Back to Journals » Clinical Ophthalmology » Volume 11
Wound and surface temperatures in vivo in torsional and longitudinal modalities of ultrasound in coaxial microincisional cataract surgery
Received 26 September 2016
Accepted for publication 23 December 2016
Published 27 January 2017 Volume 2017:11 Pages 249—255
DOI https://doi.org/10.2147/OPTH.S123222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Swapna Nair,1 Ramachandran Unnikrishnan Nair2
1Department of Cataract and Refractive Surgery, 2Department of Vitreoretinal Services, Chaithanya Eye Hospital and Research Institute, Trivandrum, Kerala, India
Background: To study phacoemulsification probe shaft/wound and corneal surface/tip temperatures in vivo during longitudinal, torsional and combined phacoemulsification modes and their relationship to machine parameters.
Design: This was a prospective study at Chaithanya Eye Hospital and Research Institute, Trivandrum, India (tertiary).
Participants: Twenty-two eyes of 22 patients were randomized into six groups depending on the grade of nuclear sclerosis (NS) and the type of ultrasound used: Group 1, torsional in NS2; Group 2, torsional in NS3; Group 3, torsional with intermittent longitudinal in NS2; Group 4, torsional with intermittent longitudinal in NS3; Group 5, longitudinal in NS2; Group 6, longitudinal in NS3.
Methods: Patients underwent phacoemulsification by torsional, longitudinal or combined modalities. A thermal camera was used to measure phaco probe temperatures.
Main outcome measures: The mean probe shaft and tip temperatures were documented for different ultrasound modalities.
Results: The mean shaft and tip temperatures were: Group 1, 29.22°C±0.71°C and 28.4°C±0.88°C; Group 2, 32.12°C±0.62°C and 31.88°C±0.84°C; Group 3, 30.25°C±0.71°C and 29.35°C±0.62°C; Group 4, 31.95°C±0.65°C and 32.01°C±1.31°C; Group 5, 23°C and 27.6°C and Group 6, 23°C and 29.68°C, respectively. In all groups using longitudinal ultrasound, the phaco tip surface temperatures were higher than the shaft temperatures, except in Group 3. Shaft temperatures were higher than tip temperatures in cases using torsional phaco, except in Group 4. The mean temperature difference between groups was significant only for shaft temperatures (P=0.001). On thermal imaging, for torsional phaco, the rise in temperature of the probe from shaft to tip was dependent on the amplitude of ultrasound applied (P=0.009).
Conclusion: The shaft temperatures were higher than over the phaco tip during torsional phacoemulsification.
Keywords: thermal imaging, cataract, phacoemulsification, microincision, torsional ultrasound
Introduction
The ultrasound used for phacoemulsification generates heat. The clinical manifestations of heat injury in the anterior chamber may be obvious or subtle depending on the quantity of heat generated. The machines presently in use are extremely efficient with low energy production,1 precise followability and fast emulsification.
We still see a few uneventful cases of phacoemulsification in low to moderate grade of cataracts with well-dilated pupils and no intraoperative iris issues showing chaffing of the subincisional iris intraoperatively that persists as an iris discoloration. Some patients may have corneal sectional edema that sometimes persists beyond the second postoperative week and may be associated with a foreign body sensation. This is possibly related to heat generation during routine phacoemulsification.
The temperature of the eye has been studied in various physiologic and pathologic contexts using thermal imaging in various subspecialities of ophthalmology including the ocular surface and orbit using thermal imaging cameras.2,3 Accuracy of the thermal imaging camera when compared to thermometry is highlighted in various studies.4,5 This study was performed to quantify heat generation during phacoemulsification using thermal imaging.4
Methods
The institutional review board of the hospital, Chaithanya Eye Hospital and Research Institute Institutional Ethics Committee, approved the study. The research adhered to the tenets of the Declaration of Helsinki. Recording of surgery, thermal imaging and its use for academic purpose were explained to the subjects prior to the surgery. An informed consent for research was obtained from all the patients. Out of patients posted for surgery over a period of 2 days, 22 patients with a nuclear sclerosis (NS) of grades 2 and 3 participated in the study. The patients were allotted to six groups based on the level of NS and the modality of phacoemulsification used. Unequal randomization was used in the ratio of 1:2:4 for longitudinal:combined:torsional groups in grade 3 NS and 1:4:8 for NS grade 2, with residual patients allocated to the torsional group in both grades of cataract. The eyes undergoing surgery in these 22 patients were imaged using an infrared camera during phacoemulsification. All the surgeries were performed by one surgeon (SN).
Surgeries were performed using the Alcon Infiniti Intrepid phacoemulsification system (Alcon, Fortworth, TX, USA) with the 0.9 mm miniflared Kelman tip. All eyes underwent coaxial microincisional cataract surgery (coaxial MICS) with the modality of ultrasound used being the variable. A new tip was used for each case. The power settings for torsional varied from an initial amplitude of 0 to a maximum of 100. During intermittent phaco as a part of combined phaco, continuous ultrasound was available between 0% and 100% with a 9 ms on time. For longitudinal ultrasound, a linear power setting between 0% and 50% was used. The room temperature was 20°C–22°C, and the irrigating solution used had a temperature of 19°C. Sodium hyaluronate 10 mg/mL was used for all cases (stored below room temperature as recommended by the manufacturer). It coats poorly and, therefore, passes out of the eye as a bolus during initial cortex aspiration prior to burrowing of the nucleus. This precluded its presence during phacoemulsification when the temperatures were measured. A 2.2 mm clear corneal incision was used in all cases with the same type of keratome. The phaco tip and the sleeve size corresponding to this microincision were used.
Imaging
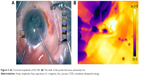
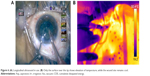
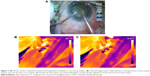
A thermal camera (ThermaCAM, FLIR Systems) kept at a constant distance from the eye was used to capture images during fixed stages of surgery where the requirement for ultrasound energy exists undeniably, for example, during initial nuclear burrowing, chopping and fragment emulsification. Sensitivity of the thermal camera was 0.1°C. FLIR infrared cameras are factory calibrated and have an electronic stabilization circuitry that maintains this calibration over temperature variations. They are annually recalibrated. In our study, prior to surgery, the camera was used to record the temperature of known objects such as ice water and boiling water and the human body temperature to verify its functioning. The thermal camera was controlled by the camera technician at a distance of 50 cm from the eye. The emissivity was kept at 0.97. The thermal camera records surface temperatures over the ocular surface, of which we analyzed those over the wound site and at the surface overlying the phaco tip. When analyzing the image using the proprietary software, the cursor positioned over the required point gives the temperature reading at that point. During the intraoperative imaging, since the camera is focused on the eye irrigated by cold saline that appears blue, any subtle variations in the probe temperature show up as a contrasting yellow. The pictures were analyzed using the accompanying software (Flir QuickReport 1.2, 2009; FLIR Systems) to study the temperatures at the site of contact of the phaco shaft with the corneal incision and at the surface over the free end of the tip. The maximum temperatures recorded for these sites for each surgery were noted, as was the basal temperature during each surgery. The increase in temperature was derived from these readings.
The machine parameters including the set minimum and maximum power, aspiration flow rate, average amplitude and cumulative dissipated energy, derived from machine metrics, were noted. All eyes were subject to postoperative slit-lamp examination.
Statistical analysis
Statistical Package for the Social Sciences ver. 21.0 was used to analyze the data. The means of the maximum and minimum temperatures at the shaft and surface over the tip were calculated, and the difference of means and its statistical significance was assessed using one-way analysis of variance. The shaft and surface (tip) temperatures between group pairs were assessed using Student’s t-test and one-sample t-test. The shaft temperature was correlated with the average amplitude and aspiration flow rate. The normality of data was tested with the Shapiro–Wilk test. For data with normal distribution, independent sample t-test was used to test significance. For data where the distribution was not normal, nonparametric Mann–Whitney U test was used to test significance. A P-value of 0.05 was considered as significant.
Results
Out of 22 patients, 14 underwent torsional phacoemulsification – nine for grade 2 NS (Group 1, tors NS2) and five for grade 3 NS (Group 2, tors NS3). The distribution of patients in each group is shown in Figure 1.
Among the groups (Figure 2), the maximum mean temperature of the shaft near the wound was seen in Group 2 with a mean of 32.12°C±1.39°C (max 33.8°C) and the least temperature was observed in both longitudinal phaco groups (5 and 6). The values of mean temperature of the surface over tip (Figure 3) were in the late 20 s for all groups except grade 3 nuclei that underwent torsional and combined torsional longitudinal ultrasound (Group 2 and Group 4 had tip temperatures >30°C).
In all groups using longitudinal ultrasound, the surface temperatures were higher than shaft temperatures, except in Group 3 (Table 1). Shaft temperatures were higher than tip in cases using torsional phaco, except Group 4. The mean temperature difference between groups was significant only for shaft temperatures. When comparing the shaft temperatures of all groups using torsional phacoemulsification (groups 1–4) with groups not using torsional mode (groups 5, 6), the shaft temperatures were significantly higher in torsional modalities (Table 2), except in Group 4.
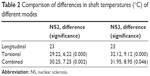  | Table 2 Comparison of differences in shaft temperatures (°C) of different modes |
The shaft temperatures of all groups had no statistical correlation with the average amplitude of ultrasound or aspiration flow (Figure 4; Table 3). But on thermal imaging, for torsional phaco, the rise of temperature of the probe from the shaft to the surface over the tip was dependent on the amplitude of ultrasound applied. The shaft and surface temperatures for different modalities are shown in the thermal images (Figures 5–7).
  | Table 3 Pearson correlation and significance of shaft temperatures with average amplitude and aspiration flow rate |
Discussion
Phacoemulsification may produce wound burns due to increased temperature at the wound site. In modern phacoemulsification, temperatures rarely rise to levels causing wound burns (incidence 0.037%).1 In this context, it is interesting to note that ophthalmic viscosurgical devices have been attributed both the role of protectors of endothelium from thermal damage as well as notorious heat enhancers due to outflow occlusion.6,7 We chose thermal imaging with an infrared camera to study temperatures during cataract surgery, as both invasive thermal documentation with thermocouples and noninvasive imaging with the camera have equitable results.4,5 The incision temperature recordings by the two methods vary only by 0.5°C, bringing about the conclusion that surface temperatures faithfully reflect activity in the anterior chamber. All the patients underwent coaxial MICS with a 2.2 mm clear corneal incision, as this is the protocol followed in our institute. Based on this, the probe and sleeve size were constant for all the patients, so were the fluidics. The only variables were firstly, the modality of ultrasound, namely, torsional (ozil), longitudinal phaco and combined longitudinal and torsional, and secondly, the grade of cataract. This was to know if the modality of ultrasound used produced temperature variations in different parts of the phaco tip.
In our study, we found the shaft temperatures to be higher during thermal imaging of torsional phacoemulsification cases compared to longitudinal phacoemulsification. In spite of this, visible wound burns were not seen in any of our cases. This could be because higher temperatures at the shaft may be required for a visible shrinking of corneal collagen at the wound site. Such high temperatures are not seen during cataract surgery due to the high efficacy of the system in emulsifying the nucleus in a short period of time. Our study closely relates to what Schmutz and Olson had observed in vitro with their thermal images showing high temperatures at the shaft in torsional ultrasound.8 They had also mentioned a larger rise in temperature at 90° to the bend of the Kelman tip, which, with the tip in the bevel up position, would translate to more heat at the sidewalls of the wound. In our study, most of the temperature effects were seen on the upper and lower lips of the wound. An explanation could be that the Kelman tip is held by most surgeons at approximately 300°–350°, with the bevel facing the left side. This might be an unconscious reflex to avoid proximity of the bend tip to the posterior capsule and also to prevent direct blasts of ultrasound energy on the overlying endothelium. In this position, the points of maximum rise of temperature would correspond to the upper and lower lips of the wound.
In most studies using thermal imaging to study probe temperatures, owing to the fact that a majority have been performed in vitro, 100% torsional ultrasound is usually compared to 100% longitudinal ultrasound.8,9 It has been argued that extremes of power may be required to generate visible changes in the probe that can be captured by the thermal camera, but this is not the case during in vivo imaging.10 This might be attributed to the fact that the anterior chamber environment is made cooler than the body temperature by the refrigerated irrigating solution, enhancing the visibility of subtler thermal changes shot by the ThermaCAM. In our study, shaft temperatures in any setting using torsional ultrasound on an average were at least 6° higher than in longitudinal ultrasound. In the study by Jun et al,9 though the shaft temperatures were always higher than the surface temperatures in all modalities, they were always more for longitudinal ultrasound, which can be due to the higher frequency and stroke length of longitudinal mode than the torsional mode. A plausible explanation for the tip temperatures of longitudinal phaco being high in our study is the jackhammer effect of longitudinal phaco, being its mainstay,11 keeps the tip temperatures high.
The biologic effects of such a difference in the pattern of heat produced by the two modalities also need to be viewed in the context of the hardness of the nucleus, the size of the incision and movements of the probe that may alter irrigation inflow.12 On cross-tabulating microscopic corneal and iris changes at the wound section with probe temperatures and machine parameters,13 we found that microscopic tissue changes at the section were associated with torsional phacoemulsification, and the difference was not statistically significant. We believe further studies with larger sample size are required to analyze these clinical correlations and to categorize these.
Our study showed that in vivo thermal imaging is a viable modality for monitoring the temperature changes during coaxial MICS.14 Further studies would enhance the utilization of this process to quantify the heat generated and accurately define the safe threshold for different ultrasound modalities. Assessing other surgical parameters such as varied tip design using thermal imaging would provide data on the safety of such innovations.
Disclosure
The authors report no conflicts of interest in this work.
References
Sorenson T, Chan CC, Bradley M, Braga-Mele R, Olson RJ. Ultrasound induced corneal incision contracture study in the United States and Canada. J Cataract Refract Surg. 2012;38(2):227–233. | ||
Alió J, Padron M. (1982) Influence of age on the temperature of the anterior segment of the eye. Measurements by infrared thermometry. Ophthalmic Res. 1982;14(3):153–159. | ||
Alio J, Padron M. Normal variations in the thermographic pattern of the orbito-ocular region. Diagn Imaging. 1982;51(2):93–98. | ||
Innocenti B, Diciotti S, Bocchi L, et al. A comparison between internal and surface temperature measurement techniques during phacoemulsification cataract surgery: thermocamera versus thermocouple. J Appl Biomater Biomech. 2008;6(3):151–156. | ||
Corvi A, Innocenti B, Menucci R. Thermography used for analysis and comparison of different cataract surgery procedures based on phacoemulsification. Physiol Meas. 2006;27(4):371–384. | ||
Jurowski P, Gos R, Kusmierczyk J, Owczarek G, Gralewicz G. Quantitative thermographic analysis of viscoelastic substances in an experimental study in rabbits. J Cataract Refract Surg. 2006;32(1):137–140. | ||
Floyd M, Valentine J, Coombs J, Olson RJ. Effect of incisional friction and ophthalmic viscosurgical devices on the heat generation of ultrasound during cataract surgery. J Cataract Refract Surg. 2006; 32(7):1222–1226. | ||
Schmutz JS, Olson RJ. Thermal comparison of infiniti ozil and signature ellips phacoemulsification systems. Am J Ophthalmol. 2010;149(5): 762–767. | ||
Jun B, Berdahl JP, Kim T. Thermal study of longitudinal and torsional ultrasound phacoemulsification: tracking the temperature of the corneal surface, incision and handpiece. J Cataract Refract Surg. 2010;36(5):832–837. | ||
Han YK, Miller KM. Heat production: longitudinal versus torsional phacoemulsification. J Cataract Refract Surg. 2009;35(10):1799–1805. | ||
Zacharias J. Role of cavitation in the phacoemulsification process. J Cataract Refract Surg. 2008;34(5):846–852. | ||
Osher RH, Injev P. Thermal study of bare tips with various system parameters and incision sizes. J Cataract Refract Surg. 2006;32(5):867–872. | ||
Vasavada AR, Vasavada V, Vasavada VA, et al. Comparison of effect of torsional and microburst longitudinal ultrasound on clear corneal incision during phacoemulsification. J Cataract Refract Surg. 2012;38(5):833–839. | ||
Rose AD, Kanade V. Thermal imaging study comparing phacoemulsification with the sovereign with whitestar system to the legacy with advantec and neosonix system. Am J Ophthalmol. 2006;141(2):322–326. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.