Back to Journals » International Medical Case Reports Journal » Volume 17
A Case of Dermatomyositis with Coexistence of Positive Anti-MDA5 Antibodies and Anti-SSA/RO52 Antibodies, Combined with Necrotic Skin Ulcers
Authors Sun T , Hu ZH, He JS, Chen YC, Gao YX
Received 16 October 2023
Accepted for publication 1 December 2023
Published 6 January 2024 Volume 2024:17 Pages 9—15
DOI https://doi.org/10.2147/IMCRJ.S441691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Tian Sun,1 Zhong Hao Hu,1 Jin Shi He,1 Yu Chi Chen,2 Yong Xiang Gao2
1Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan Province, People’s Republic of China
Correspondence: Yu Chi Chen, Email [email protected]; Yong Xiang Gao, Email [email protected]
Background: Dermatomyositis (DM) is an idiopathic inflammatory myopathy that is clinically challenging to diagnose and has a poor prognosis. It is characterized by symmetric proximal muscle weakness, muscle tenderness, dysphagia, characteristic skin rash (heliotrope rash, Gottron’s sign), elevated muscle enzyme levels, abnormal electromyography, and muscle biopsy findings. DM with positive anti-MDA5 antibodies is mainly characterized by Gottron’s sign, skin ulcers, facial erythema, mechanic’s hands, and V-sign. In this case, the patient presented with the rare manifestation of severe necrotic skin ulcers in association with Gottron’s sign, prompting us to report this case.
Case Presentation: A 45-year-old female was admitted to the hospital with systemic joint pain, fatigue, multiple ulcers, and purulent discharge on both hands. Her myositis-specific antibody profile revealed positive anti-MDA5 and anti-SSA/RO52 antibodies. Treatment included a combination of glucocorticoids, immunosuppressants, gastric and liver protection, infection control, and wound care. After two weeks of treatment, the patient showed improvement in symptoms. However, on the 24th day of hospitalization, the wound at the right elbow joint ruptured and became infected, requiring debridement and skin grafting in the appropriate department.
Conclusion: There has been limited research and reported cases of dermatomyositis with coexistence of positive anti-MDA5 and anti-SSA/RO52 antibodies combined with severe skin ulcers. Therefore, we present this rare case and emphasize the need for close follow-up on pulmonary involvement and skin ulcer progression, as well as timely implementation of new treatment strategies to actively improve the prognosis.
Keywords: dermatomyositis, MDA5 antibodies, SSA/RO52 antibodies, immunosuppressants
Introduction
Dermatomyositis (DM) with coexistence of positive anti-MDA5 antibodies and anti-SSA/RO52 antibodies (MDA5+DM) is a rare subtype of dermatomyositis. European studies have shown that the prevalence of this disease varies regionally, with rates ranging from 1.3% to 10% in European populations and 11% to 60% in Asian countries, with a higher incidence among females.1,2 Numerous clinical studies have demonstrated that MDA5+DM can lead to rapidly progressive interstitial lung disease (RP-ILD) and rare complications such as mediastinal emphysema, macrophage activation syndrome, and spontaneous muscle hemorrhage.3 The presence of anti-MDA5 antibodies is closely associated with specific symptoms in DM patients, such as skin ulcers and palmoplantar papules.4 The prognosis of MDA5+DM is typically poor, highlighting the urgent need for further research on its clinical diagnosis and treatment.
Unlike typical dermatomyositis, MDA5+DM is distinct and carries a risk of developing complications independent of immunosuppressive therapy. MDA5-DM+ILD patients are susceptible to pulmonary infections. As demonstrated in this case report, severe skin infections often accompany skin ulcers.5 The necrotic skin ulcers in this case exhibited a rapidly progressive and life-threatening nature. Approximately six days after admission, the wounds on the upper limbs progressively increased in number, expanded rapidly, and deepened quickly, even reaching the bone, putting the patient at risk of disability.
Case Report
A 45-year-old Tibetan female (actual age corrected from 35 years, as stated incorrectly in the medical records) presented with significant swelling and pain in the bilateral metacarpophalangeal and elbow joints. She reported muscle weakness in all four limbs, with slight swelling and discomfort in both knee joints and restricted range of motion in all four limbs, particularly in the right upper limb. She experienced morning stiffness lasting more than one hour and exhibited a right hand deformity with a right angle bend at the proximal interphalangeal joint of the ring finger. Eyelid swelling was also observed. Physical examination revealed 5–6 ulcerated wounds, approximately 1*2 cm in size, with yellow viscous secretions on the metacarpophalangeal and interphalangeal joints of both hands. These wounds showed a trend of increasing number as well as rapid expansion and deepening. There was a longitudinal ulcerated wound, approximately 8*2 cm in size, on the right elbow joint. The periorbital area showed erythema, and scattered skin rash was observed on the back. The patient did not report any ulcers or itching. Additional observations noted severe deformity, multiple ulcerations with purulent discharge, and necrotic ulcers on the metacarpophalangeal and interphalangeal joints of both hands, indicative of mechanic’s hands. The patient exhibited cyanosis of the lips, hair loss, symmetrical chest without deformities, and occasional dry and moist rales in both lungs. The spine appeared normal, and multiple joints throughout the body were tender with restricted movement. Local skin temperature was elevated.
The patient was admitted with approximately normal liver and renal function, high blood lipids, creatine kinase: 40 U/L, blood count: lymphocyte count: 0.63*10^9/L, percentage of lymphocytes: 7%, monocytes: 0.28*10^9/L, neutrophil count: 7.98*10^9/L, CRP: 83.2 mg/L. The patient’s diagnosis was not clear, and a sample of his serum was sent to an external clinical Laboratory, myositis-specific antibody profile report suggested positive anti-MDA5 antibody (Figure 1), titre: 1:300 (fibronectin/CBA method),6,7 positive anti-SSA/RO52 antibody.8 Skin biopsy: the tissue was covered with slightly hyperplastic squamous epithelium, subepithelial fibrous hyperplasia, vitellosis, and culture of multidrug-resistant organisms (Escherichia coli, Staphylococcus aureus) in the mesenchyme, perivascular area, and wound secretion from the joints around the skin appendages. Chest CT scan half a month later showed interstitial lung lesions (Figure 2), and the patient was found to be suffering from new oral ulcers around 20 days. Twenty-four days later, the patient’s medical condition was controlled and stabilised, and the erythema and ulcers had subsided locally with no new eruptions, and the medical problems were basically resolved, but the patient’s right elbow lesion was large and deep, and in order to deal with all the lesions that were unable to heal on their own, the patient was transferred to the Department of Burns and Plastic Surgery of the Sichuan Provincial People’s Hospital, where he was proposed to have a debridement and skin grafting. In conclusion, the patient’s symptoms, signs, examinations and tests were highly consistent with the symptoms of this disease.9 Based on these results, we diagnosed a double-positive dermatomyositis with anti-MDA5 and anti-SSA/RO52 antibodies.
 |
Figure 1 Myositis-specific antibody profile report. |
 |
Figure 2 Patient’s chest CT report. |
Typical Skin Ulcers
On the day of admission (2023-07-01), the patient had ulceration and crusting at the distal interphalangeal joint of the left second finger, proximal interphalangeal joint of the left fourth finger, metacarpophalangeal joints of the right second and fifth fingers. The affected areas showed exfoliation and erythema of the superficial skin, with the presence of yellow viscous secretions upon compression. There was a dark red rash at the bottom of the joint of the left foot at the first toe and on the outer side of the metatarsophalangeal joint of the left foot. A longitudinal ulcerated wound on the right elbow joint had crust formation and measured approximately 8*2cm (prior to admission, the patient had received “cauterization treatment” at a local Tibetan medicine clinic for the right elbow, but there was no significant improvement). Irregularly shaped scattered skin rashes were observed on the back, approximately 4*3cm in size, and there was noticeable erythema around both eye sockets (Figure 3).
 |
Figure 3 Patient’s skin condition on 2023-07-01. |
On the 6th day of admission (2023-07-06), the muscles surrounding the distal interphalangeal joint of the left second finger, proximal interphalangeal joint of the left fourth finger, metacarpophalangeal joints of the right second and fourth fingers, and the proximal and distal interphalangeal joints of the right fifth finger were affected. The white joint capsule was exposed, accompanied by exfoliation and erythema of the superficial skin. Yellow viscous secretions were observed upon compression. The crusts on the longitudinal ulcerated wound of the right elbow joint broke, and yellow viscous secretions spontaneously flowed out. A new ulcerated wound, measuring 1*2 cm, had developed on the left elbow joint without purulent discharge. A fresh red rash, approximately 2*1 cm in size, appeared on the right knee joint. The condition of the skin rashes in other areas remained the same as before (Figure 4).
 |
Figure 4 Patient’s skin condition on 2023-07-06. |
On the 11th day of admission (2023-07-11), the patient had severe purulent discharge at the site of the longitudinal ulcerated wound on the right elbow joint. The patient was advised to seek treatment at the dermatology department of our hospital for dressing changes. Some of the wounds were debrided and dressed by the dermatology department. There were scattered bruises near the ulcerated wound on the left elbow joint, and the condition of the skin rashes in other areas remained the same as before, with no new developments at the moment (Figure 5).
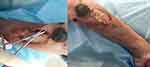 |
Figure 5 Patient’s skin condition on 2023-07-11. |
On the day of discharge (2023-07-25), the patient had ulceration at the distal interphalangeal joint of the left second finger, metacarpophalangeal joint of the right second finger, and proximal interphalangeal joint of the left fourth finger. The white joint capsule was exposed at the metacarpophalangeal joints of the right second and fourth fingers, as well as the proximal and distal interphalangeal joints of the right fifth finger. No secretions or erythema were observed at these sites (Figure 6).
 |
Figure 6 Patient’s skin condition on 2023-07-25. |
Discussion and Conclusions
We present a rare case of double-positive dermatomyositis with anti-MDA5 antibody and anti-SSA/RO52 antibody, which poses significant diagnostic and therapeutic challenges. Cutaneous manifestations of dermatomyositis include Gottron’s sign, rash, V sign, among others. Gottron’s sign is a bright red scaly rash slightly above the skin on the extensor side of the joints, mostly on the backs of the interphalangeal joints, auricles, finger bellies, trunk, V region of the chest, and trunk, and in a few cases, skin ulcers may be present. However, we report a case of severe necrotizing skin ulcer combined with a pathogenic infection and interstitial lung lesion, which is a rare occurrence. Therefore, we mainly document the skin symptoms during hospitalization, and the prognosis of the patient is yet to be determined.
Gottron’s sign is a typical manifestation of dermatomyositis, characterized by erythematous or purplish-red plaques on the metacarpophalangeal and interphalangeal joints, accompanied by scaling and ulceration. Pathologically, local lymphoedema is observed, while skin ulcers typically present with loss of dermis and epidermis, often accompanied by loss of subcutaneous tissue.10,11 Hua Cao et al reported that 38.4% of patients with ulcerative Gottron’s sign developed necrosis out of 5 patients with DM and 21 patients with CADM. The probability of anti-MDA5 antibody positivity in ulcerative Gottron’s sign was generally higher than that in the no-ulcer group, and only 3–19% of DM patients would develop skin ulcers with severe pain, and even face the risk of disability at a later stage.12,13 In this case, the necrosis of the skin ulcer may even invade the bone layer, which is rare. It is now believed that this disease is mainly caused by type I interferon (IFN), and the expression of serum IFN-α is increased in patients with anti-MDA5-positive dermatomyositis. More importantly, the genetic signature of type I IFN is evident in peripheral blood mononuclear cells (PBMC) and skin tissues of these patients, and the discovery of autoantibodies that directly stimulate the production of IFN-γ supports this view.14,15
MDA5 has been recognized as a specific autoantigen in dermatomyositis (DM), and the presence of anti-MDA5 antibodies is associated with the correlation between skin ulcers and interstitial lung disease (ILD). Therefore, the investigation of cutaneous symptoms of the disease may aid in the diagnosis of ILD.16,17 Interstitial pulmonary fibrosis is observed in a significantly higher number of patients with anti-SSA/Ro52 and anti-MDA5 antibodies compared to those with only anti-MDA5 antibodies, and it is associated with a relatively better prognosis. Conversely, patients with only MDA5 antibodies have a relatively worse prognosis.18,19 The resurgence of anti-MDA5 antibody levels is closely linked to disease recurrence, and high levels of creatine kinase, elevated CRP levels, anti-Ro52 positivity, and high anti-MDA5 titres (++∼+++) are independent risk factors for rapidly progressive ILD (RPILD).20,21
Currently, there are no established official guidelines for the treatment of such diseases. The treatment of dermatomyositis typically involves the use of glucocorticoids either alone or in combination with immunosuppressive agents. The adjustment of dosage is based on the degree of improvement in the patient’s clinical symptoms. In the case at hand, the treatment plan involved the administration of high-dose intravenous methylprednisolone sodium succinate, intravenous cyclophosphamide, and oral tacrolimus to alleviate symptoms.22 This regimen was supplemented with gastric protection, liver protection, anti-infection measures, timely debridement, and dressing changes. After two weeks of treatment, the patient’s inflammatory markers decreased, and there was an improvement in mood and sleep. While ulcers on the fingers and rashes on the face and back persisted without oozing pus, there were no new rashes in other areas. Additionally, symptoms such as joint pain and wound discharge were significantly reduced. However, there was a need for surgical debridement of the wound at the right elbow joint. Current evidence suggests that cyclophosphamide (CYC) is more effective for refractory skin lesions, while rituximab (RTX) in combination with immunosuppressants is beneficial for those with combined RP-ILD.23–25 A recent retrospective study indicated that 71.43% (25/35) of patients with anti-MDA5 DM responded to RTX treatment for ILD, with more than half experiencing improvement in skin rashes. However, 37.14% of patients developed infections after RTX use.26 Cyclosporine and tacrolimus are considered third-line options or are used when myopathy is controlled, but other immunosuppressants fail to control skin symptoms. Additionally, sildenafil may aid in pain relief and ulcer healing,27 while ECMO therapy may be considered for late onset of acute respiratory failure secondary to RP-ILD.28
In this case, the patient’s necrotizing skin ulcer is characterized by rapid progression, and the risk of later disability is extremely high. During the course of treatment, we regularly review the patient’s serum level, CT scan and skin changes, and adjust the treatment plan in a timely manner, so as to prevent the patient’s condition from deteriorating. At the same time, the whole course of the disease also enlightens us that once a patient is found to have similar skin symptoms in the future, it is necessary to detect myositis-specific antibody profiles early. The prognosis of these patients is generally poor, and how can the treatment of this rare type of dermatomyositis, which is characterized by severe skin ulcers, reduce the risk of skin and lung infection while using high-dose corticosteroids and immunosuppressants? This is something we need to consider in the future.
Ethics Approval and Consent to Participate
The article provides a case report. Therefore, our Ethical Committee’s approval was not required.
Consent for Publication
Written informed consent was obtained from the patient, including the use of photographs of the face.
Author Contributions
Every author contributed significantly to the work reported, whether it was in the design, execution, acquisition of data, analysis, and interpretation, or in all of these areas; they also participated in the article’s drafting, revision, or critical review; they approved the final version that was published; they agreed on the journal to which the article was submitted; and they agreed to take responsibility for every aspect of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Betteridge Z, Tansley S, Shaddick G, et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J Autoimmun. 2019;101:48–55. doi:10.1016/j.jaut.2019.04.001
2. Nombel A, Fabien N, Coutant F. Dermatomyositis With Anti-MDA5 Antibodies: bioclinical Features, Pathogenesis and Emerging Therapies. Front Immunol. 2021;12:773352. doi:10.3389/fimmu.2021.773352
3. Yang J, Yan B. Rare complications of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis: time to nip them in the bud. Front Immunol. 2022;13:1009546. doi:10.3389/fimmu.2022.1009546
4. Sontheimer RD. MDA5 autoantibody-another indicator of clinical diversity in dermatomyositis. Ann Transl Med. 2017;5(7):160. doi:10.21037/atm.2017.03.94
5. Li J, Liu Y, Li Y, et al. Associations between anti-melanoma differentiation-associated gene 5 antibody and demographics, clinical characteristics and laboratory results of patients with dermatomyositis: a systematic meta-analysis. J Dermatol. 2018;45(1):46–52. doi:10.1111/1346-8138.14092
6. Keppeke GD, Satoh M, Kayser C, et al. A cell-based assay for detection of anti-fibrillarin autoantibodies with performance equivalent to immunoprecipitation. Front Immunol. 2022;13:1011110. doi:10.3389/fimmu.2022.1011110
7. Coutant F, Bachet R, Pin JJ, Alonzo M, Miossec P. Monoclonal antibodies from B cells of patients with anti-MDA5 antibody-positive dermatomyositis directly stimulate interferon gamma production. J Autoimmun. 2022;130:102831. doi:10.1016/j.jaut.2022.102831
8. Gui X, Shenyun S, Ding H, et al. Anti-Ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatology (Oxford). 2022;61(11):4570–4578. doi:10.1093/rheumatology/keac090
9. Castellanos-Gonzalez M, Bris BB, Marsol IB, et al. Predictive factors for anti-MDA5 antibody in patients with dermatomyositis: a retrospective multicenter study. J Dtsch Dermatol Ges. 2023;21(7):741–748. doi:10.1111/ddg.15089
10. Cox NH, Coulson JH. Diagnosis of skin disease. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook’s Textbook of Dermatology. Vol. 1. Hoboken: Blackwell Publishing Ltd; 2010.
11. Fernandez-Flores A, Cassarino DS. Gottron Papules Show Histopathologic Features of Localized Lymphedema. Am J Dermatopathol. 2017;39(7):518–523. doi:10.1097/DAD.0000000000000701
12. Thuner J, Coutant F. IFN-γ: an overlooked cytokine in dermatomyositis with anti-MDA5 antibodies. Autoimmun Rev. 2023;22(10):103420. doi:10.1016/j.autrev.2023.103420
13. Hu H, Yang H, Liu Y, Yan B. Pathogenesis of Anti-melanoma Differentiation-Associated Gene 5 Antibody-Positive Dermatomyositis: a Concise Review With an Emphasis on Type I Interferon System. Front Med Lausanne. 2022;8:833114. doi:10.3389/fmed.2021.833114
14. Cao H, Xia Q, Pan M, et al. Gottron Papules and Gottron Sign with Ulceration: a Distinctive Cutaneous Feature in a Subset of Patients with Classic Dermatomyositis and Clinically Amyopathic Dermatomyositis. J Rheumatol. 2016;43(9):1735–1742. doi:10.3899/jrheum.160024
15. Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78(4):776–785. doi:10.1016/j.jaad.2017.12.010
16. Narang NS, Casciola-Rosen L, Li S, Chung L, Fiorentino DF. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res (Hoboken). 2015;67(5):667–672. doi:10.1002/acr.22498
17. Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol. 2017;176(2):395–402. doi:10.1111/bjd.14882
18. Xu A, Ye Y, Fu Q, et al. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology (Oxford). 2021;60(7):3343–3351. doi:10.1093/rheumatology/keaa786
19. Wang H, Chen X, Du Y, et al. Mortality risk in patients with anti-MDA5 dermatomyositis is related to rapidly progressive interstitial lung disease and anti-Ro52 antibody. Arthritis Res Ther. 2023;25(1):127. doi:10.1186/s13075-023-03100-z
20. Waldman R, DeWane ME, Lu J. Dermatomyositis: diagnosis and treatment. J Am Acad Dermatol. 2020;82(2):283–296. doi:10.1016/j.jaad.2019.05.105
21. You H, Wang L, Wang J, et al. Time-dependent changes in RPILD and mortality risk in anti-MDA5+ DM patients: a cohort study of 272 cases in China. Rheumatology (Oxford). 2023;62(3):1216–1226. doi:10.1093/rheumatology/keac450
22. Tsuji H, Nakashima R, Hosono Y, et al. Multicenter Prospective Study of the Efficacy and Safety of Combined Immunosuppressive Therapy With High-Dose Glucocorticoid, Tacrolimus, and Cyclophosphamide in Interstitial Lung Diseases Accompanied by Anti-Melanoma Differentiation-Associated Gene 5-Positive Dermatomyositis. Arthritis Rheumatol. 2020;72(3):488–498. doi:10.1002/art.41105
23. Huang K, Vinik O, Shojania K, et al. Clinical spectrum and therapeutics in Canadian patients with anti-melanoma differentiation-associated gene 5 (MDA5)-positive dermatomyositis: a case-based review. Rheumatol Int. 2019;39(11):1971–1981. doi:10.1007/s00296-019-04398-2
24. Nishi K, Ogura M, Tamai N, et al. Successful rituximab treatment for severe rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive juvenile dermatomyositis: a case report and literature review. Pediatr Rheumatol Online J. 2022;20(1):60. doi:10.1186/s12969-022-00723-5
25. Yen TH, Tseng CW, Wang KL, Fu PK. Combination Therapy with Rituximab, Tofacitinib and Pirfenidone in a Patient with Rapid Progressive Interstitial Lung Disease (RP-ILD) Due to MDA5 Antibody-Associated Dermatomyositis: a Case Report. Medicina. 2021;57(12):1358. doi:10.3390/medicina57121358
26. Koichi Y, Aya Y, Megumi U, et al. A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol. 2017;27(3):536–540. doi:10.3109/14397595.2015.1014140
27. Collantes-Rodríguez C, Jiménez-Gallo D, Ossorio-García L, Villegas-Romero I, Linares-Barrios M. Image Gallery: cutaneous ulcers in anti-MDA5 dermatomyositis successfully treated with sildenafil. Br J Dermatol. 2020;182(1):e1. doi:10.1111/bjd.18376
28. Gu Q, Diao M, Hu W, Huang M, Zhu Y. Case Report: extracorporeal Membrane Oxgenation for Rapidly Progressive Interstitial Lung Disease Associated With Clinically Amyopathic Dermatomyositis in a Post-partum Woman. Front Med Lausanne. 2021;8:742823. doi:10.3389/fmed.2021.742823
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
