Back to Journals » Drug Design, Development and Therapy » Volume 14
Xanthohumol Inhibits TGF-β1-Induced Cardiac Fibroblasts Activation via Mediating PTEN/Akt/mTOR Signaling Pathway
Authors Jiang C, Xie N, Sun T, Ma W, Zhang B, Li W
Received 16 September 2020
Accepted for publication 18 November 2020
Published 7 December 2020 Volume 2020:14 Pages 5431—5439
DOI https://doi.org/10.2147/DDDT.S282206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Chuanhao Jiang,1,2,* Ning Xie,3,* Taoli Sun,4 Wanjun Ma,2 Bikui Zhang,2 Wenqun Li2
1Department of Laboratory Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, People’s Republic of China; 2Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha 410011, People’s Republic of China; 3Department of Breast Cancer Medical Oncology, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya Medical School, Central South University, Changsha, Hunan 410013, People’s Republic of China; 4Key Laboratory Breeding Base of Hu’nan Oriented Fundamental and Applied Research of Innovative Pharmaceutics, College of Pharmacy, Changsha Medical University, Changsha, Hunan 410219, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenqun Li Email [email protected]
Background: Xanthohumol (Xn) is the most abundant prenylated flavonoid in Hops (Humulus lupulus L.), and exhibits a range of pharmacological activities. This study aimed to investigate the effect of Xn on TGF-β 1-induced cardiac fibroblasts activation and elucidate the underlying mechanism.
Materials and Methods: The cellTiter 96® AQueous one solution cell proliferation assay kit was adopted to determine the cell viability of cardiac fibroblasts, and the proliferation was detected through 5-ethynyl-2ʹ-deoxyuridine (EdU) incorporation assay. The α-SMA protein expression was measured by using immunofluorescence and Western blotting. Western blotting was conducted to test the protein expressions of collagen I and III, PTEN, p-Akt, Akt, p-mTOR, mTOR, p-Smad3, Smad3 and GAPDH. The mRNA levels of α-SMA, collagen I and III were determined by quantitative real-time polymerase chain reaction (PCR).
Results: Xn inhibited the TGF-β 1-induced proliferation, differentiation and collagen overproduction of cardiac fibroblasts. TGF-β 1 induced the down-regulated PTEN expression, Akt and mTOR phosphorylation. These effects of TGF-β 1 were suppressed by Xn, while blocking of PTEN reduced Xn-mediated inhibitory effect on cardiac fibroblasts activation induced by TGF-β 1.
Conclusion: Xn inhibits TGF-β 1-induced cardiac fibroblasts activation via mediating PTEN/Akt/mTOR signaling pathway.
Keywords: xanthohumol; Xn, cardiac fibrosis, cardiac fibroblasts, TGF-β 1, PTEN/AKT/mTOR pathway
Introduction
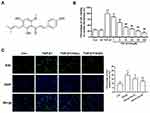
Hops (Humulus lupulus L.), a raw material to preserve beer, has been widely applied in the brewing industry throughout the world. Meanwhile, Hops was also used as a medicinal plant since ancient times for treating anxiety, insomnia, mild pain and dyspepsia.1 These traditional medicine values of hops are attributed to its richness in multiple prenylated flavonoids, whereas xanthohumol (Xn) is the most abundant prenylated flavonoid in hops as shown in Figure 1A. A range of pharmacological activities of Xn has been uncovered, such as anticancer, antioxidant, antibacterial, antiviral, anti-inflammatory, and antiplasmodial and antifungal activity. Among them, most of previous studies focused on anticancer activity of Xn, which has been summarized in our recently review.2 Moreover, we firstly demonstrated that Xn inhibited the proliferation of gastric cancer cells.3 In addition to anticancer activities, emerging evidence indicates the cardiovascular protective effects of Xn. It has been reported that Xn-fortified beer intake significantly attenuates the pulmonary vascular remodeling, and Xn can inhibit arrhythmia in rat ventricular myocytes.4
Cardiac fibrosis is regarded as the common pathological process that occurred in many cardiovascular diseases including myocardial infarction, hypertension, aortic stenosis, myocarditis and idiopathic dilated cardiomyopathy.5 To the best of our knowledge, the effects of Xn on cardiac fibrosis have not been clarified to date. Cardiac fibroblasts, accounting for 60%–70% of the cells in the human heart, play a key role in cardiac fibrosis. Under pro-fibrotic cytokine (TGF-β1) stimuli, cardiac fibroblasts are activated as indicated by enhanced proliferation, differentiation to myofibroblasts and collagen deposition.6 In view of the inhibition of Xn on hepatic stellate cells activation during liver fibrosis,7 we speculated that Xn can prevent the TGF-β1-induced cardiac fibroblasts activation.
It has been suggested that the antiangiogenic and anti-lymphocytic leukemia activity of Xn were mediated by inhibiting Akt phosphorylation.8,9 Akt was viewed as the key protein in PTEN/Akt/mTOR signaling pathway that has been reported to involve in cardiac fibroblasts activation.10 These studies impelled us to further explore the inhibition of Xn on cardiac fibroblasts activation whether involved PTEN/Akt/mTOR signaling pathway. Collectively, this study aimed to investigate the protective effect of Xn on TGF-β1-induced cardiac fibroblasts activation, and explore the underlying mechanism (PTEN/Akt/mTOR signaling pathway).
Materials and Methods
Reagents
Xn with the purity ≥96% (HPLC) was purchased from Sigma-Aldrich, St Louis, Missouri. Recombinant Human TGF-β1 was purchased from Peprotech Tebu-bio, Offenbach, Germany.
Cell Culture
The hearts of neonatal male Sprague-Dawley rats (1- to 2-days-old) were used to isolate primary rat cardiac fibroblasts. The cells were cultured in DMEM containing 10% FBS at 37 °C under 5% CO2. The passages between 2 and 4 of cells were used for the following experiments. The detail procedure and immunofluorescence identify of cardiac fibroblasts were performed according to previous article.11 Optimum time point and concentration of TGF-β1 (5 ng/mL, 24 h) treated cardiac fibroblasts has been obtained in our previous article.11
The rats were treated and cared for in accordance with the National Institutes of Health Guide (NIH publications № 8023). These animals were purchased from Laboratory Animal Center, Xiangya School of Medicine, Central South University (Changsha, China), and approved by the Medicine Animal Welfare Committee of Xiangya School of Medicine (Ref No. SYXK-2015/0017).
Cell Viability Assay
The cell viability was determined by cellTiter 96® AQueous one solution cell proliferation assay kit (Promega, USA). Cardiac fibroblasts cultured in a 96-well plate were incubated with 10 μL MTS for 1 h at 37 °C. The absorbance at 450 nm wavelength was tested in a microplate reader (Biotek Instruments, Winooski, VT).
EdU Incorporation Assay
The EdU (5-ethynyl-20-deoxyuridine) incorporation assay kit (Ribobio, Guangzhou China) was used to determine cell proliferation ability. The detail procedure was performed according to previous article.11 The ratio of EdU positive cells (green fluorescence) to total DAPI positive cells (blue fluorescence) was calculated as EdU incorporation rate.
Immunofluorescence Assay
Cardiac fibroblasts after treatment were incubated with 4% formaldehyde, 0.1% Triton X-100 and 5% BSA in proper order. Cells were stained with α-SMA (1:1000, #ab32575, abcam, Cambridge, UK) overnight, and incubated with secondary antibody for 1 hour followed with PBS. DAPI was used to stain cell nuclei. After washing in PBS, Carl Zeiss Axio VertA1 microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) was used to visualize cells.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Trizol reagent (Invitrogen) was used to obtain total RNA. The following transcription reaction and quantitative analysis were performed according to our previous article.11 Primers used to amplify the fragments of α-SMA, collagen I, collagen III and GAPDH were α-SMA-F 5′-CTATTCCTTCGTGACTACT-3′, α-SMA-R 5′- ATGCTGTT ATAGGTGGTT-3′; collagen I-F 5′-CCAACTGAACGTGACCAAAAA CCA-3′, collagen I-R 5′- GAAGG TGCTGGGTAGGGAAGTAGGC-3′; collagen III-F 5′- ATTCTGCCAC CCTGAACTCAAGAGC-3′, collagen III-R 5′- TCCATGTAGGCAATGC TGTTTTTGC-3′; GAPDH-F 5′- TGGCCTCCAAGGAGTAAGAAAC-3′, GAPDH-R 5′- GGCCTCTC TCTTGCTCTCAGTATC-3′.
Western Blot Analysis
Fresh myocardiums were lysed in RIPA buffer containing 0.1% PMSF (BOSTER Biological Technology; Wuhan, China). Proteins were separated by SDS-PAGE using 8–10% gradient gel and transferred onto 0.45 μm PVDF membranes. The expressions of target protein were normalized to GAPDH protein. Antibodies against α-SMA (#19245), PTEN (#9188), p-Akt (#9271), Akt (#9272), p-mTOR (#5536), mTOR (#2972), GAPDH (#2118) and horseradish peroxidase-linked anti-mouse or anti-rabbit IgG (#7074) were purchased from Cell Signaling Technology (Boston, USA). Antibodies against collagen-I (ab6308), collagen-I (ab6310), p-Smad3 (ab193297) and Smad3 (ab208182) were purchased from Abcam (Cambridge, UK).
Statistical Analysis
The results were presented as means ± S.E.M. Data comparison of multiple groups were performed by one-way ANOVA and Student-Newman-Keuls test. Results were considered statistically significant when P < 0.05.
Results
Xn Inhibited the TGF-β1-Induced Cardiac Fibroblasts Proliferation
TGF-β1, the pro-fibrotic growth factor, plays a key role in cardiac fibrosis. Therefore, TGF-β1 is commonly used to induce cardiac fibroblasts activation. The activated cardiac fibroblasts can be indicated by enhanced proliferation, differentiation to myofibroblasts and collagen deposition. To investigate the effect of Xn on cardiac fibroblasts proliferation, cell viability and EdU incorporation assay were conducted. Result showed that TGF-β1 promoted the cell viability of cardiac fibroblasts, which can be inhibited by Xn (1, 5, 10, 20, 50 and 100 μM) in a dose dependent manner, with an IC50 of 8.86 μM. However, the Xn (100 μM) alone exerted no effect on cells viability, suggesting the non-toxic of Xn (Figure 1A and B). Thus, two concentrations distributed around its IC50 value (5 and 10 µM), named Xn (L) and Xn (H), were selected for following experiments. The percentage of EdU-positive cells reflects the cell proliferation ability. As shown in Figure 1C, Xn reduced the percentage of EdU‑positive cells under TGF-β1 treatment, suggesting the anti-proliferation role of Xn in cardiac fibroblasts activation.
Xn Suppressed the TGF-β1-Induced Differentiation and Collagen Overproduction of Cardiac Fibroblasts
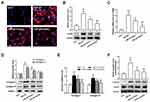
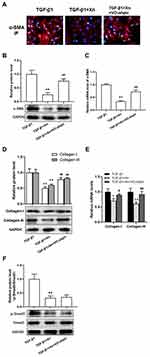
In addition to proliferation, the differentiation and collagen overproduction are the important pathological features of activated cardiac fibroblasts. To determine the effect of Xn on the differentiation and collagen overproduction, α-SMA and Collagen levels were investigated. The TGF-β1-induced up-regulation of α-SMA protein and mRNA expressions indicated the differentiation to myofibroblasts, which was suppressed by Xn (Figure 2A–C). Moreover, collagen overproduction mainly refers to collagen types I and III, their protein and mRNA were therefore measured. As shown in Figure 2D and E, Xn inhibited the up-regulation of Collagen-I and III expression induced by TGF-β1. Smad signaling pathway is the classical down-stream signaling of TGF-β1. Therefore, we further determined phosphorylation level of Smad3, and found Xn inhibited the TGF-β1-induced p-Smad3 upregulation (Figure 2F). These results indicated that Xn can suppress the cardiac fibroblasts differentiation and collagen overproduction induced by TGF-β1.
Xn Inhibited the PTEN Down-Regulation and Akt/mTOR Phosphorylation Induced by TGF-β1 in Cardiac Fibroblasts
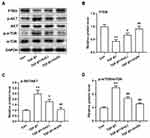
To explore the underlying mechanism, and in consideration of the key role of PTEN/Akt/mTOR signaling pathway in cardiac fibroblasts activation, the PTEN expression and Akt/mTOR phosphorylation were determined. Results showed that TGF-β1 induced the down-regulation of PTEN, phosphorylation of Akt and mTOR, whereas Xn reduced the effect of TGF-β1 on PTEN/Akt/mTOR signaling pathway (Figure 3A–D). The results indicated the involvement of PTEN/Akt/mTOR signaling pathway in the inhibition of Xn on cardiac fibroblasts activation.
Blocking of PTEN Reduced Xn-Mediated Inhibitory Effect on TGF-β1-Induced Cardiac Fibroblasts Activation
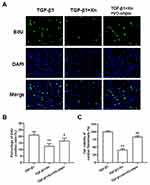
To further determine the role of PTEN/Akt/mTOR pathway in the inhibition of Xn on cardiac fibroblasts activation, activated cardiac fibroblasts were treated with VO-ohpic (a PTEN specific inhibitor) in the presence of Xn. As shown in Figure 4A–C, VO-ohpic can reverse the inhibition of Akt/mTOR phosphorylation induced by Xn. Correspondingly, VO-ohpic can abolish the protective effect of Xn against TGF-β1-induced proliferation (Figure 5A–C), differentiation and collagen overproduction (Figure 6A–E). However, VO-ohpic exhibited no effect on phosphorylation level of Smad3 (Figure 6F). Collectively, these results demonstrated that Xn inhibited TGF-β1-induced cardiac fibroblasts activation through regulating PTEN/Akt/mTOR signaling pathway.
Discussion
Cardiac fibrosis is viewed as a common end-stage pathologic manifestation that occurred in many cardiovascular diseases, such as hypertension, myocardial infarction, myocarditis, aortic stenosis and idiopathic dilated cardiomyopathy.5 Initially, cardiac fibrosis acts as an adaptive compensatory response to increased cardiac stress, which if untreated, may become maladaptive over time and results in heart failure and rising mortality.12 Cardiac fibroblasts play a key role in cardiac fibrosis. During the process of cardiac fibrosis, normal cardiac fibroblasts proliferate and are activated to myofibroblast phenotype.13 The increased myofibroblasts produce a large number of extracellular matrix (ECM), which breaks the balance of synthesis and degradation of ECM, finally leading to cardiac fibrosis.14 Moreover, the activation of cardiac fibroblasts is regulated by a variety of neurohumoral factors, such as TGF-β1, Ang II, ET-1 and PDGF. Among these cytokines, TGF-β1 is viewed as pro-fibrotic cytokine to induce cardiac fibroblasts proliferation, differentiation and ECM production during cardiac fibrosis.15 Therefore, pharmacological interventions targeting TGF-β1-induced cardiac fibroblasts activation is important to identify new therapeutic options for treating cardiovascular diseases.
A large number of compelling experimental evidences indicate the protective role of flavonoids against cardiovascular diseases. For instance, Kaempferol, a flavonoid mainly derived from the rhizome of Kaempferia galanga L, has been reported to alleviate Ang II–induced cardiac fibrosis.16 As a natural phytoestrogen isolated from soy extract, Genistein can prevent the cardiac fibrosis induced by pressure overload.17 Therefore, looking for protective candidate from flavonoids against cardiac fibrosis may be a novel approach for treating cardiovascular disease.
Xn, he most abundant prenylated flavonoid with 0.1%-1% of dry weight in hops (Humulus lupulus L.), is also a constituent of beer with the concentrations up to 0.96 mg/L.2 Increased evidence indicates the protective role of Xn against cardiovascular diseases. It has been reported that Xn attenuates atherosclerosis by reducing arterial cholesterol content.18 Xn can suppress aberrant ryanodine receptor Ca2+ release in rat ventricular myocytes, exhibited clinically desirable antiarrhythmic properties.19 Recently, the beneficial effect of Xn on pulmonary vasculature and right ventricular function was revealed.4 More importantly, feeding of Xn attenuated the liver fibrosis induced by non-alcoholic steatohepatitis and CCl4 via inhibiting the hepatic stellate cell proliferation and inflammation.20,21 In this study, we found that Xn could reduce the cell viability and percentage of EdU positive cells, suggesting its inhibition on TGF-β1-induced proliferation of cardiac fibroblasts. Meanwhile, the TGF-β1-induced up-regulation of α-SMA and Collagen-I/III expression was decreased after Xn treatment, indicating Xn could suppress the cardiac fibroblasts differentiation and collagen overproduction. Collectively, these finding demonstrated the inhibition of Xn on cardiac fibroblasts activation. Encouraged by these results obtained, we further explored the mechanism underlying the protective effect of Xn against cardiac fibroblasts activation.
During liver fibrosis, Xn inhibited the transcription factor NF-κB activation, and decreased the NF-κB-dependent pro-inflammatory gene expression such as MCP-1 in hepatic stellate cells.7 In addition to NF-κB, several studies have indicated that Xn inhibits cancer cells proliferation and induces apoptosis through Akt.8,9 It is well known that the Akt is regulated by PI3K, and the PI3K/Akt signaling pathway plays a key role in a variety of pathological processes.22 Moreover, PTEN is a negative regulator of the PI3K/AKT signaling pathway, and inhibition of PTEN has been associated with cardiac fibrosis. PTEN overexpression suppressed the Ang-II-induced cardiac fibroblasts activation via reducing the Akt phosphorylation.23 Akt phosphorylation can activate or inhibit down-stream target proteins to regulate cell proliferation, differentiation and apoptosis, while the mTOR is a typical down-stream protein of Akt that involves in cardiac fibroblasts activation.24 Here, we found that TGF-β1 induced the down-regulated PTEN expression, Akt and mTOR phosphorylation. These effects of TGF-β1 were inhibited by Xn, while blocking of PTEN reduced Xn-mediated inhibitory effect on TGF-β1-induced cardiac fibroblasts activation, indicating the involvement of PTEN/Akt/mTOR signaling pathway in the protective role of Xn against cardiac fibroblasts activation.
To our knowledge, the mechanism underlying regulation of TGF-β1 on PTEN expression in cardiac fibroblasts has not been elucidated. The related studies in other cell types may have reference value. For example, TGF-β1 could activate PTEN/Akt/mTOR signaling pathway in airway smooth muscle cells by up-regulating miR-181a and miR-620, and PTEN is a direct target of miR-181a and miR-620.25,26 In hepatic stellate cells, TGF-β1 regulated PTEN/Akt/mTOR pathway through miR‑141.27 These recent studies indicate that TGF-β1 regulates PTEN expression via miRNAs rather than directly bind to PTEN, which may be a direction for clarifying the detail mechanism of this study.
It has been reported that TGF-β1 binds to TGF-β receptors (TGF-βR) on the cellular membrane, and leads to the activation of down-stream signal transduction pathways. Smad signaling pathway is the classical down-stream signaling of TGF-β1, and we found that Xn inhibited the TGF-β1-induced phosphorylation of Smad3. Moreover, several non-Smad signaling pathways are gradually uncovered in recent years. The PTEN/AKT/mTOR signaling pathway is one of the non-Smad signaling pathways.28 This study found Xn inhibited the TGF-β1 induced cardiac fibroblasts activation via PTEN/AKT/mTOR signaling pathway. However, inhibition of PTEN exhibited no effect on the Smad3 phosphorylation of cardiac fibroblasts under TGF-β1 and Xn treatment. These results suggested Smad signaling pathway and PTEN/AKT/mTOR signaling pathway are independent of each other, and both of them are involved in the protective effect of Xn.
This study provided supporting evidence for protective role of Xn against cardiac fibrosis, but there are several barriers from basic research to clinical practice. The low bioavailability is one of the important limitations,29 the future studies on chemical structure modification and optimization of physical and chemical properties may improve the bioavailability of Xn. Moreover, the low extractive yield is another important factor restricting clinical application.30 Learning from the development of Paclitaxel, further studies can focus on improvement of total synthesis yield.
In conclusion, our research firstly determined the effect of Xn on cardiac fibroblasts, and provided critical evidence that Xn significantly inhibited TGF-β1-induced cardiac fibroblasts activation via mediating PTEN/Akt/mTOR signaling pathway. These findings provide a scientific basis supporting the cardiovascular protective activity of Xn, and suggest Xn may be a novel therapeutic agent for cardiac fibrosis prevention. However, this study lacks in vivo experiment regarding the protective effect of Xn on cardiac fibrosis. It is well known that PTEN/AKT/mTOR pathway is a central regulating mechanism contributing to autophagy,31 therefore whether the cardioprotective effect of Xn involves autophagy deserves further investigation. In addition, lack of a control group in the second batch of experiment (TGF-β1, TGF-β1+Xn, TGF-β1+Xn+ VO-ohpic) and the small sample size may also be the limitations of this study.
Ethics Approval
The animals were treated and cared for in accordance with the National Institutes of Health Guide (NIH publications № 8023). These animals were purchased from Laboratory Animal Center, Xiangya School of Medicine, Central South University (Changsha, China), and approved by the Medicine Animal Welfare Committee of Xiangya School of Medicine (Ref No. SYXK-2015/0017).
Acknowledgments
This study was supported by grants of the National Natural Scientific Foundation of China (No. 81703518, 81673614, 81973406, 81701577), Hunan Provincial Natural Scientific Foundation (No. 2018JJ3571, 2019JJ50849, 2020JJ4823, 2020JJ8064), Scientific Research Project of Hunan Provincial Health and Family Planning Commission (No. B20180253, B2019089), and Fundamental Research Funds for the Central Universities of Central South University (No. 2020zzts822).
Disclosure
The authors declare no conflicts of interest.
References
1. Hamm AK, Manter DK, Kirkwood JS, Wolfe LM, Cox-York K, Weir TL. The effect of hops (Humulus lupulus L.) extract supplementation on weight gain, adiposity and intestinal function in ovariectomized mice. Nutrients. 2019;11(12):3004. doi:10.3390/nu11123004
2. Jiang CH, Sun TL, Xiang DX, Wei SS, Li WQ. Anticancer activity and mechanism of xanthohumol: a prenylated flavonoid from hops (Humulus lupulus L.). Front Pharmacol. 2018;9:530. doi:10.3389/fphar.2018.00530
3. Wei S, Sun T, Du J, Zhang B, Xiang D, Li W. Xanthohumol, a prenylated flavonoid from hops, exerts anticancer effects against gastric cancer in vitro. Oncol Rep. 2018;40(6):3213–3222. doi:10.3892/or.2018.6723
4. Silva AF, Faria-Costa G, Sousa-Nunes F, et al. Anti-remodeling effects of xanthohumol-fortified beer in pulmonary arterial hypertension mediated by ERK and AKT inhibition. Nutrients. 2019;11(3):583. doi:10.3390/nu11030583
5. Creemers EE, van Rooij E. Function and therapeutic potential of noncoding RNAs in cardiac fibrosis. Circ Res. 2016;118(1):108–118. doi:10.1161/CIRCRESAHA.115.305242
6. Saucerman JJ, Tan PM, Buchholz KS, McCulloch AD, Omens JH. Mechanical regulation of gene expression in cardiac myocytes and fibroblasts. Nat Rev Cardiol. 2019;16(6):361–378. doi:10.1038/s41569-019-0155-8
7. Dorn C, Kraus B, Motyl M, et al. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol Nutr Food Res. 2010;54(Suppl S2):S205–13. doi:10.1002/mnfr.200900314
8. Dell’Eva R, Ambrosini C, Vannini N, Piaggio G, Albini A, Ferrari N. AKT/NF-kappaB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007;110(9):2007–2011. doi:10.1002/cncr.23017
9. Benelli R, Vene R, Ciarlo M, Carlone S, Barbieri O, Ferrari N. The AKT/NF-kappaB inhibitor xanthohumol is a potent anti-lymphocytic leukemia drug overcoming chemoresistance and cell infiltration. Biochem Pharmacol. 2012;83(12):1634–1642. doi:10.1016/j.bcp.2012.03.006
10. Yang W, Wu Z, Yang K, et al. BMI1 promotes cardiac fibrosis in ischemia-induced heart failure via the PTEN-PI3K/Akt-mTOR signaling pathway. American Journal of Physiology-Heart and Circulatory Physiology. 2019;316(1):H61–H69. doi:10.1152/ajpheart.00487.2018
11. Li W-Q, Li X-H, Wu Y-H, et al. Role of eukaryotic translation initiation factors 3a in hypoxia-induced right ventricular remodeling of rats. Life Sci. 2016;144:61–68. doi:10.1016/j.lfs.2015.11.020
12. Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–574. doi:10.1007/s00018-013-1349-6
13. van Putten S, Shafieyan Y, Hinz B. Mechanical control of cardiac myofibroblasts. J Mol Cell Cardiol. 2016;93:133–142. doi:10.1016/j.yjmcc.2015.11.025
14. Gonzalez A, Schelbert EB, Diez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71(15):1696–1706. doi:10.1016/j.jacc.2018.02.021
15. Tarbit E, Singh I, Peart JN, Rose’Meyer RB. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail Rev. 2019;24(1):1–15. doi:10.1007/s10741-018-9720-1
16. Liu Y, Gao L, Guo S, et al. Kaempferol alleviates angiotensin II-induced cardiac dysfunction and interstitial fibrosis in mice. Cell Physiol Biochem. 2017;43(6):2253–2263. doi:10.1159/000484304
17. Qin W, Du N, Zhang L, et al. Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. Br J Pharmacol. 2015;172(23):5559–5572. doi:10.1111/bph.13002
18. Hirata H, Yimin Y, Segawa S, et al. Xanthohumol prevents atherosclerosis by reducing arterial cholesterol content via CETP and apolipoprotein E in CETP-transgenic mice. PLoS One. 2012;7(11):e49415. doi:10.1371/journal.pone.0049415
19. Arnaiz-Cot JJ, Cleemann L, Morad M. Xanthohumol modulates calcium signaling in rat ventricular myocytes: possible antiarrhythmic properties. J Pharmacol Exp Ther. 2017;360(1):239–248. doi:10.1124/jpet.116.236588
20. Dorn C, Heilmann J, Hellerbrand C. Protective effect of xanthohumol on toxin-induced liver inflammation and fibrosis. Int J Clin Exp Pathol. 2012;5(1):29–36.
21. Yang M, Li N, Li F, et al. Xanthohumol, a main prenylated chalcone from hops, reduces liver damage and modulates oxidative reaction and apoptosis in hepatitis C virus infected Tupaia belangeri. Int Immunopharmacol. 2013;16(4):466–474. doi:10.1016/j.intimp.2013.04.029
22. Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8(3):187–198. doi:10.2174/156800908784293659
23. Nie L, Zhao JH, Wang J, Song R, Zhu SJ. Effect of phosphatase and tensin homologue on chromosome 10 on angiotensin II-mediated proliferation, collagen synthesis, and Akt/P27 signaling in neonatal rat cardiac fibroblasts. Biomed Res Int. 2016;2016:2860516. doi:10.1155/2016/2860516
24. Xu X, Jiang R, Chen M, et al. Puerarin decreases collagen secretion in angII-induced atrial fibroblasts through inhibiting autophagy via the JNK-Akt-mTOR signaling pathway. J Cardiovasc Pharmacol. 2019;73(6):373–382. doi:10.1097/FJC.0000000000000672
25. Lv X, Li Y, Gong Q, Jiang Z. TGF-beta1 induces airway smooth muscle cell proliferation and remodeling in asthmatic mice by up-regulating miR-181a and suppressing PTEN. Int J Clin Exp Pathol. 2019;12(1):173–181.
26. Chen H, Guo SX, Zhang S, Li XD, Wang H, Li XW. MiRNA 620 promotes TGF-β1-induced proliferation of airway smooth muscle cell through controlling PTEN/AKT signaling pathway. Kaohsiung J Med Sci. 2020;36(11):869–877. doi:10.1002/kjm2.12260
27. Liang H, Wang X, Si C, et al. Downregulation of miR141 deactivates hepatic stellate cells by targeting the PTEN/AKT/mTOR pathway. Int J Mol Med. 2020;46(1):406–414. doi:10.3892/ijmm.2020.4578
28. Lodyga M, Hinz B. TGF-beta1 - A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2020;101:123–139. doi:10.1016/j.semcdb.2019.12.010
29. Venturelli S, Burkard M, Biendl M, Lauer UM, Frank J, Busch C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition. 2016;32(11–12):1171–1178. doi:10.1016/j.nut.2016.03.020
30. Dresel M, Dunkel A, Hofmann T. Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer. J Agric Food Chem. 2015;63(13):3402–3418. doi:10.1021/acs.jafc.5b00239
31. Shi X, Liu Y, Zhang D, Xiao D. Valproic acid attenuates sepsis-induced myocardial dysfunction in rats by accelerating autophagy through the PTEN/AKT/mTOR pathway. Life Sci. 2019;232:116613. doi:10.1016/j.lfs.2019.116613
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.