Back to Journals » International Journal of Women's Health » Volume 16
A Case Report of Malignant Perivascular Epithelioid Cell Tumors of the Uterus and Literature Review
Authors Hu D, Miao M, Zhou H, Gu X, Wang X, Teichmann AT, Wang Q , Yang Y
Received 7 December 2023
Accepted for publication 9 March 2024
Published 15 April 2024 Volume 2024:16 Pages 619—628
DOI https://doi.org/10.2147/IJWH.S453226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Daifeng Hu,1,* Mengyue Miao,1,* Hui Zhou,1 Xia Gu,1,2 Xuedan Wang,3 Alexander Tobias Teichmann,1 Qin Wang,1 Youzhe Yang1,2
1Sichuan Provincial Center for Gynaecology and Breast Diseases, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, People’s Republic of China; 2Academician (Expert) Workstation of Sichuan Province, Luzhou, 646000, People’s Republic of China; 3Department of Pathology, The Affiliated Hospital of Southwest Medical University, Luzhou, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qin Wang; Youzhe Yang, The Affiliated Hospital of Southwest Medical University, NO. 25 Taiping Street, Jiangyang District, Luzhou, 646000, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Perivascular epithelioid cell tumors (PEComas) are rare mesenchymal tumors originating from perivascular epithelioid cells. In gynecological system, the uterus is one of the most common sites affected by PEComas. Most PEComas are benign, and patients usually have a good prognosis. However, malignant uterus PEComa is rare, and better comprehensive epidemiological investigations are needed. To date, there are a few reported cases of uterus PEComa. We herein report a rare case of malignant PEComa occurred in the uterine corpus and cervix, possibly accompanied by pulmonary lymphangioleiomyomatosis (PLAM). In addition, 55 cases of malignant uterus PEComa were picked out and collected in the data base of PubMed and Medline. On the one hand, the age of onset, population distribution, clinical manifestations, metastatic sites and routes of metastasis were analysed. On the other hand, a summary of the epidemiology, pathogenesis, diagnosis, and treatments of uterus PEComa was given.
Keywords: perivascular epithelioid cell tumors, uterus, malignant, rare case, cervix
Introduction
Perivascular epithelioid cell tumors (PEComas) are a group of rare mesenchymal origin tumors derived from perivascular epithelioid cells that grow in nests and sheets around blood vessels, expressing features of melanotic and smooth muscle differentiation.1 PEComas have unpredictable biological behavior. Most of them are benign and can occur anywhere in the body.2 The classifications and corresponding sites are shown in Table 1.3,4 Some PEComas occurring in a non-classical anatomical distribution are called perivascular epithelioid cell tumors not otherwise specified (PEComas-NOS). PEComas-NOS does not meet any criteria for a normal disease entity as defined by histopathologic findings, while other types exhibit unique histological and immunohistochemical characteristics. In the female reproductive system, the uterus is the most common site affected by PEComas-NOS, and this can be defined as uterus PEComa. Uterine corpus is also frequently affected by uterus PEComa, while cases occurring in the cervix are rare.5–8
 |
Table 1 Classifications and Sites of Onset of PEComas |
Clinical symptoms of uterus PEComa are non-specific and include abnormal uterine bleeding, pelvic pain, pelvic mass and presumed fibroids.9 More rarely, patients may present with uterine rupture or hemoperitoneum, especially in the setting of pregnancy.10–12 Because of the rarity of the uterus PEComa, there are almost no epidemiological studies of this disease. The guidelines for diagnosis and treatment for this disease have not yet been well established both domestically and internationally. Currently, surgical treatment is the best choice for PEComas. However, whether the surgery can cure the disease depends on the severity of the disease at the time of the patient’s visit, so the screening and early diagnosis of the disease is very important.13
To date, there are about 150 cases of gynecological PEComas according to the data base of PubMed and Medline in English, with one-third of them are malignant uterus PEComa. This article reports a case of rare malignant PEComa occurring simultaneously in the cervix and uterine corpus, reviews the relevant literature in recent years, and discusses the epidemiology, pathological characteristics, biological behavior, diagnosis and treatment methods of malignant uterus PEComa, in order to improve people’s understanding of this disease.
Case Presentation
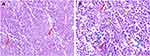
The patient was a 56-year-old woman who presented with symptoms of vaginal bleeding after sexual intercourse, accompanied by lower abdominal pain. The symptoms lasted for about one month, then she sought medical help and was admitted to our hospital. There was no special mention of medical history. Physical examination found a mass about 4 cm in diameter at the top of the vagina. Transvaginal ultrasound and Color Doppler flow imaging (CDFI) examinations were given to the patient to detect the locations and characteristics of the mass, which showed a hypoechoic mass 4.7 × 4.2 × 4.3 cm on the anterior wall of the uterus and a hypoechoic mass 2.3 × 3.7 × 4.5 cm at the cervix with abundant blood flow signals (Figure 1). Then, we removed a part of the lump for biopsy through the vaginal route. Hematoxylin and eosin (H&E) staining revealed that the tumor was composed of epithelioid cells primarily and spindle cells were found which were growing in a nested and alveolar pattern around small blood vessels. The tumor cells also exhibited transparent cytoplasm and showed evidence of cellular pleomorphism, pathological nuclear division, tumor necrosis and intravascular tumor emboli (Figure 2). Immunohistochemical staining for multiple biomarkers was conducted and the results showed positive immunoreactivity for HMB-45, Melan-A, SMA, Desmin, Vim, P53 (+, 40%) and Ki-67 (+, 60%) and negative for S100, S0X10, TFE3, PCK, P63, ER and LCA in the tumor cell cytoplasm, which matched with a PEComa. The level of Ki-67 is associated with increased risk of invasion and recurrence, which means this tumor was highly malignant. Taken these findings together, the tumor could be diagnosed as uterine and cervical malignant PEComa. Typical immunohistochemical indicators were shown in Figure 3.
 |
Figure 1 The locations of the tumor on transvaginal ultrasound apparatus (A, white arrows) and Color Doppler flow imaging (CDFI) results (B–D), red and light blue arrows). |
 |
Figure 3 The tumor cells showed immunohistochemical positivity for Melan-A (A) and Desmin (B). |
Further imageological examinations were performed to find out whether the tumor cells had infiltrated and/or metastasized to other organs or sites. Thickening of the tissues in the uterus, cervix and vagina was observed. A cauliflower-like mass about 8.5 × 5.6 cm in size was found by Magnetic Resonance Imaging (MRI) (Figure 4). In addition, multiple thin-walled cysts (10–30 mm) were detected in both lungs by chest computed tomography (CT) (Figure 5).
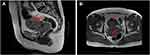 |
Figure 4 The location and TWI signal ((A): T2WI, (B) T1WI) of the tumor on MRI. The red arrows indicate the locations of the tumor. |
To completely remove the tumor and clarify its stage, the modified wide hysterectomy combined with bilateral salpingo-oophorectomy and bilateral pelvic lymphadenectomy was performed on the patient (Figure 6). Macroscopically, the uterine body and cervix fused partially to form a mass of approximately 8 × 4.5 × 4 cm without clear boundary (Figure 6A), which contains yellow mucus and necrotic component (Figure 6B). The enlarged lymph node was also observed near the right common iliac artery during operation.
Postoperative pathology revealed that tumor cells grow in patches around blood vessels with infiltrative growth. The cells showed obvious cellular pleomorphism and exhibited pathological nuclear division, tumor necrosis, and intravascular tumor emboli. The depth of tumor infiltration was greater than 1/3 of the cervical stroma and more than 1/2 of uterine myometrium, involving the vaginal vault and right parametrial tissues, with visible vascular invasion. There was evidence of cancer metastasis in the right external iliac lymph node.
Malignant uterus PEComa belongs to a rare tumor type in the female reproductive system. Most reported cases occurred in the corpus of uterus, while a few occurred in the cervix. The physiology after surgery in this case revealed the presence of malignant PEComa in both the uterine body and cervix. It is possible that the tumors originated from the uterine body and the cervix simultaneously. In addition, the patient’s chest computed tomography exhibited typical pulmonary lymphangioleiomyomatosis (PLAM) features. Due to the fact that both PLAM and malignant uterus PEComa belong to the PEComas family, they may have common pathogenic mechanisms. Unfortunately, the patient voluntarily gave up receiving more examinations and treatments. Thus, we failed to obtain lung tissue for pathological examination to confirm whether the patient had PLAM or not. Therefore, the diagnosis of PLAM cannot be ruled out at present.
Literature Review
For the review part, articles published in English in the electronic medical data bases of PubMed and Medline from January 1, 2005, to September 1, 2023, were selected. Various terms “perivascular epithelioid cell tumor”, “perivascular epithelioid cell neoplasm”, and “PEComa” were used as keywords in the article title and abstract. “uterine”, “uterus”, “cervix”, “cervical” as keywords of site of onset were included. The main subject terms are “perivascular epithelioid cell neoplasms”, “uterus”, “cervix”. The types of the articles were mainly focused on case reports and small-scale studies. A summary of finally selected articles (Table S1) was given in Supporting Information.
Epidemiology
Since malignant uterus PEComa is rare, there are almost no epidemiological investigations of this disease. The retrieved literature showed that the age of onset had a normal distribution (P > 0.05). The average age and median age of patients during consultation were 47.1 and 48, respectively, with a 95% confidence interval of (43.3, 50.9). Asians accounted for the largest proportion (60%) of this disease, followed by Europeans (20%) and Americans (18.1%). One-way analysis of variance (ANOVA) found that there was no statistical difference in the average age at disease onset among these populations (P > 0.05). In addition, the incidence of tuberous sclerosis complex (TSC) mutation is unusual in malignant uterus PEComa, accounting for only 14.5%.
The clinical manifestations of malignant uterus PEComa include abnormal uterine bleeding (41.8%), abdominal pain (25.4%), intra-abdominal hemorrhage (10.9%) and palpable abdominal mass (7.2%). In 12.7% of cases, patients had no symptoms and were found incidentally during surgery or physical examination. Malignant uterus PEComa can occur in different locations of the uterus, including the uterine corpus (80.0%), cervix (14.5%), broad ligament (5.4%), round ligament (3.6%), and both corpus and cervix (3.6%).
According to preoperative imaging examinations and postoperative pathological analysis, malignant PEComas originating in the uterus can metastasize to multiple tissues and organs, such as ovary (46.2%), pelvic lymph node (46.2%), lung (30.8%), vagina (23.1%) and intestine (15.4%). This suggests that there may be various ways of metastasis in malignant uterus PEComa, including implantation metastasis, lymphatic metastasis, and hematologic metastasis, etc. 25.5% patients developed metastasis after non-radical surgery, with the lung being the most common metastatic site (85.7%), followed by the liver (28.6%) and bone (21.4%). Non-radical surgery may make it easier for tumors to metastasize through the blood to distant sites, thus leading to adverse outcomes.
Pathogenesis
The occurrence of malignant uterus PEComa in tissues is controversial. So far, several hypotheses about the origin of PEComas have been proposed, such as originating from neural crest cells,14 smooth muscle cells,15 and pericytic cells.16 These assumptions are based on observations of pathological and molecular characteristics, and further experimental evidence is needed to support specific hypotheses and uncover the origin of PEComas. Molecular genetic studies have found that the onset of some malignant uterus PEComa are related to mutations in the genes of tuberous sclerosis complex 1 (TSC1) and/or tuberous sclerosis complex 2 (TSC2). A mutation in either of these two genes can lead to TSC, which is an autosomal dominant disorder that affects multiple organ systems.17
TSC1 encodes Hamartin, and TSC2 encodes tuberin. They bind with Tre2-Bub2-Cdc16 (TBC) 1 domain family, member 7 (TBC1D7) to form the regulatory complex called mammalian target of rapamycin complex 1 (mTORC1), which negatively regulates the mTOR pathway through a G protein called Rheb.18,19 Rheb inhibits cell proliferation and metabolism. If either of these two genes are deactivated, it will lead to abnormal cell proliferation.20,21 Specifically, evidence of transcription factor E3 (TFE3) gene fusions was found in the malignant uterus PEComa without TSC1/2 mutations.22 In addition, there have been reported of a rarer gene rearrangement, RAD51 paralog B (RAD51B) rearrangement, which has only been observed in malignant uterus PEComa.9,23 These may indicate that there are alternative pathways for the occurrence of malignant uterus PEComa.
Some cases have also reported increased expression of estrogen receptor (ER) and progesterone receptor (PR) in patients with malignant uterus PEComa, with a more significant increase in ER, and hormone therapy has been effective.24–26Whether malignant uterus PEComa is a hormone-dependent disease is still lacking in research.
Diagnosis
Malignant uterus PEComa is a rare group of tumor that is often confused with other uterine mesenchymal tumors, such as endometrial stromal sarcoma and leiomyosarcoma. Patients may experience a range of similar symptoms, taking vaginal bleeding, abdominal pain, compression syndrome and palpable abdominal mass as examples. Currently, there are several cases that the early diagnosis of cervical PEComa has been achieved through the use of routine cervical smears.6,27,28 However, this method is not applicable for the diagnosis of PEComa originated from uterine body. Uterus PEComa can be diagnosed by histological and immunohistochemical features, but preoperative diagnosis can be challenging.
Microscopic observation reveals that cells of uterus PEComa are usually epithelioid or spindled, which are often arranged in nests and sheets. Most of them have typical transparent, eosinophilic and granular cytoplasm and tend to grow around blood vessels.9 Melanocytic markers (HMB-45 and/or Melan-A) and myogenic markers (SMA and/or Desmin) are special immunohistochemical indicators for diagnosing uterus PEComa.29–31 However, when HMB-45 and Melan-A are negative or locally positive, PNL2 may be a more useful biomarker for uterus PEComa.32,33 If both histological and immunohistochemical results are consistent with uterus PEComa, further assessments are also needed to determine the malignancy of uterus PEComa. This is tightly related to subsequent treatment strategies. Folpe et al classified the uterus PEComa as follows: “benign”, “uncertain malignant potential” and “malignant”.31 The classification criteria can be found in Table 2.
 |
Table 2 Classification of Uterus PEComa |
Treatments
Because malignant uterus PEComa is very rare in clinical practice, the management strategies have not yet been well established globally, and the efficacies of various treatment regimens are inconsistent. Until now, the number of cases reported in the database is small, and there are various treatment methods. However, the follow-up time is limited, so we cannot perform statistical analysis to find the best treatment plan.
According to the reported literature, primary surgical excision is the golden standard for the treatment of malignant uterus PEComa. The selection of the surgical extent should be individualized based on the patient’s health condition and the tumor’s biological behavior, with the ultimate goal of achieving a tumor-free margin. After radical surgery, patients have a good survival rate, which greatly reduces the recurrence or metastasis of tumors.
Radical hysterectomy with negative margin is considered the best surgical method for malignant uterus PEComa.34 If the fertility function of the patient should be preserved, only removing the tumor seems to be a good option for low-grade malignant uterus PEComa. However, the possibility of recurrence of this treatment strategy is high, and another surgery is needed.35 Adjuvant therapy should be provided to patients after surgery, especially for those with locally advanced or metastatic malignant uterus PEComa,34 although the efficacy of chemotherapy and radiotherapy is uncertain.
Chemotherapy drugs, including ifosfamide, doxorubicin, vincristine, taxol, gemcitabine, apatinib and everolimus, as well as different combinations of them, are used to treat malignant uterus PEComa. Among them, mTOR inhibitors and VEGFR inhibitors have the highest disease control rates for advanced PEComas.36 Inhibition of the mTOR pathway combined with inhibition of the VEGFR pathway may be a useful strategy for metastatic uterus PEComa. Mechanistically, the occurrence of malignant uterus PEComa in some patients may be related to TSC mutations, causing overactivation of mTOR and abnormal cell proliferation. Everolimus and sirolimus are specific inhibitors of mTOR, and targeting treatment with mTOR inhibitors has important clinical value.37,38 Interestingly, mTOR inhibitor for a patient with a mutation in the TFE3 gene but without TSC mutation was also effective.39 However, gastrointestinal PEComa patient with TFE3 mutations did not respond to everolimus. But after switching to apatinib, the patient’s condition was improved partially.40 Overexpression of VEGF is a prognostic factor in patients with solid tumors and is associated with increased risk of metastasis and reduced overall survival.41 mTOR inhibitors are considered the most active drugs, but a small number of patients is resistant to them.42,43 Thus, VEGFR inhibitors and chemotherapy regimens based on gemcitabine or anthracyclines could be used as options for patients with drug resistance of mTOR inhibitors whether preoperative neoadjuvant chemotherapy or postoperative supportive chemotherapy.36 A few reports have found that combining mTOR inhibitors with VEGFR inhibitors can alleviate advanced uterus PEComa.41,44,45 For a small number of malignant uterus PEComas expressing estrogen and progesterone receptors, hormonal therapy may be a good option.24–26 Due to the rarity of the disease, it requires further confirmation of effectiveness of combination therapy through large-scale prospective studies.
There are a few cases of patients receiving radiation therapy.34,36,46–49 Radiation therapy may show clinical efficacy in local disease control, but these reports did not provide complete radiation treatment strategies. Theoretically, radiotherapy is reasonable and effective for malignant uterus PEComa. Firstly, malignant uterus PEComa has rich capillaries with higher oxygen content and relatively weak resistance to ionization radiation.50 Secondly, the tumor cells have a high mitotic index. They are exposed to more radiation during the mitosis (M) phase of cell division, causing DNA damage which is difficult to repair, resulting in apoptosis and mitotic catastrophe.51 Radiotherapy can be combined with angiostatin in the treatment of malignant tumors. This combination therapy does not increase the deleterious effects but can improve the tumor eradication rate.52
Discussion and Conclusion
We herein reported a case of rare malignant uterus PEComa. The patient presented with abnormal uterine bleeding and abdominal pain, and an enlarged uterus could be palpated in the lower abdomen. These symptoms and signs are similar to other uterine tumors, and often result in misdiagnosis before surgery. Postoperative pathology showed that the uterus PEComa exhibited four worrisome features, including a maximum tumor diameter of approximately 8 cm, tumor necrosis and vascular invasion seen under the microscope, infiltration of the uterine myometrium and cervical stroma, and pathologic nuclear division of tumor cells. The level of Ki-67 (60%), which indicates the rate of cancer cell proliferation, proved this disease was highly malignant and might have a poor prognosis.
Molecular genetic research on malignant uterus PEComa has found that some patients have mutations in the TSC gene, which is related to TSC. The 2012 International Tuberous Sclerosis Complex Consensus Group released diagnostic criteria for TSC, including genetic diagnostic criteria and clinical diagnostic criteria. Experts suggest that the diagnosis of TSC can be made by identifying pathogenic mutations in TSC1 or TSC2 or by considering the concomitance of the patient’s major and minor clinical features.53 Patient in this case did not show any specific skin, dental, oral and nail lesions during the physical examination. CT scans did not reveal any lesions such as cardiac rhabdomyoma, multiple renal cysts, nonrenal hamartoma and vascular adipose tumors. Therefore, molecular testing for TSC1 and TSC2 genes was recommended, and the results could be used as a basis for the prescription of mTOR inhibitor.
More rarely, this patient might develop PEComa lesions in both lungs, which were different from uterus PEComa. Lymphangioleiomyomatosis (LAM) is a type of PEComas that typically occurs in the lungs, causing progressive cystic destruction of lung parenchyma.54 It is a rare condition, and usually affects females.55 The patient’s chest CT result revealed multiple thin-walled cystic changes in both lungs. Pneumologist identified these lesions as a suspicious PLAM because of the typical PLAM imaging features, such as thin cyst wall, bilateral, diffuse distribution, round shape, size ranging from 2 to 30 millimeters, and devoid of internal structure.56 Some people believe that it is sufficient to diagnose LAM solely based on the features of high resolution CT (HRCT),57 especially when there is pathological evidence of pelvic masses.58 According to literature, PLAM often occurs together with uterus PEcoma,59 and the uterus may be the origin site of LAM.60–62 However, for a definite diagnosis, other people believe that it is necessary to obtain tissue or cytological examination for verification.
In the review part, 55 cases of malignant uterus PEComa were collected and analyzed. A summary of the epidemiology, pathogenesis and diagnostic methods for malignant uterus PEComa was given. The average age at onset is 47.1 years old. Asians are most likely to develop this disease (60%) compared with other races. Clinical manifestations include abnormal uterine bleeding (41.8%), abdominal pain (25.4%), intra-abdominal hemorrhage (10.9%), and palpable abdominal masses (7.2%), while 12.7% are asymptomatic. Malignant PEComas originating in the uterus most likely metastasizes to the ovary (46.2%) and pelvic lymph nodes (46.2%). After surgery, the main site of metastasis is lung (85.7%). The origin and pathogenesis of malignant uterus PEComa are not clear, and about 14.5% of patients are found TSC1 and/or TSC2 gene mutation. A few patients have their onset related to TFE3 gene fusion and RAD51B gene rearrangement. Preoperative diagnosis is difficult, and a definite diagnosis depends on histological and immunohistochemical results.
In addition, treatment plans and follow-up information of patients with malignant uterus PEComa over the past 20 years were collected, hoping to provide better clues for standardizing the treatment strategies of this disease. To date, there is no specific management guideline globally, and multi-sample studies are crucial for developing the best management strategy for this rare disease. Currently, primary surgical excision is the gold standard for treatment. Postoperative chemotherapy combined with radiotherapy is needed to maximize the therapeutic effect, and treatment needs to be individualized.63 There is evidence that chemotherapy regimen using mTOR inhibitors in combination with VEGFR inhibitors may be beneficial for the treatment of this disease.
Even there are a few reported cases of PEComas, due to the rarity of malignant uterus PEComa, further research should be conducted to clarify their clinical characteristic, management strategies and prognosis.
Data Sharing Statement
This case report contains clinical data from the medical records in the Affiliated Hospital of Southwest Medical University. Additional information is available from the corresponding author Q. Wang or Y. Yang upon reasonable request.
Ethics Approval
The study was conducted in accordance with the Declaration of Helsinki. The Affiliated Hospital of Southwest Medical University has approved the release of case details.
Consent Statement
The patient gave her consent for publication of the case report.
Funding
This study was supported by the grants from the Luzhou Science and Technology Department Applied Basic Research program (No: 2022-WYC-196), the Sichuan Province Science and Technology Department of foreign (border) high-end talent introduction project (No: 2023ZHYZ0009), the Doctoral Research Initiation Fund of the Affiliated Hospital of Southwest Medical University (No. 18061) and the Fund for High-level Talents in Luzhou City (No. 02/00180095, 02/00180117).
Disclosure
None of the authors declare any conflict of interest.
References
1. Gadducci A, Zannoni GF. Perivascular epithelioid cell tumors (PEComa) of the female genital tract: a challenging question for gynaecologic oncologist and pathologist. Gynecol Oncol Rep. 2020;33:100603. doi:10.1016/j.gore.2020.100603
2. Czarnecka AM, Skoczylas J, Bartnik E, et al. Management strategies for adults with locally advanced, unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa): challenges and solutions. Cancer Manage Res. 2023;15:615–623. doi:10.2147/cmar.S351284
3. Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19(5):359–368. doi:10.1016/j.anndiagpath.2015.06.003
4. Fletcher CD, Unni K, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC press; 2002.
5. Azad NS, Aziz AB, Pervez S, et al. Uterine perivascular epithelioid cell tumour presenting as a cervical mass. J Pak Med Assoc. 2006;56(2):83–84.
6. Tang X, Feng M, Shen Y, et al. Perivascular epithelioid cell tumor of the uterine cervix identified on the liquid-based cytology: a case report. Diagn. Pathol. 2023;18(1):7. doi:10.1186/s13000-023-01290-3
7. Zhang C, Pan F, Qiao J, et al. Perivascular epithelioid cell tumor of the cervix with malignant potential. Int J Gynaecol Obstet off Organ Int Fed Gynaecol Obstet. 2013;123(1):72–73. doi:10.1016/j.ijgo.2013.06.013
8. Sharmila V, Balakrishnan P, Arun Babu T. PEComa of Uterine Cervix. Ind J Surg Oncol. 2021;12(4):686–687. doi:10.1007/s13193-021-01416-3
9. Bennett JA, Braga AC, Pinto A, et al. Uterine PEComas: a morphologic, immunohistochemical, and molecular analysis of 32 tumors. Am J Surg Pathol. 2018;42(10):1370–1383. doi:10.1097/pas.0000000000001119
10. Nitahara K, Sasaki M, Ichikawa S, et al. Rupture of perivascular epithelioid cell neoplasm at 34 weeks’ gestation: a nonendometriosis case of spontaneous hemoperitoneum in pregnancy. J Obst Gynaecol Res. 2019;45(3):709–713. doi:10.1111/jog.13870
11. Babayev E, Fay KE, Horowitz JM, et al. Perivascular epithelioid cell tumors (PEComa) in pregnancy with uterine rupture and ongoing abdominal gestation: a case report. Case Rep Wom Health. 2020;25:e00172. doi:10.1016/j.crwh.2020.e00172
12. Nguyen JMV, Ghandehari H, Parra-Herran C, et al. Uterine rupture: an unusual presentation of a uterine perivascular epithelioid cell tumor (PEComa). Intern J Gynecolog Can. 2020;30(12):2008–2011. doi:10.1136/ijgc-2020-001837
13. D’Augè TG, Giannini A, Bogani G, et al. Prevention, screening, treatment and follow-up of gynecological cancers: state of art and future perspectives. Clin Exp Obstet Gynecol. 2023;50(8). doi:10.31083/j.ceog5008160
14. Gammill LS, Bronner-Fraser M. Neural crest specification: migrating into genomics. Nat Rev Neurosci. 2003;4(10):795–805. doi:10.1038/nrn1219
15. Stone CH, Lee MW, Amin MB, et al. Renal angiomyolipoma: further immunophenotypic characterization of an expanding morphologic spectrum. Arch Pathol Lab Med. 2001;125(6):751–758. doi:10.5858/2001-125-0751-ra
16. Shen J, Shrestha S, Yen YH, et al. Pericyte antigens in angiomyolipoma and PEComa family tumors. Med Oncol. 2015;32(8):210. doi:10.1007/s12032-015-0659-y
17. Henske EP, Jóźwiak S, Kingswood JC, et al. Tuberous sclerosis complex. Nat Rev Dis Prim. 2016;2:16035. doi:10.1038/nrdp.2016.35
18. Garami A, Zwartkruis FJ, Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Molecular Cell. 2003;11(6):1457–1466. doi:10.1016/s1097-2765(03)00220-x
19. Dibble CC, Elis W, Menon S, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Molecular Cell. 2012;47(4):535–546. doi:10.1016/j.molcel.2012.06.009
20. Benvenuto G, Li S, Brown SJ, et al. The tuberous sclerosis-1 (TSC1) gene product hamartin suppresses cell growth and augments the expression of the TSC2 product tuberin by inhibiting its ubiquitination. Oncogene. 2000;19(54):6306–6316. doi:10.1038/sj.onc.1204009
21. Huang J, Dibble CC, Matsuzaki M, et al. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol. 2008;28(12):4104–4115. doi:10.1128/mcb.00289-08
22. Schoolmeester JK, Dao LN, Sukov WR, et al. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39(3):394–404. doi:10.1097/pas.0000000000000349
23. Agaram NP, Sung YS, Zhang L, et al. Dichotomy of genetic abnormalities in PEComas with therapeutic implications. Am J Surg Pathol. 2015;39(6):813–825. doi:10.1097/pas.0000000000000389
24. Armah HB, Parwani AV. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus with late renal and pulmonary metastases: a case report with review of the literature. Diagn Pathol. 2007;2:45. doi:10.1186/1746-1596-2-45
25. Le P, Garg A, Brandao G, et al. Hormonal manipulation with letrozole in the treatment of metastatic malignant pecoma. Curr Oncol. 2014;21(3):e518–20. doi:10.3747/co.21.1849
26. Okamoto S, Komura M, Terao Y, et al. Pneumothorax caused by cystic and nodular lung metastases from a malignant uterine perivascular epithelioid cell tumor (PEComa). Respirat Med Case Rep. 2017;22:77–82. doi:10.1016/j.rmcr.2017.06.011
27. Tajima S, Koda K. Perivascular epithelioid cell tumor of the uterine cervix identified on a conventional cervical smear. Diagn Cytopathol. 2015;43(12):1011–1016. doi:10.1002/dc.23369
28. Stone JL, Batty T, Nicklin J. Cervical perivascular epithelioid cell tumour (PEComa) of the uterine cervix: cytological findings in a cervical smear. Cytopathology. 2013;24(4):272–273. doi:10.1111/j.1365-2303.2012.00989.x
29. Bonetti F, Pea M, Martignoni G, et al. PEC and sugar. Am J Surg Pathol. 1992;16(3):307–308. doi:10.1097/00000478-199203000-00013
30. Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48(1):75–82. doi:10.1111/j.1365-2559.2005.02316.x
31. Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29(12):1558–1575. doi:10.1097/01.pas.0000173232.22117.37
32. Gulavita P, Fletcher CDM, Hirsch MS. PNL2: an adjunctive biomarker for renal angiomyolipomas and perivascular epithelioid cell tumours. Histopathology. 2018;72(3):441–448. doi:10.1111/his.13369
33. Valencia-Guerrero A, Pinto A, Anderson WJ, et al. PNL2: a useful adjunct biomarker to HMB45 in the diagnosis of uterine perivascular epithelioid cell tumor (PEComa). Internat J Gynecolog Pathol. 2020;39(6):529–536. doi:10.1097/pgp.0000000000000653
34. Jeon IS, Lee SM. Multimodal treatment using surgery, radiotherapy, and chemotherapy in a patient with a perivascular epithelioid cell tumor of the uterus. J Pediat Hematol. 2005;27(12):681–684. doi:10.1097/01.mph.0000193475.06870.d5
35. Yamamoto E, Ino K, Sakurai M, et al. Fertility-sparing operation for recurrence of uterine cervical perivascular epithelioid cell tumor. Rare Tumors. 2010;2(2):e26. doi:10.4081/rt.2010.e26
36. Sanfilippo R, Jones RL, Blay JY, et al. Role of Chemotherapy, VEGFR Inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas). Clin Can Res. 2019;25(17):5295–5300. doi:10.1158/1078-0432.Ccr-19-0288
37. Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28(5):835–840. doi:10.1200/jco.2009.25.2981
38. Italiano A, Delcambre C, Hostein I, et al. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Anna Oncol. 2010;21(5):1135–1137. doi:10.1093/annonc/mdq044
39. Purwar R, Soni K, Shukla M, et al. TFE3-associated perivascular epithelioid cell tumor with complete response to mTOR inhibitor therapy: report of first case and literature review. World J Surg Oncol. 2022;20(1):62. doi:10.1186/s12957-021-02462-5
40. Xu J, Gong XL, Wu H, et al. Case report: gastrointestinal PEComa With TFE3 rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol. 2020;10:582087. doi:10.3389/fonc.2020.582087
41. Liapi A, Mathevet P, Herrera FG, et al. VEGFR Inhibitors for uterine metastatic perivascular epithelioid tumors (PEComa) Resistant to mTOR Inhibitors. A case report and review of literature. Front Oncol. 2021;11:641376. doi:10.3389/fonc.2021.641376
42. Kwon BS, Suh DS, Lee NK, et al. Two cases of perivascular epithelioid cell tumor of the uterus: clinical, radiological and pathological diagnostic challenge. Eur J Med Res. 2017;22(1):7. doi:10.1186/s40001-017-0248-y
43. Kopparthy P, Murphy M. Rapid and durable response with nab-sirolimus after everolimus failure in a patient with perivascular epithelioid cell tumors (PEComas) of the uterus. Cureus. 2021;13(5):e14951. doi:10.7759/cureus.14951
44. Gao F, Huang C, Zhang Y, et al. Combination targeted therapy of VEGFR inhibitor, sorafenib, with an mTOR inhibitor, sirolimus induced a remakable response of rapid progressive Uterine PEComa. Cancer Biol Ther. 2016;17(6):595–598. doi:10.1080/15384047.2016.1167290
45. Sui C, Wu J, Mei D, et al. Uterine perivascular epithelioid tumors (PEComas) with lung metastasis showed good responses to mTOR and VEGFR inhibitors: a case report. Front Oncol. 2022;12:797275. doi:10.3389/fonc.2022.797275
46. Fukunaga M. Perivascular epithelioid cell tumor of the uterus: report of four cases. Internat J Gynecolog Pathol. 2005;24(4):341–346. doi:10.1097/01.pgp.0000168515.83557.89
47. Ciarallo A, Makis W, Hickeson M, et al. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus: serial imaging with F-18 FDG PET/CT for surveillance of recurrence and evaluation of response to therapy. Clin. Nucl. Med. 2011;36(4):e16–9. doi:10.1097/RLU.0b013e31820ae032
48. Natella V, Merolla F, Giampaolino P, et al. A huge malignant perivascular epithelioid cell tumor (PEComa) of the uterine cervix and vagina. Pathol Res Pract. 2014;210(3):186–188. doi:10.1016/j.prp.2013.10.003
49. Ascione A, Martignoni G, d’Amati G, et al. Extremely late-onset pulmonary metastasis from uterine PEComa. Pathologica. 2022;114(4):312–315. doi:10.32074/1591-951x-762
50. Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26(312):638–648. doi:10.1259/0007-1285-26-312-638
51. Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tum Biol. 2010;31(4):363–372. doi:10.1007/s13277-010-0042-8
52. Mauceri HJ, Hanna NN, Beckett MA, et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–91. doi:10.1038/28412
53. Northrup H, Krueger DA, Northrup H. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49(4):243–254. doi:10.1016/j.pediatrneurol.2013.08.001
54. McCormack FX, Travis WD, Colby TV, et al. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med. 2012;186(12):1210–1212. doi:10.1164/rccm.201205-0848OE
55. Harknett EC, Chang WY, Byrnes S, et al. Use of variability in national and regional data to estimate the prevalence of lymphangioleiomyomatosis. QJM. 2011;104(11):971–979. doi:10.1093/qjmed/hcr116
56. McCarthy C, Gupta N, Johnson SR, et al. Lymphangioleiomyomatosis: pathogenesis, clinical features, diagnosis, and management. Lancet Respir Med. 2021;9(11):1313–1327. doi:10.1016/s2213-2600(21)00228-9
57. Johnson SR, Cordier JF, Lazor R, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Europ resp J. 2010;35(1):14–26. doi:10.1183/09031936.00076209
58. Gupta N, Finlay GA, Kotloff RM, et al. Lymphangioleiomyomatosis diagnosis and management: high-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. Am J Respir Crit Care Med. 2017;196(10):1337–1348. doi:10.1164/rccm.201709-1965ST
59. Hayashi T, Kumasaka T, Mitani K, et al. Prevalence of uterine and adnexal involvement in pulmonary lymphangioleiomyomatosis: a clinicopathologic study of 10 patients. Am J Surg Pathol. 2011;35(12):1776–1785. doi:10.1097/PAS.0b013e318235edbd
60. Clay MR, Gibson P, Lowell J, et al. Microscopic uterine lymphangioleiomyomatosis perivascular epithelioid cell neoplasm: a case report with the earliest manifestation of this enigmatic neoplasm. Internat J Gynecolog Pathol. 2011;30(1):71–75. doi:10.1097/PGP.0b013e3181efe08d
61. Prizant H, Sen A, Light A, et al. Uterine-specific loss of Tsc2 leads to myometrial tumors in both the uterus and lungs. Molecul Endocrinol. 2013;27(9):1403–1414. doi:10.1210/me.2013-1059
62. Ando H, Ogawa M, Watanabe Y, et al. Lymphangioleiomyoma of the uterus and pelvic lymph nodes: a report of 3 cases, including the potentially earliest manifestation of extrapulmonary lymphangioleiomyomatosis. Internat J Gynecolog Pathol. 2020;39(3):227–232. doi:10.1097/pgp.0000000000000589
63. Di Donato V, Giannini A, Bogani G. Recent advances in endometrial cancer management. J Clin Med. 2023;12(6):2241. doi:10.3390/jcm12062241
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.