Back to Journals » Cancer Management and Research » Volume 10
Young age is an independent adverse prognostic factor in early stage breast cancer: a population-based study
Authors Zhang X, Yang J , Cai H, Ye Y
Received 6 March 2018
Accepted for publication 23 July 2018
Published 27 September 2018 Volume 2018:10 Pages 4005—4018
DOI https://doi.org/10.2147/CMAR.S167363
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Xiao Zhang,1,* Jian Yang,2,* Haoyang Cai,2 Yifeng Ye1
1Department of Breast Surgery, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu 611731, China; 2Center of Growth, Metabolism, and Aging, Key Laboratory of Bio-Resources and Eco-Environment, College of Life Sciences, Sichuan University, Chengdu, Sichuan 610064, China
*These authors contributed equally to this work
Objective: To compare the prognosis of young breast cancer patients with the older ones.
Patients and methods: Utilizing the Surveillance, Epidemiology, and End Results database, we identified 150,588 female breast cancer patients diagnosed during 2003–2014, including 6,668 patients younger than 35 years and 143,920 patients aged between 35 and 60 years. Kaplan–Meier analysis was performed to compare the prognosis of these two groups. Univariate and multivariate Cox proportional hazard models were utilized to identify independent prognostic factors and calculate the HR and 95% CI. Subgroup analysis was performed stratified according to the lymph node status and estrogen receptor (ER) status.
Results: The young patients presented with more aggressive clinicopathological characteristics, including larger tumor size (P<0.001), more lymph node metastasis (P<0.001), higher grade (grades III and IV, P<0.001), more ER/progesterone receptor absence (P<0.001), and more human epidermal growth factor receptor 2 overexpression (P<0.001). The patients younger than 35 years presented with inferior breast cancer-specific survival (BCSS) and overall survival (OS) (log-rank, P<0.001) in comparison with the older ones. In the multivariable Cox proportional hazard regression analysis, young age remained to be an independent adverse prognostic factor in operable breast cancer in terms of BCSS (HR, 1.200; 95% CI, 1.110–1.297; P<0.001) and OS (HR, 1.111; 95% CI, 1.032–1.196; P=0.005). In the subgroup analysis, young age remained a significant adverse prognostic factor in N0 (BCSS), N1, and ER-positive subgroups (P<0.05).
Conclusion: Young age is an independent adverse prognostic factor in operable breast cancer. Young patients may receive more intensive treatment than older ones.
Keywords: breast cancer, young, characteristics, survival
Introduction
Breast cancer is the most common malignant disease and the second cause of cancer-specific death among women in the US.1 Patients diagnosed at a young age, especially younger than 35 years, are relatively rare, accounting for only 1.8% of total number of cases diagnosed among women each year.2
Whether young age is an independent adverse prognostic factor in breast cancer remains controversial. There is a lot of evidence showing that young breast cancer patients are more likely to present with more aggressive clinicopathological characteristics, including larger tumor size, more involved lymph nodes, higher grade, higher proliferation index, more lymphovascular invasion, absence of estrogen receptor (ER)/progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) overexpression.3–12 Compared with older ones, the young breast cancer patients have inferior prognosis.4–6,10,11,13–18 However, some studies have come to the opposite conclusion. Those authors advocated that young age was not an independent adverse prognostic factor in breast cancer patients.9,19–22 Gajdos et al9 compared the prognosis of 101 cases of women under age 36 and 631 cases of women aged 36 years and older with stage 0–III breast cancer, and found that the cumulative 5-year local and distant disease-free survival was significantly worse for patients younger than 36 years. However, after controlling the covariates including tumor size, nodal involvement, chemotherapy, and tamoxifen, age was no longer significantly associated with local or distant disease-free survival. However, these studies have some limitations. For example, these patients were diagnosed many years ago, but treatments have improved a lot during this time. Moreover, most of these studies did not contain HER2 status, which is an important independent prognostic factor. Finally, these studies were retrospective and poorly represented the situation of the real world.
Because whether young age is an independent adverse prognostic factor in breast cancer remains uncertain, and it is important for considering tailored treatment program, we aimed to conduct a population-based study to compare the prognosis of young and older operable breast cancer patients utilizing Surveillance, Epidemiology, and End Results (SEER) database.
Materials and methods
Ethics statement
This is a database-based retrospective study, and hence informed consent and approval by institutional ethics committee are not required. Data-Use Agreement for the SEER 1973-2014 Research Data File was completed.
Patients
We used SEER*Stat (version 8.3.4) to download data from the SEER 18 registries research database, which covers approximately 28% of the US population. The SEER 18 database contains data from the SEER 13 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, rural Georgia, and the Alaska Native Tumor Registry) and the registries of greater California, Kentucky, Louisiana, New Jersey, and greater Georgia. We generated a case listing according to the following criteria: histologically confirmed breast cancer, invasive ductal cancer (ICD-O-3 8500), female, age at diagnosis less than 60 years old, diagnosed between 2003 and 2014, breast cancer as the first and only malignancy, unilateral, histological grades I–IV, T1mic-T3, N0–N3, M0, and received surgical treatment. We excluded patients whose tumors were not totally removed. Tumors with invasion of subcutaneous tissue and/or chest wall were not included in this study (T4). In total, 150,588 patients were eligible, including 6,668 cases of younger than 35 years and 143,920 cases aged between 35 and 60 years.
Variables
The variables included age at diagnosis, race (White, Black, others, or unknown), laterality (left, right), breast-adjusted AJCC sixth T (T1, T2, or T3), breast-adjusted AJCC sixth N (N01, N1, N2, or N3), breast-adjusted AJCC sixth stage (I, IIA, IIB, IIIA, or IIIC), Grade (I, II, III, or IV), ER status (positive, negative, borderline, or unknown), PR status (positive, negative, borderline, or unknown), and HER2 status (positive, negative, borderline, or unknown). In the multiple imputation, ER and PR borderline was classified as positive. Breast cancer-specific survival (BCSS) and overall survival (OS) were defined as the time from diagnosis to death due to breast cancer and any cause, respectively.
Statistical analysis
All statistical analyses were performed using R (version 3.2.4, R Project for Statistical Computing, Vienna, Austria) and SPSS 22 (IBM SPSS Statistics, Chicago, IL, US). The clinicopathological characteristics of two groups were compared using chi-squared test.
The Kaplan–Meier method was performed to generate BCSS and OS survival curves, and the log-rank test was used to compare the BCSS and OS of two groups. Univariate and multivariate Cox proportional hazard regression models were utilized to identify independent prognostic factors and calculate the HR and 95% CI. Propensity score matching method was performed to diminish the effect of unbalance of baseline clinicopathological characteristics between these two groups. In order to reduce bias caused by the missing values, we used SPSS 22 to perform multiple imputation on the missing values, which generated five imported datasets. We then performed Cox proportional hazards models in five imported datasets and combined the results to produce merged HR.
We carried out subgroup analysis stratified by lymph node status and ER status. In each subgroup, Kaplan–Meier method and log-rank test were performed to compare the BCSS and OS of younger with older groups. Additionally, univariate and multivariate Cox proportional hazards models were used to calculate the HR and 95% CI after controlling for the potential confounder factors.
In order to explore the reasonable cutoff age to define “young age”, we treated age as an ordered factor and categorized in each two-year period. Multivariate Cox proportional hazard model was performed to calculate the HR and 95% CI of each group compared with the reference group (age 59–60 years). The spline function of R package was used to line smooth the hazard plots.
All P-values were two-sided, and P<0.05 was considered as statistically significant.
Results
Patient characteristics
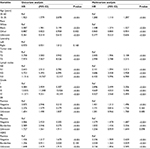
In total, 150,588 patients with early-stage invasive ductal breast cancer diagnosed from 2003 to 2014 were included in this study. Among them, 6,668 patients (4.4%) were younger than 35 years and 143,920 patients (95.6%) were between 35 and 60 years. Demographic and clinicopathological characteristics of this study are summarized in Table 1. The distribution of important clinicopathological characteristics was significantly different between the two groups. Compared with the older group, the younger group presented with more aggressive tumor characteristics, including larger tumor size (T3, 10.2% vs 4.5%, P<0.001), more lymph node metastasis (48.1% vs 35.3%, P<0.001), higher grade (grades III and IV, 64.9% vs 43.1%, P<0.001), more ER absence (35.2% vs 22.9%, P<0.001) and PR absence (42.9% vs 31.3%, P<0.001), and more HER2 overexpression (11.1% vs 7.9%, P<0.001). In addition, the younger group had a higher proportion of triple-negative (9.6% vs 5.7%, P<0.001) and HER2-overexpression (2.9% vs 2.3%, P<0.001) subtypes.
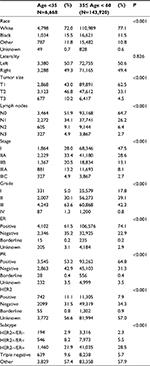  | Table 1 Patient characteristics of the study population Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2. |
Comparison of survival between the younger and older groups
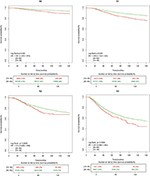
Kaplan–Meier analysis was performed to compare the prognosis between the younger and older groups. As shown in Figure 1, the younger group had significantly worse survival compared with the older group, in terms of BCSS (HR, 1.923, 95% CI, 1.779–2.078, P<0.001) and OS (HR, 1.684, 95% CI, 1.564–1.812, P<0.001). The five-year BCSS rates were 89.5% (95% CI, 88.6%–90.3%) in the younger group and 94.3% (95% CI, 94.1%–94.4%) in the older group. Similarly, OS rates were 88.5% (95% CI, 87.6%–89.4%) and 93.1% (95% CI, 92.9%–93.2%) in the two groups, respectively. The 10-year BCSS and OS rates were 81.2% (95% CI, 79.8%–82.7%) and 79.5% (95% CI, 78.0%–81.0%) in the younger group, and 90.0% (95% CI, 89.8%–90.3%) and 87.4% (95% CI, 87.1%–87.7%) in the older group, respectively.
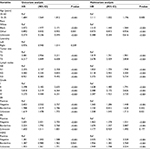
Univariate and multivariate Cox proportional hazard regression models were utilized to identify independent prognostic factors and calculate HR in early-stage breast cancer. In the univariate model, age, race, tumor size, lymph node status, tumor grade, ER status, PR status, and HER2 status were significantly associated with BCSS, as summarized in Table 2. After controlling the above factors, in the multivariate model, age, race, tumor size, lymph nodes, grade, ER status, PR status, and HER2 status remained significantly related to BCSS. Specially, compared with the older group (between 35 and 60 years), the younger group (between 15 and 35 years) had worse BCSS (HR, 1.200, 95% CI, 1.110–1.297, P<0.001). As presented in Table 3, some factors including age, race, tumor size, lymph nodes, tumor grade, ER status, PR status, and HER2 status were significantly related to OS in the univariate model. In the multivariate model, ER borderline status and PR unknown status were no longer significantly associated with OS, while young age was a significant worse prognostic predictor for OS (HR, 1.111, 95% CI, 1.032–1.196, P<0.001).
Propensity score matching method was utilized to give more convincing evidence for the survival difference between the younger and older groups. As shown in Table S1, the distribution of important clinicopathological characteristics of matched patients was not significantly different between the two groups. Kaplan–Meier analysis was performed to compare the survival between the younger and older groups. As shown in Figure 2, the younger group had significantly worse survival compared with the older group, both in terms of BCSS (HR, 1.196, 95% CI, 1.072–1.335, P=0.001) and OS (HR, 1.119, 95% CI, 1.009–1.240, P=0.032).
Results of multivariate Cox proportional hazard regression analysis of multiple imputed datasets were consistent with those presented in Tables 2 and 3. As presented in Table S2, the younger patients had inferior BCSS (HR, 1.214, 95% CI, 1.120–1.316, P<0.001) and OS (HR, 1.123, 95% CI, 1.041–1.211, P=0.003) compared with the older ones.
Subgroup analysis of BCSS and OS
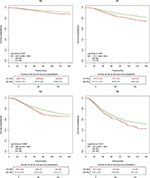
Since lymph node status and ER status are important prognostic factors in breast cancer, and the distribution of these two factors was significantly different between the younger and older groups, we conducted subgroup analyses of survival by stratifying lymph node status and ER status. As shown in Figures 3 and 4, when stratified by lymph node status, the younger group had worse BCSS compared with the older group in N0 (P=0.031) and N1 subgroups (P<0.001), and worse OS in N1 subgroup (P=0.009).
As presented in Figures 5 and 6, in the ER-positive subgroup, the younger group showed worse BCSS (HR, 1.354, 95% CI, 1.208–1.518, P≤0.001) and OS (HR, 1.187, 95% CI, 1.065–1.323, P=0.002) compared with the older group. However, in the ER-negative subgroups, there was no significant difference in BCSS and OS between the two groups (HR, 1.085, 95% CI, 0.970–1.214, P=0.152 and HR, 1.044, 95% CI, 0.939–1.161, P=0.427, respectively).
Explore the reasonable cutoff age to define “young age”
To explore a reasonable age to define young age breast cancer, we grouped patients by age of two years, and then multivariate Cox proportional hazard regression model was utilized to compare the prognosis of patients in different groups with controls.
Line smoothing of HR plots was performed to show the trend that HR increased when age decreased. As demonstrated in Figure 7, in terms of both BCSS and OS, in patients younger than 35 years, HR increased significantly with decreasing age. However, in patients aged between 35 and 60 years, HR was not associated with the change of age.
Discussion
Breast cancer in young patients is thought to be a special subgroup, and young age is an important factor for personalizing the treatment of breast cancer.23 There is no definite definition of “young age”. Although age is a continuum and any cutoff is arbitrary, many studies chose 354,6,9,15,17,18,24–26 or 4010,27,28 as threshold to distinguish young and old age. Han et al5 suggested that the risk of death in patients younger than 35 years increased dramatically, whereas patients aged between 35 and 40 years had similar risk of death in comparison with older premenopausal patients. In our study, in patients younger than 35 years, the risk of death increased significantly with decreasing age, whereas there was no such trend in patients aged between 35 and 60 years. In this case, it is reasonable to define younger than 35 years as “young age” in this study. Meanwhile, we chose patients aged between 35 and 60 years as the control group, since patients older than 60 years are considered postmenopausal as per the National Comprehensive Cancer Network (NCCN) guidelines.
Many studies suggest that breast cancer in young patients is associated with more advanced stage, such as higher T and N grade. Meanwhile, tumors in young patients always present with more aggressive tumor biology, including higher histological grade, higher proliferation index, more lymphovascular invasion, more triple negative, and HER2-enriched subtypes.3–12 The trend of advanced stage may be attributed to the lack of effective screening in mammography because of the dense breast tissue in young women. Additionally, the incidence of breast cancer in young women is relatively low, and hence clinicians are more inclined to treat the masses in breasts of young women as benign. The aggressive tumor biology also causes more lymph nodes involvement in young patients. Keegan et al12 analyzed 5,606 breast cancer patients aged between 15 and 39 years, and found that young patients had higher proportions of ER+/HER2+, triple-negative, and ER−/HER2+ subtypes. Anders et al11 compared the microarray data from 200 cases of young and 211 cases of older breast cancer patients, and suggested that young patients illustrated lower ER and PR mRNA expression, and higher HER2 and EGFR expression. In our study, the results are consistent with the previous studies that more aggressive clinicopathological characteristics were observed in younger breast cancer patients. However, the underlying mechanism of this phenomenon remains uncertain. Further studies are needed to elucidate the molecular biologic feature of breast cancer arising in young women, and new treatment approaches may be developed.
Currently, whether young breast cancer patients have worse prognosis in comparison with older patients remains controversial. Some researchers suggested that there was no difference in prognosis between young and older patients.21,26,29–31 In some studies, young patients even presented with better prognosis.32,33 However, there are more evidences that young breast cancer patients have worse prognosis compared with older ones. Since young breast cancer patients have more aggressive clinicopathological characteristics, some researchers suggested that the poor prognosis of young patients was attributed to these adverse factors.9,12,19,20,22,34 Anders et al22 compared the microarray data of 140 young breast cancer (≤45 years old) and 252 older breast cancer (≥65 years old), and found that there was greater proportion of aggressive intrinsic subtypes in the young breast cancer group. However, age alone did not provide an additional biologic complexity above breast cancer subtype and grade. In addition, treatment decisions should be driven by subtype and grade, not by age. However, a lot of studies reported that young age remained an independent adverse prognostic factor after controlling the adverse clinicopathological factors.4–6,10,13–18,27 Cancello et al4 suggested that after controlling the multiple factors, including tumor diameter, nodal involvement, ER and PR expression, Ki-67 labeling index, HER2 overexpression, vascular invasion, grade, histotype, and molecular subtypes, breast cancer patients younger than 35 years old (315 cases, 11%) presented a significantly increased risk of recurrence and death (HR=1.65, 95% CI=1.3–2.1 and HR=1.78, 95% CI=1.12–2.85, respectively) compared with older patients (2,655 cases, 89%). These conflicting results may be due to the following factors: 1) the inconsistent definition of “young age” and different control groups; 2) the small number of patients involved; 3) different therapy strategies; 4) lack of HER2 status, which is an important prognostic factor; and 5) heterogeneity of patients in terms of races. In this study, we conducted a population-based analysis that included 6,668 cases of breast cancer patients younger than 35 years and 143,920 patients aged between 35 and 60 from the SEER 18 database. This large cohort represented a large proportion of the population in the US and could provide more comprehensive results for the real-world situation. Furthermore, all patients were diagnosed with breast cancer in the latest 12 years, which reflected the results of state-of-the-art therapy strategies, and HER2 status was recorded in many cases. Additionally, we included patients younger than 60 years as the control group, the difference of comorbidity which could influence therapy strategies between these two groups was not significant, and according to the NCCN guidelines, the patients of both groups were premenopausal. We carried out the Kaplan–Meier analysis and found that compared with the older ones, the young patients presented with inferior prognosis in terms of both BCSS and OS. Additionally, after controlling the potential confounders, including tumor size, lymph node status, tumor grade, ER/PR, and HER2 status, young age remained an independent adverse prognostic factor in terms of both BCSS and OS. However, the underlying mechanism remains unclear, and further studies are needed to uncover the underlying key pathways and molecules, based on which new prognostic molecular biomarkers and therapy strategies may be developed. Additionally, since young age is an independent adverse prognostic factor, young breast cancer patients may receive more intensive treatment.
In the subgroups defined by different clinicopathological factors, young age may influence prognosis in different ways. Some studies revealed that young age could influence prognosis negatively in lymph node negative breast cancer,31,35 while others reported that young age was not related to prognosis.15,17 In the present study, subgroup analysis was performed based on lymph node status, and patients younger than 35 years presented with inferior prognosis in N0 (BCSS) and N1 (both BCSS and OS) subgroups. The inconsistency of these studies may be attributed to the different definition of young age, different criteria of molecular subtype, and ethnic disparity. When stratified by ER status, the impact of young age on prognosis was different. Some studies5,6,17 suggested that in the ER-positive group, young patients presented with poorer prognosis than the older ones, while in the ER-negative group, there was no significant difference in prognosis between these two groups. On the other hand, Cancello et al4 reported that the young patients presented with inferior prognosis in the luminal B, HER2-enriched, and triple-negative subtypes compared with the older ones. In the present study, young patients were significantly related to increased risk of BCSS and OS only in the ER-positive subgroup, and there was no survival difference in the ER-negative subgroups. The underlying mechanism may be that young patients always present with higher level of estrogen, which can stimulate the growth of ER-positive tumors. This implies that young patients may need more intensive endocrine therapy.
Our study has some limitations. Firstly, the treatment information, which is important for determining the prognosis, is not available in the public-accessed SEER database. In our study, we included patients aged from 35 to 60 years as the control group; there may be few differences in adjuvant therapies between these two groups.27 Secondly, the HER2 status was not available until 2010; this value was defined as unknown in the data before 2010. Lastly, since some important clinicopathological factors such as comorbidities, Ki-67, lymphovascular invasion, receipt of chemotherapy, and endocrine therapy were not available, this study may have unknown bias.
In conclusion, the young patients with operable breast cancer presented with more aggressive clinicopathological factors than the older patients. Furthermore, young age was an independent adverse prognostic factor in operable breast cancer. In the clinical practice, young patients may need more intensive treatment. Further research is required to elucidate the underlying mechanism and to find new biomarkers and therapy strategies.
Acknowledgments
We thank all the colleagues of the College of Life Sciences and Department of Breast Surgery for their support.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. | ||
Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol. 2010;21(10):1974–1981. | ||
Han W, Kang SY, Korean Breast Cancer Society. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010;119(1):193–200. | ||
Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4(11):e7695. | ||
Maru D, Middleton LP, Wang S, Valero V, Sahin A. HER-2/neu and p53 overexpression as biomarkers of breast carcinoma in women age 30 years and younger. Cancer. 2005;103(5):900–905. | ||
Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13(2):273–279. | ||
Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190(5):523–529. | ||
Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208(3):341–347. | ||
Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26(20):3324–3330. | ||
Keegan TH, Derouen MC, Press DJ, Kurian AW, Clarke CA. Occurrence of breast cancer subtypes in adolescent and young adult women. Breast Cancer Res. 2012;14(2):R55. | ||
Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea--a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25(17):2360–2368. | ||
de La Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341(8852):1039–1043. | ||
Han W, Kim SW, Park IA, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004;4:82. | ||
El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. | ||
Kim JK, Kwak BS, Lee JS, et al. Do very young Korean breast cancer patients have worse outcomes? Ann Surg Oncol. 2007;14(12):3385–3391. | ||
Dubsky PC, Gnant MF, Taucher S, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3(1):65–72. | ||
Jmor S, Al-Sayer H, Heys SD, et al. Breast cancer in women aged 35 and under: prognosis and survival. J R Coll Surg Edinb. 2002;47(5):693–699. | ||
Henderson IC, Patek AJ. Are breast cancers in young women qualitatively distinct? Lancet. 1997;349(9064):1488–1489. | ||
Anderson BO, Senie RT, Vetto JT, Wong GY, Mccormick B, Borgen PI. Improved survival in young women with breast cancer. Ann Surg Oncol. 1995;2(5):407–415. | ||
Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29(1):e18–20. | ||
Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. | ||
Maggard MA, O’Connell JB, Lane KE, Liu JH, Etzioni DA, Ko CY. Do young breast cancer patients have worse outcomes? J Surg Res. 2003;113(1):109–113. | ||
Wang J, Wang J, Li Q, et al. Young breast cancer patients who develop distant metastasis after surgery have better survival outcomes compared with elderly counterparts. Oncotarget. 2017;8(27):44851–44859. | ||
Tang J, Wu CC, Xie ZM, Luo RZ, Yang MT. Comparison of Clinical Features and Treatment Outcome of Breast Cancers in Young and Elderly Chinese Patients. Breast Care. 2011;6(6):435–440. | ||
Tang LC, Jin X, Yang HY, et al. Luminal B subtype: a key factor for the worse prognosis of young breast cancer patients in China. BMC Cancer. 2015;15:201. | ||
Johnson RH, Hu P, Fan C, Anders CK. Gene expression in “young adult type” breast cancer: a retrospective analysis. Oncotarget. 2015;6(15):13688–13702. | ||
Rapiti E, Fioretta G, Verkooijen HM, et al. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur J Cancer. 2005;41(10):1446–1452. | ||
Barchielli A, Balzi D. Age at diagnosis, extent of disease and breast cancer survival: a population-based study in Florence, Italy. Tumori. 2000;86(2):119–123. | ||
Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. Factors influencing the effect of age on prognosis in breast cancer: population based study. BMJ. 2000;320(7233):474–478. | ||
Chia KS, Du WB, Sankaranarayanan R, et al. Do younger female breast cancer patients have a poorer prognosis? Results from a population-based survival analysis. Int J Cancer. 2004;108(5):761–765. | ||
La Rosa F, Patavino VM, Epifani AC, Petrinelli AM, Minelli L, Mastrandrea V. Ten-year survival and age at diagnosis of women with breast cancer from a population-based study in Umbria, Italy. Tumori. 1996;82(5):441–443. | ||
Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. | ||
Boyages J, Chua B, Taylor R, et al. Use of the St Gallen classification for patients with node-negative breast cancer may lead to overuse of adjuvant chemotherapy. Br J Surg. 2002;89(6):789–796. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.