Back to Journals » Infection and Drug Resistance » Volume 13
Zinc Chelator N,N,N′,N′-Tetrakis(2-Pyridylmethyl)Ethylenediamine Reduces the Resistance of Mycobacterium abscessus to Imipenem
Authors He S, Zou Y, Zhan M, Guo Q, Zhang Y, Zhang Z, Li B, Zhang S, Chu H
Received 13 June 2020
Accepted for publication 5 August 2020
Published 18 August 2020 Volume 2020:13 Pages 2883—2890
DOI https://doi.org/10.2147/IDR.S267552
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Siyuan He,1,2,* Yuzhen Zou,1,2,* Mengling Zhan,1,2,* Qi Guo,1,2 Yongjie Zhang,1,2 Zhemin Zhang,1 Bing Li,1 Shaoyan Zhang,1 Haiqing Chu1,3
1Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China; 2Tongji University School of Medicine, Shanghai 200092, People’s Republic of China; 3Shanghai Key Laboratory of Tuberculosis, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haiqing Chu; Shaoyan Zhang
Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, No. 507 Zhengmin Road, Shanghai 200433, People’s Republic of China
Tel/ Fax +86 21 6511 5006
Email [email protected]; [email protected]
Purpose: Imipenem is one of the very few effective options for treating Mycobacterium abscessus (M. abscessus) infections; the development of imipenem resistance is a major health concern.
Materials and Methods: The susceptibility of 194 clinical M. abscessus isolates to imipenem was determined. The ability of imipenem to synergize with N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), a zinc chelator and a metallo-β-lactamases (MBLs) inhibitor, to inhibit M. abscessus growth was also assessed.
Results: M. abscessus exhibited an elevated resistance to imipenem (MIC50 = 16 mg/L, MIC90 = 64 mg/L). A combination of TPEN and imipenem synergized to inhibit the growth of 100% of imipenem-resistant and 79.2% of imipenem-resistance intermediate isolates; no synergy was observed treating imipenem-sensitive isolates. A remarkable decrease in the MIC50 (from 16 to 4 mg/L) and MIC90 (from 64 to 8 mg/L) of imipenem was observed when it was combined with TPEN; the portion of imipenem-resistant isolates also decreased (from 48.4% to 0%). Consistent with these results demonstrating synergy, a time-kill assay showed the addition of TPEN significantly improved the bactericidal activity of imipenem toward M. abscessus. Similarly, EDTA (a potent MBLs inhibitor) promoted the anti-M. abscessus activity of imipenem in a disk assay, corroborating the effect of TPEN and supporting the role of MBLs in imipenem resistance exhibited by some isolates.
Conclusion: These findings demonstrate that TPEN can reduce the resistance of M. abscessus to imipenem and suggest that the inhibition of MBLs activity is the underlying mechanism.
Keywords: Mycobacterium abscessus, TPEN, imipenem resistance, metallo-β-lactamases
Introduction
Mycobacterium abscessus (M. abscessus) is a rapidly growing non-tuberculous mycobacterium that causes progressive inflammatory lung damage and a significant decline in lung function in patients with structural lung diseases such as cystic fibrosis and bronchiectasis.1 Pulmonary M. abscessus infections are notoriously difficult to treat, necessitating prolonged multidrug therapy due to the intrinsic resistance of the organism to most anti-mycobacterial drugs.2 The increasing prevalence of pulmonary disease due to M. abscessus supports the urgent need for more effective treatment regimens.
Due to its broad spectrum of antibacterial activity, imipenem is currently recommended as one drug of choice for treating M. abscessus pulmonary disease. Meta-analysis involving 14 eligible studies showed that treatment with imipenem correlated with clinical success.3 Imipenem combined with other antibiotics exerted a synergistic or additive effect, which contributed to this success.4–6 Developing resistance to imipenem, however, is a major health concern. Recently, for example, two groups reported the high MIC50 (16 mg/L) and MIC90 (16–32 mg/L) of imipenem for M. abscessus.7,8 Moreover, bacterial resistance to imipenem in some studies was more than 60%, clearly limiting the clinical application of imipenem in treating M. abscessus infections.9–11
N,N,N’,N’-tetrakis (2-pyridylmethyl) ethane-1,2-diamine (TPEN) is a specific zinc chelator and metallo-β-lactamases (MBLs) inhibitor shown to increase the sensitivity of certain bacteria (eg, K. pneumoniae, S. maltophilia and E. meningoseptica) to carbapenems in vitro and in vivo by suppressing MBLs activity.12,13 There are no reports, however, concerning TPEN and the resistance of M. abscessus to imipenem. In the study described herein, one hundred ninety-four clinical, M. abscessus isolates were collected from patients with lung diseases; the activity and MIC of imipenem toward the isolates were determined. The MIC of imipenem and the number of resistant isolates decreased remarkably in the presence TPEN, presumably as a result of the inhibitory effect of TPEN on MBLs activity. To our knowledge, this is the first study to explore the ability of TPEN to increase the susceptibility of M. abscessus to imipenem treatment. The results improve our understanding of imipenem resistance and suggest new strategies to enhance treatment of M. abscessus infections.
Materials and Methods
Ethics Statement
As the study only concerned laboratory testing of mycobacteria without the direct involvement of human subjects, ethics approval was not sought.
Bacteria
One hundred and nighty-four clinical isolates were collected as described previously from the sputum and bronchoalveolar lavage fluid samples of patients with lung infections in Shanghai Pulmonary Hospital.11,14 Isolates were initially screened for non-tuberculosis mycobacteria by growth in MGIT960 culture medium and resistance to p-nitrobenzoic acid. M. abscessus was identified by sequencing the rpoB and erm(41) genes. Isolates were stored at −80°C in trypticase soy broth containing 40% glycerol. Fresh cultures were started for each experiment. The antibiotic-sensitive reference strain, M. abscessus ATCC 19977 was purchased from the American Type Culture Collection (ATCC; Manassas, VA) and grown to mid-log phase in Middle brook 7H9 medium (Becton Dickinson, Cockeysville, MD) supplemented with 0.2% glycerol, 10% oleic acid-albumin-dextrose-catalase (OADC) enrichment (Becton Dickinson) and 0.05% Tween 80 (BBL Life Sciences; 7H9sB).
Chemical Reagents
Imipenem (GlpBio, Montclair, CA, USA), sulbactam, avibactam, relebactam and TPEN (MedChemExpress, Monmouth Junction, NJ, USA) were used alone or in combination. Stock solutions of imipenem (2048 μg/mL), sulbactam (9132.5 μg/mL), avibactam (9132.5 μg/mL), and relebactam (9132.5 μg/mL) were prepared in sterile water. TPEN, dissolved in 100% DMSO (32 mg/mL stock solution), was diluted in sterile water to obtain the desired working concentration.
Susceptibility Testing
The sensitivity of M. abscessus to imipenem with or without TPEN was determined by the microdilution method in cation-adjusted Muller Hinton Broth (BD Bioscience, Drachten, The Netherlands); the results were assessed after 3 days. The breakpoints of imipenem MICs were interpreted according to Clinical and Laboratory Standards Institute (CLSI)-M24-A2 where ≤4 mg/L = susceptible, 8–16 mg/L = resistance intermediate, and ≥32 mg/L = resistant. Mycobacterium peregrinum (ATCC700686) served as the control reference strain.
Synergy Testing
Synergy between imipenem and TPEN was assessed by the broth microdilution checkerboard method using the M. abscessus reference strain ATCC19977 and 61 clinical isolates (30 imipenem-resistant, 23 imipenem-resistance intermediate and 8 imipenem-susceptible). All synergy tests were performed in cation-adjusted Muller Hinton Broth. Drug interactions were interpreted according to the fractional inhibitory concentration index (FICI). The fractional inhibitory concentration (FIC) was calculated using the formula FIC = (MICa combination/MICA alone) + (MICb combination/MICB alone). The minimum FIC for each combination was defined as the FIC index (FICI).8 Drug interactions were classified as: synergistic, FICI = ≤0.5; no interaction, FICI = >0.5–4.0; or antagonistic, FICI = >4.0.
Time–Kill Kinetics (TK) Assays
TK assays were performed using the M. abscessus reference strain ATCC19977 and an imipenem-resistant clinical isolate, A189. Five milliliter tubes of 7H9sB containing imipenem or TPEN alone, or both drugs in various combination at 0.5×MIC, 1×MIC or 2×MIC were inoculated with 5×105 CFUs of bacteria growing exponentially, and the bacteria were incubated at 37°C with shaking (100 rpm). The bacteria were enumerated after 0, 1, 2, 3 and 5 days incubation by plating serial dilutions of culture aliquots on lysogeny broth agar plates; CFUs were counted after an additional 5 days incubation at 37°C. All assays were performed in triplicate. A bactericidal effect was defined as ≥2 log reduction in CFUs.
Exploration of the Relationship Between MBLs and Imipenem Resistance in M. abscessus
β-lactamase-dependent degradation is the major mechanism conferring microbial resistance to imipenem. EDTA, like TPEN, is a well-documented MBLs inhibitor. The growth of imipenem-resistant, -resistance intermediate and -sensitive isolates surrounding imipenem disks impregnated or not impregnated with EDTA on agar plates was assessed in order to substantiate the potential role of MBLs in conferring imipenem resistance upon M. abscessus. Middlebrook 7H10 agar plates (7.0 cm) were prepared in our laboratory using dehydrated products from Becton Dickinson. Bacteria were grown to mid-log phase, and fresh cultures were used to prepare suspensions at a McFarland turbidity of 0.5. Two OXOID imipenem antimicrobial susceptibility disks (10 μg; Thermo Fisher Scientific, Waltham, MA, USA) were placed >30 mm apart onto 7H10 agar plates, 0.03125 M EDTA was added to one of the disks, and the plates were incubated for 4 days at 37 oC. A zone ≥5 mm diameter around the imipenem-EDTA disk compared to the imipenem disk alone was considered positive for MBLs.15 The ability of additional β-lactamase inhibitors, which do not target MBLs (ie, sulbactam, avibactam and relebactam), to synergize with imipenem and inhibit the growth of an imipenem-resistant isolate was accessed in a similar fashion.
Results
Imipenem Susceptibility Profile of Clinical M. abscessus Isolates
The MICs of imipenem for 194 M. abscessus isolates ranged from 4 to 256 mg/L, MIC50 = 16 mg/L, MIC90 = 64 mg/L (Figure 1). Of all the isolates, 183 (94.3%) were not susceptible to imipenem; 87 (44.8%) of these were intermediately resistant and 96 (49.5%) were totally resistant. This demonstrates the relatively high resistance rate of clinical M. abscessus isolates to imipenem.
 |
Figure 1 MIC of imipenem for 194 clinical M. abscessus isolates. |
Imipenem and TPEN Act Synergistically
The sensitivity of ATCC 19977 and the sixty-one clinical isolates to imipenem and TPEN tested separately was determined initially. The MIC50 and MIC90 of imipenem and TPEN were 16 and 64 mg/L, and 64 and 128 mg/L, respectively. Subsequently, synergy between imipenem and TPEN was assessed. Using a combination of TPEN and imipenem, synergy was demonstrable in 100% (30/30) of the imipenem-resistant and 79.2% (19/24) of the imipenem-resistance intermediate isolates; no synergy was observed in the imipenem-sensitive isolates (Table 1). The data for each isolate is shown in Table S1. Imipenem exhibited a remarkable decrease in MIC50 (from 16 to 4 mg/L) and MIC90 (from 64 to 8 mg/L) when used in combination with TPEN. The proportion of imipenem-resistant isolates also decreased from 48.4% to 0% (Figure 2). Furthermore, when TPEN was tested in combination with a variety of other antibiotics commonly used to treat M. abscessus infections (ie, clarithromycin, amikacin, cefoxitin, moxifloxacin, linezolid and tigecycline), no synergy was found (Table S2). Therefore, synergy between TPEN and imipenem appears specific.
 |
Table 1 Imipenem and TPEN Act Synergistically to Inhibit the Growth of Imipenem-Resistant M. abscessus* |
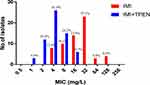 |
Figure 2 Susceptibility of M. abscessus isolates (n=62) to imipenem with or without TPEN. The data above each bar are the percentage of isolates. |
Time–Kill Studies
Consistent with the results demonstrating synergy, the addition of TPEN increased the bactericidal activity of imipenem in contrast to exerting only a weak bacteriostatic effect when TPEN was added alone. Bactericidal synergy in TK assays was defined as ≥2 log reduction in CFUs compared to 2×MIC imipenem alone. The bactericidal effect of IMI + TPEN 2×MIC was evident on day 3 for ATCC19977 (Figure 3A), while it was demonstrable from days 2 to 5 for the randomly selected imipenem-resistant clinical isolate A189 (Figure 3B). As such, the combination of TPEN and imipenem appeared to exert a stronger synergistic effect on resistant isolates. Presumably, the re-growth of both ATCC19977 and A189 observed in TK assays after 3 days culture was due to the predominant bactericidal role of imipenem and its degradation in vitro.
Relationship Between MBLs and Imipenem Resistance in M. abscessus
The growth of 30 imipenem-resistant, 23 imipenem-resistance intermediate and 8 imipenem-sensitive isolates on agar plates around imipenem disks impregnated with EDTA further substantiated the presence of MBLs and their role in imipenem resistance. A zone of growth inhibition ≥5 mm larger than the zone surrounding a disk impregnated with imipenem alone was detected in all imipenem-resistant isolates (MIC ≥16 mg/L), 50% of the intermediately resistant isolates (MIC=8 mg/L), and none of the imipenem-sensitive isolates (Table 2, Figure 4A). These results suggest that the resistance of M. abscessus isolates to imipenem is due to MBLs, which are inhibited by the presence of EDTA.
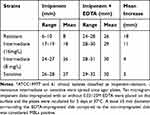 |
Table 2 Zone of Inhibition Surrounding Imipenem Disk with or without EDTA* |
Sulbactam, avibactam and relebactam are novel β-lactamase inhibitors.16 Experiments were undertaken to determine whether these inhibitors could synergize with imipenem to inhibit the growth of M. abscessus in a manner analogous to EDTA. Growth of an imipenem-resistant isolate (A247) on agar plates around imipenem disks infused with sulbactam, avibactam or relebactam was equivalent to growth around a disk infused with imipenem alone (Figure 4B). A significant difference in the zone of inhibition demonstrating synergy was only seen surrounding imipenem disks impregnated with EDTA shown for comparison purposes. These findings indicate that MBLs specifically enhance the resistance of M. abscessus to imipenem, other β-lactamases are incapable.
Discussion
Imipenem is one of the most reliable antibiotics used to treat M. abscessus infections.17,18 Recently, however, imipenem-resistant M. abscessus has emerged; the underlying resistance mechanism remains to be delineated fully. The present study confirmed the high rate of M. abscessus resistance to imipenem based upon analysis of a large number of clinical isolates obtained in mainland China. Imipenem resistance exhibited by these isolates correlated with the presence of MBLs. The presence of TPEN, a zinc chelator which inhibits MBL activity, dramatically reduced the resistance of M. abscessus to imipenem.
β-lactam antibiotics are important chemotherapeutic agents used to treat M. abscessus infections. One study even proposed treating such infections with two β-lactams, ie, ceftazidime and imipenem, to enhance killing activity.19 Imipenem has garnered considerable attention recently due to its association with improved clinical prognoses.3 The anti-M. abscessus activity exhibited by imipenem in vitro, however, is variable. In the current study, 49.5% of 194 isolates were resistant to imipenem (MIC50 = 16 mg/L), a result which is consistent with previous studies.7–11 Notably, a high imipenem MIC cannot be equated with poor clinical outcomes since imipenem shows elevated intracellular antimicrobial activity.20 Moreover, it exhibits synergism or an additive effect when used in combination with other antibiotics.5,6 Therefore, resistance to imipenem does not limit its clinical application in treating M. abscessus infections.
Degradation by β-lactamase is the major mechanism conferring microbial resistance to imipenem.21 MBLs are pan-reactive enzymes that can degrade most classes of β-lactams including antibiotics of last-resort such as carbapenems. The MBLs are a group of enzymes that exhibit catalytic active in the presence of Zn2+ ions. TPEN is a cell-permeable, zinc-specific chelator that functions as a broad-spectrum antimicrobial agent.22 TPEN can modulate human monocyte functions that include phagocytosis, oxidative burst, and TNF-α and IL-6 production in response to Escherichia coli, Staphylococcus aureus and Streptococcus pneumoniae.23 Additionally, it prevented death in a mouse model of lethal aspergillus infection.24 As such, TPEN exhibits antimicrobial activity both in vitro and in vivo. As a zinc chelator, TPEN is also a potent MBLs inhibitor. Recently, Princip and colleagues found TPEN reversed the MBL-mediated resistance of K. pneumoniae, S. maltophilia and E. meningoseptica to meropenem both in vitro and in vivo.13 Azumah and colleagues reported that TPEN also rendered carbapenem-resistant Enterobacteriaceae (CRE) susceptible to meropenem.25 Notably, TPEN exhibited the greatest activity, assessed in terms of a decrease in the MIC of meropenem, compared to a variety of metal chelators tested.24 The relationship between imipenem resistance and the presence of MBLs in M. abscessus is proposed for the first time herein. TPEN synergized with imipenem to diminish the growth of imipenem-resistant M. abscessus in both synergy testing and TK assays. Synergy was more pronounced in resistant isolates potentially capable of producing MBLs. Presumably, the increase in CFUs observed in TK assays after 2–3 days culture in the presence of imipenem and TPEN was due to the predominant bactericidal role and degradation of imipenem in vitro.25
To date, no drug used clinically affects MBL activity. TPEN is a heavy metal chelator which, unlike comparable chelators such as DTPA and EDTA, is cell permeable. Thus, it promises to be more effective in treating M. abscessus, a typical facultative intracellular bacterium. Free intracellular zinc levels were significantly lowered using TPEN.23 Immunosuppressed mice infected with A. fumigatus demonstrated a 60% survival rate when treated with 5 mg/kg TPEN daily; doses ≤10 mg/kg were well tolerated.26,27 As such, TPEN may be an effective drug in treating imipenem-resistant M. abscessus by virtue of its ability to permeate cells and inhibit MBLs activity.
Experiments involving the extraction and purification of the metalloenzyme proteins from M. abscessus are currently ongoing in the laboratory in an effort to demonstrate the direct interaction of TPEN with MBLs. Additional experiments are dedicated to determining the capacity of TPEN to increase the anti-microbial activity of imipenem in animal models of M. abscessus infection.
Conclusion
TPEN reduces the resistance of M. abscessus to imipenem by inhibiting MBLs activity.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Ethics Approval
Ethical approval was not required; the isolates were collected for routine diagnostic testing.
Acknowledgments
We sincerely thank Dr. Stephen H. Gregory (Providence, Rhodes Island, USA) who helped write and edit this manuscript.
Author Contributions
All authors made significant contributions to the work reported here in terms of: study conception, design and execution; data acquisition, analysis and interpretation; drafting, revising and/or critically reviewing the article; providing final approval of the version submitted for publication; agreeing upon the journal to which the article was submitted; and agreeing to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Swenson C, Zerbe CS, Fennelly K. Host variability in NTM disease: implications for research needs. Front Microbiol. 2018;9:2901. doi:10.3389/fmicb.2018.02901
2. Hunt-Serracin AC, Parks BJ, Boll J, Boutte CC. Mycobacterium abscessus cells have altered antibiotic tolerance and surface glycolipids in artificial cystic fibrosis sputum medium. Antimicrob Agents Chemother. 2019;63:7. doi:10.1128/AAC.02488-18
3. Kwak N, Dalcolmo MP, Daley CL, et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J. 2019;54:1. doi:10.1183/13993003.01991-2018
4. Brown-Elliott BA, Wallace RJ
5. Le Run E, Arthur M, Mainardi JL. In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/AAC.01915-18
6. Miyasaka T, Kunishima H, Komatsu M, et al. In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int J Antimicrob Agents. 2007;30(3):255–258. doi:10.1016/j.ijantimicag.2007.05.003
7. Brown-Elliott BA, Rubio A, Wallace RJ
8. Cheng A, Tsai YT, Chang SY, et al. In vitro synergism of rifabutin with clarithromycin, imipenem, and tigecycline against the Mycobacterium abscessus complex. Antimicrob Agents Chemother. 2019;63:4. doi:10.1128/AAC.02234-18
9. Chua KYL, Bustamante A, Jelfs P, Chen SC-A, Sintchenko V. Antibiotic susceptibility of diverse Mycobacterium abscessus complex strains in New South Wales, Australia. Pathology. 2015;47(7):678–682. doi:10.1097/PAT.0000000000000327
10. Lee MC, Sun PL, Wu TL, et al. Antimicrobial resistance in Mycobacterium abscessus complex isolated from patients with skin and soft tissue infections at a tertiary teaching hospital in Taiwan. J Antimicrob Chemother. 2017;72(10):2782–2786. doi:10.1093/jac/dkx212
11. Li B, Yang S, Chu H, et al. Relationship between antibiotic susceptibility and genotype in Mycobacterium abscessus clinical isolates. Front Microbiol. 2017;8:1739. doi:10.3389/fmicb.2017.01739
12. Wachino J, Yamaguchi Y, Mori S, et al. Structural insights into recognition of hydrolyzed carbapenems and inhibitors by subclass B3 metallo-beta-lactamase SMB-1. Antimicrob Agents Chemother. 2016;60(7):4274–4282. doi:10.1128/AAC.03108-15
13. Principe L, Vecchio G, Sheehan G, et al. Zinc chelators as carbapenem adjuvants for metallo-beta-lactamase-producing bacteria: in vitro and in vivo evaluation. Microb Drug Resist. 2020. doi:10.1089/mdr.2020.0037
14. Ye M, Xu L, Zou Y, et al. Molecular analysis of linezolid-resistant clinical isolates of Mycobacterium abscessus. Antimicrob Agents Chemother. 2019;63:2.
15. Gao Q, Wu S, Xu T, Zhao X, Huang H, Hu F. Emergence of carbapenem resistance in Bacteroides fragilis in China. Int J Antimicrob Agents. 2019;53(6):859–863. doi:10.1016/j.ijantimicag.2019.02.017
16. Papp-Wallace KM, Bonomo RA. New beta-lactamase inhibitors in the clinic. Infect Dis Clin North Am. 2016;30(2):441–464. doi:10.1016/j.idc.2016.02.007
17. Brown-Elliott BA, Killingley J, Vasireddy S, Bridge L, Wallace RJ
18. Jones LA, Doucette L, Dellon EP, Esther CR, McKinzie CJ. Use of inhaled imipenem/cilastatin in pediatric patients with cystic fibrosis: a case series. J Cyst Fibros. 2019;18(4):e42–e44. doi:10.1016/j.jcf.2019.04.017
19. Pandey R, Chen L, Manca C, et al. Dual beta-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio. 2019;10(1). doi:10.1128/mBio.02895-18.
20. Rominski A, Schulthess B, Muller DM, Keller PM, Sander P. Effect of beta-lactamase production and beta-lactam instability on MIC testing results for. Mycobacterium Abscessus J Antimicrob Chemother. 2017;72(11):3070–3078. doi:10.1093/jac/dkx284
21. Nordmann P, Poirel L. Emerging carbapenemases in gram-negative aerobes. Clin Microbiol Infect. 2002;8(6):321–331. doi:10.1046/j.1469-0691.2002.00401.x
22. Leonardelli F, Macedo D, Dudiuk C, et al. In vitro activity of combinations of zinc chelators with amphotericin B and posaconazole against six Mucorales species. Antimicrob Agents Chemother. 2019;63:5. doi:10.1128/AAC.00266-19
23. Mayer LS, Uciechowski P, Meyer S, Schwerdtle T, Rink L, Haase H. Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics. 2014;6(7):1288–1295. doi:10.1039/c4mt00051j
24. Hein KZ, Takahashi H, Tsumori T, et al. Disulphide-reduced psoriasin is a human apoptosis-inducing broad-spectrum fungicide. Proc Natl Acad Sci U S A. 2015;112(42):13039–13044. doi:10.1073/pnas.1511197112
25. Azumah R, Dutta J, Somboro AM, et al. In vitro evaluation of metal chelators as potential metallo-beta-lactamase inhibitors. J Appl Microbiol. 2016;120(4):860–867. doi:10.1111/jam.13085
26. Laskaris P, Atrouni A, Calera JA, et al. Administration of zinc chelators improves survival of mice infected with Aspergillus fumigatus both in monotherapy and in combination with caspofungin. Antimicrob Agents Chemother. 2016;60(10):5631–5639. doi:10.1128/AAC.00324-16
27. Adler M, Dinterman RE, Wannemacher RW. Protection by the heavy metal chelator N,N,N’,N’-tetrakis (2-pyridylmethyl)ethylenediamine (TPEN) against the lethal action of botulinum neurotoxin A and B. Toxicon. 1997;35(7):1089–1100. doi:10.1016/S0041-0101(96)00215-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


