis. Among the features of cancer cells that characterize them is a metabolic shift towards anaerobic glycolysis, and LDH is a key enzyme in this process. Many patients with advanced cancer have high levels of circulating LDH.30 Elevated LDH levels were the outcome of tumor necrosis due to increased tumor glycolytic activity and hypoxia. Fewer people have elevated LDH levels to benefit from immunotherapy.31 Mingxia Cheng et al32 found that whether LDH is elevated prior to immunotherapy can be a promising biomarker for forecasting the efficacy of immunotherapy. Patients with metastatic cervical cancer who received combination immunotherapy were associated with poorer survival rates in the high LDH group. Laurent Dercle et al33 found that LDH was an independent marker of prognosis in melanoma and non-melanoma solid tumors and lower LDH may be significantly correlated with superior OS. Raised serum LDH was a key predictor of resistance to PD-1 inhibitor immunotherapy in patients with primary cancers. In a phase I clinical trial examining LDH and efficacy in patients with advanced ESCC treated with camrelizumab, it was found that patients with elevated LDH levels had lower tumor remission rates (P=0.02) and shorter PFS (P=0.002) and OS (P<0.0001). This implied that elevated LDH may be associated with tumor progression and that LDH may be a useful predictor of prognosis in patients with ESCC.34 A retrospective study found that advanced ESCC patients with lower LDH at baseline had better PFS and OS.35 Our study discovered a better prognosis in ESCC patients with lower LDH prior to treatment with camrelizumab. The correlation between LDH and the prognosis of ESCC immunotherapy has been less well studied. We have identified the critical value of LDH based on the ROC curve in our study. We not only found that pre-immunotherapy LDH was linked to DCR but also demonstrated that lower LDH was related to better PFS and OS. Therefore, we believed that LDH was associated with the prognosis and DCR of ESCC patients treated with camrelizumab.
We developed a nomogram for variables independently correlated with OS to more accurately predict the prognosis of esophageal cancer patients after immunotherapy. LDH and PLR (Platelet to Lymphocyte Ratio) were some of the common blood and biochemical indicators in our clinic, and the collection and organization of the data were convenient, quick, simple, and efficient. Tumor staging was determined based on the size of the primary tumor, lymph node involvement, and whether it has spread to other organs in the body. It described the severity and extent of involvement of the malignancy.36 We combined the above three indicators to construct a nomogram predicting the prognosis of patients with advanced ESCC at 6 months, 12 months, and 18 months after camrelizumab. The C-index of the model was 0.819. Further validation proved that The nomogram offers a favorable predictive accuracy and potential for clinical application.
In our research, LDH and PLR as independent prognostic factors in the prognosis of esophageal cancer immunotherapy. However, it was not clear whether these two indicators can be used together to predicate the prognosis of ESCC. Therefore, based on the results of OS, we combined LDH with PLR to develop an L-P score to predict the efficacy of PD-1 inhibitor immunotherapy. There have been similar studies in the past. Tetsushi Hirahara et al.37 Together, the NLR and PLR were combined to create the NLR-PLR score that serves as a new scoring system used to predict tumor response and prognosis in patients with advanced or recurrent gastric cancer receiving chemotherapy. Based on the weak correlation between LDH and PLR, we combined the two to construct a new predictor. The AUC value for LDH was 0.731 (95% CI:0.612–0.851). The AUC value for PLR was 0.842 (95% CI:0.736–0.948). The L-P score performed best as a predictor with an AUC value of 0.876 (95% CI:0.783–0.969). The AUC values indicated that LDH combined with PLR has superior predictive performance than individual indicators. This study was the first to combine inflammatory indices and LDH metrics in the prognosis of patients with locally advanced metastatic ESCC treated with camrelizumab.
We acknowledge several limitations in our study. Firstly, it was a smaller clinical retrospective study with a limited sample size, which may have influenced the findings. Secondly, retrospective studies are prone to selection bias, which can lead to imprecise outcomes. Thirdly, the nomogram was internally validated only, not externally validated. While our study has provided some value in predicting the prognosis of ESCC patients receiving immunotherapy, further validation of the L-P score in future prospective studies is needed to assess its effectiveness in predicting the prognosis of ESCC immunotherapy. In our study, we primarily focused on examining the relationship between changes in peripheral blood indices and prognosis in immunotherapy. In future research, it would be worthwhile to investigate the correlation between dynamic changes in peripheral blood before and after immunotherapy and the efficacy and prognosis of immunotherapy.
Conclusion
This study demonstrated that PLR and LDH can be used to predict the prognostic status of patients with advanced ESCC treated with camrelizumab. We not only found better OS for patients with lower PLR before immunotherapy but also better PFS and OS for patients with lower LDH. To further explore the prognosis of ESCC patients treated with camrelizumab, we constructed an L-P score based on independent prognostic factors for OS. The result of the study indicated that the L-P score is a new useful clinical predictor of the prognosis of ESCC patients receiving camrelizumab therapy. We hope that the construction of a predictive model by combining easily accessible peripheral blood indices provides some reference for better individualized clinical treatment, but still needs to be confirmed by a lot of prospective studies in the future.
Ethics Approval Statement
As a non-interventional retrospective study, the study was reviewed and approved by the Medical Ethics Committee of the Second People’s Hospital of Lianyungang. Informed consent was obtained from all participating patients. All patients’ personal information and privacy are to be kept confidential in line with the Declaration of Helsinki. (approval no. AF/SC-08/01.0).
Funding
Funding information is not applicable.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Morgan E, Soerjomataram I, Rumgay H, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649–658.e642. doi:10.1053/j.gastro.2022.05.054
2. Waters JK, Reznik SI. Update on management of squamous cell esophageal cancer. Curr Oncol Rep. 2022;24(3):375–385. doi:10.1007/s11912-021-01153-4
3. Weidenbaum C, Gibson MK. Approach to localized squamous cell cancer of the esophagus. Curr Treat Options Oncol. 2022;23(10):1370–1387. doi:10.1007/s11864-022-01003-w
4. Li Q, Liu T, Ding Z. Neoadjuvant immunotherapy for resectable esophageal cancer: a review. Front Immunol. 2022;13:1051841. doi:10.3389/fimmu.2022.1051841
5. Kelly RJ. Emerging multimodality approaches to treat localized esophageal cancer. J Natl Compr Canc Netw. 2019;17(8):1009–1014. doi:10.6004/jnccn.2019.7337
6. Hong Y, Ding ZY. PD-1 inhibitors in the advanced esophageal cancer. Front Pharmacol. 2019;10:1418. doi:10.3389/fphar.2019.01418
7. da Silva LL, Aguiar PN, Park R, Edelman Saul E, Haaland B, de Lima Lopes G. Comparative efficacy and safety of programmed Death-1 pathway inhibitors in advanced gastroesophageal cancers: a systematic review and network meta-analysis of Phase III clinical trials. Cancers. 2021;13(11):2614. doi:10.3390/cancers13112614
8. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi:10.1001/jama.2021.12836
9. Banchereau R, Leng N, Zill O, et al. Molecular determinants of response to PD-L1 blockade across tumor types. Nat Commun. 2021;12(1):3969. doi:10.1038/s41467-021-24112-w
10. Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18(6):345–362. doi:10.1038/s41571-021-00473-5
11. Zhao Q, Bi Y, Xue J, Liu Y, Zhu J, Qin S. Prognostic value of absolute lymphocyte count in patients with advanced esophageal cancer treated with immunotherapy: a retrospective analysis. Ann Translat Med. 2022;10(13):744. doi:10.21037/atm-22-2669
12. Hwang M, Canzoniero JV, Rosner S, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer. 2022;10(6):e004688. doi:10.1136/jitc-2022-004688
13. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. doi:10.1038/s41392-021-00658-5
14. Chen Y, Wen S, Xia J, et al. Association of dynamic changes in peripheral blood indexes with response to PD-1 inhibitor-based combination therapy and survival among patients with advanced non-small cell lung cancer. Front Immunol. 2021;12:672271. doi:10.3389/fimmu.2021.672271
15. Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273(3):532–541. doi:10.1097/SLA.0000000000003370
16. Gou M, Qian N, Zhang Y, et al. Construction of a nomogram to predict the survival of metastatic gastric cancer patients that received immunotherapy. Front Immunol. 2022;13:950868. doi:10.3389/fimmu.2022.950868
17. Wu X, Han R, Zhong Y, Weng N, Zhang A. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Can Cell Inter. 2021;21(1):356. doi:10.1186/s12935-021-02072-x
18. Ouyang H, Xiao B, Huang Y, Wang Z. Baseline and early changes in the neutrophil-lymphocyte ratio (NLR) predict survival outcomes in advanced colorectal cancer patients treated with immunotherapy. Int Immunopharmacol. 2023;123:110703. doi:10.1016/j.intimp.2023.110703
19. Claps G, Faouzi S, Quidville V, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. 2022;19(12):749–762. doi:10.1038/s41571-022-00686-2
20. Yan Y, Feng X, Li C, Lerut T, Li H. Treatments for resectable esophageal cancer: from traditional systemic therapy to immunotherapy. Chinese Med J. 2022;135(18):2143–2156. doi:10.1097/CM9.0000000000002371
21. Sihag S, Ku GY, Tan KS, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thoracic Cardiovasc Surg. 2021;161(3):836–843.e831. doi:10.1016/j.jtcvs.2020.11.106
22. Wang H, Xu Y, Zuo F, Liu J, Yang J. Immune-based combination therapy for esophageal cancer. Front Immunol. 2022;13:1020290. doi:10.3389/fimmu.2022.1020290
23. Salas-Benito D, Perez-Gracia JL, Ponz-Sarvise M, et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 2021;11(6):1353–1367. doi:10.1158/2159-8290.CD-20-1312
24. Rimini M, Casadei-Gardini A, Ravaioli A, et al. Could inflammatory indices and metabolic syndrome predict the risk of cancer development? Analysis from the bagnacavallo population study. J Clin Med. 2020;9(4):1177. doi:10.3390/jcm9041177
25. Jiang Y, Xu D, Song H, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis. BMJ open. 2021;11(9):e048324. doi:10.1136/bmjopen-2020-048324
26. Liu J, Gao D, Li J, Hu G, Liu J, Liu D. The predictive value of systemic inflammatory factors in advanced, metastatic esophageal squamous cell carcinoma patients treated with camrelizumab. Onco Targets Ther. 2022;15:1161–1170. doi:10.2147/OTT.S382967
27. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485–503. doi:10.1038/s41568-020-0281-y
28. Zhang X, Gari A, Li M, et al. Combining serum inflammation indexes at baseline and post treatment could predict pathological efficacy to anti‑PD‑1 combined with neoadjuvant chemotherapy in esophageal squamous cell carcinoma. J Transl Med. 2022;20(1):61. doi:10.1186/s12967-022-03252-7
29. Ma R, Yuan D, Mo C, et al. Factors affecting the ORR after neoadjuvant therapy of TP regimen combined with PD-1 inhibitors for esophageal cancer. Sci Rep. 2023;13(1):6080. doi:10.1038/s41598-023-33038-w
30. Miholjcic TBS, Halse H, Bonvalet M, et al. Rationale for LDH-targeted cancer immunotherapy. Eur J Cancer. 2023;181:166–178. doi:10.1016/j.ejca.2022.11.032
31. Van Wilpe S, Koornstra R, Den Brok M, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology. 2020;9(1):1731942. doi:10.1080/2162402X.2020.1731942
32. Cheng M, Li G, Liu Z, Yang Q, Jiang Y. Pretreatment neutrophil-to-lymphocyte ratio and lactate dehydrogenase predict the prognosis of metastatic cervical cancer treated with combination immunotherapy. J Oncol. 2022;2022:1828473. doi:10.1155/2022/1828473
33. Dercle L, Ammari S, Roblin E, et al. High serum LDH and liver metastases are the dominant predictors of primary cancer resistance to anti-PD(L)1 immunotherapy. Eur J Cancer. 2022;177:80–93. doi:10.1016/j.ejca.2022.08.034
34. Wang X, Zhang B, Chen X, et al. Lactate dehydrogenase and baseline markers associated with clinical outcomes of advanced esophageal squamous cell carcinoma patients treated with camrelizumab (SHR-1210), a novel anti-PD-1 antibody. Thoracic Cancer. 2019;10(6):1395–1401. doi:10.1111/1759-7714.13083
35. Li Y, Wang K, Zhao E, et al. Prognostic value of lactate dehydrogenase in second-line immunotherapy for advanced esophageal squamous cell carcinoma. Pathol Oncol Res. 2022;28:1610245. doi:10.3389/pore.2022.1610245
36. Patel A, West HJ. What does my stage of cancer mean? JAMA Oncol. 2020;6(8):1308. doi:10.1001/jamaoncol.2020.1592
37. Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. 2019;19(1):672. doi:10.1186/s12885-019-5903-y
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



