Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Wolf’s Isotopic Response After Herpes Zoster Infection in Chronic Lichen Sclerosus-Like Graft versus Host Disease: Case Report and Literature Review
Authors Xu W, Yu C , Le Y, Zhang J
Received 21 August 2022
Accepted for publication 23 September 2022
Published 7 October 2022 Volume 2022:15 Pages 2153—2157
DOI https://doi.org/10.2147/CCID.S387014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Wen Xu,1,* Cong Yu,1,* Yijun Le,2 Jianzhong Zhang1
1Department of Dermatology, People’s Hospital of Peking University, Beijing, People’s Republic of China; 2Musculoskeletal Tumor Center, People’s Hospital of Peking University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianzhong Zhang, Department of Dermatology, People’s Hospital of Peking University, Beijing, People’s Republic of China, Tel +86 18001315877, Email [email protected]
Abstract: Wolf’s isotopic response (WIR) refers to the occurrence of a new skin disease at the exact site of an unrelated skin disease that had previously healed, often subsequent to virus infection. Secondary cutaneous diseases that are frequently observed in WIR include granulomatous reactions, dysimmune reactions, malignancies, and infections. However, secondary chronic graft-versus-host disease (GVHD) is rare. We describe a patient with lichen sclerosus-like GVHD who developed lichen planus-like GVHD lesions secondary to herpes zoster infection.
Keywords: isotopic, graft-versus-host disease, lichen sclerosus, lichen planus
Case Report
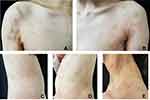
A 36-year-old female with acute lymphoblastic leukemia (B-cell type) underwent an allogeneic hematopoietic stem cell transplantation (allo-HSCT) five years ago from a human leukocyte antigen (HLA) fully matched related donor in Peking University Institute of Hematology, Peking University People’s Hospital. The conditioning regimen with hydroxyurea, cytarabine, busulfan, cyclophosphamide and semustine was used. After HSCT, graft-versus-host disease (GVHD) prophylaxis was performed with cyclosporine and prednisone for 3 months. And then, it was changed to dasatinib for consolidation. The patient did not develop acute GVHD. Eighteen months after HSCT, the patient developed oral leukoplakia and scattered gray-white patches on the bilateral ribs. Dasatinib was discontinued temporarily and was resumed in another center after a diagnosis of “chronic GVHD”. The lesions on the trunk gradually resolved. Fifty-two months after HSCT, the patient felt persistent neuralgia on the right side of the head and face, followed by erythema and clustered blisters in a band-like distribution. She was diagnosed with herpes zoster in other hospitals and received antiviral treatment with valacyclovir. The cutaneous lesions and neuralgia improved in two weeks, although postherpetic pigmentation was observed. Fifty-four months after HSCT, the patient was conscious of the appearance of dark red flat polygonal papules with an overlying network of white lines at the site of postherpetic pigmentation. She then went to our department for further examination and treatment. Physical examination: Poorly defined gray-white sclerotic plaques were observed on the neck and trunk with a slightly cigarette-paper epidermal atrophy. Some sclerotic plaques were surrounded by purple-red patches (Figure 1). A band of dark red, polygonal, flat papules with well-defined boundaries were observed on the right forehead. Hyperpigmentation was visible on the right eyelid (Figure 2). Diagnoses include chronic cutaneous GVHD, lichen sclerosus type on the trunk and lichen planus type on the head and face (Wolf’s isotopic response after herpes zoster infection). The lesions did not spread and were resolved with 5mg of oral prednisone and 0.05% halometasone monohydrate cream.
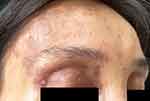 |
Figure 2 A band-like distribution of lichen planus-like lesions was observed on the right forehead, and the right eyelid was heavily pigmented. |
Discussion
In 1995, Wolf et al defined the isotopic response as the occurrence of a new skin disease at the exact site of an unrelated skin disease that had previously healed. The interval between the primary trauma and the onset of WIR is variable, ranging from weeks to years, although most cases occur during the period of active disease.1 Wolf et al have recently expanded the definition of healed skin diseases that trigger an isotopic response, which now includes “scars, pigment changes, color changes or various other minimal changes by the first disease”.2 Infection with varicella-zoster virus (VZV) or herpes simplex virus (HSV) is the most common pre-disposing skin disorder for an isotopic response.3 Of all the isotopic responses following a VZV/HSV infection, the most frequent secondary cutaneous disease is granulomatous reaction, followed by malignancies, leukaemic or lymphomatous infiltrations, dysimmune reactions, infections, comedonic-microcystic reactions, and keloid.3 Clinically, the cutaneous manifestations also exhibited various appearances. Psoriasis, bullous pemphigoid, eosinophilic dermatitis, and dermatitis neglecta have also been reported to manifest as isotopic responses.4 However, cutaneous GVHD is rarely reported as the manifestation of WIR.
The pathogenesis of WIR after herpes zoster remains unclear; potential mechanisms include viral-induced, vascular or immunologic disorder, as well as a neural-centered hypothesis (which may be the most widely accepted hypothesis). VZV infection causes changes in antigenicity of keratinocytes that may act as targets for donor effector cells.5 Other hypotheses suggest that viral infection may increase the expression of human leukocyte antigen (HLA)-II antigens and adhesion molecules on keratinocytes, or that antibodies against viral antigens may cross-react with host HLA molecules.5 VZV may also modify the normal host immune response by reversing the ratio of CD4+/CD8+ T lymphocyte and altering the activity of cytotoxic T-lymphocytes and natural-killer cells.5 The density of epidermal nerve endings is reduced at sites previously infected with VZV compared to normal skin. Nerve injury causes neuropeptide release, which leads to immune dysfunction and alters immune control in the affected dermatome. Neuroimmune destabilization in the VZV-infected site may be mediated by the continuous influence of viral DNA.4
The definitive pathophysiologic mechanisms of chronic GVHD are complex. After injury, keratinocytes may express pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), leading to the release of inflammatory factors from recipient antigen-presenting cells (APC) and the subsequent T-cell activation and proliferation. Chemotherapy, pre-transplantation conditioning, acute GVHD and other processes can damage the recipient’s thymus, resulting in defective recessive selection and the production of autoreactive T cells. The unbalanced proportions of Th1, Th2, and Th17 cells, as well as decreased amounts of the regulatory T cells, regulatory B cells and natural killer cells, contribute to B cell activation and autoantibody production through cytotoxicity, various inflammatory factors and the antigen presentation by APC. Meanwhile, macrophages are activated to generate extracellular matrix, INF-γ, TNF-α, perforin and granzymes, leading to the destruction of recipient tissues, autoantibody secretion after B cell activation and the recruitment of other effector cells. These actions lead to organ damage and tissue fibrosis, resulting in the development of chronic GVHD.6 Accordingly, epithelial cell damage and immune abnormalities after herpes zoster are the most possible causes of secondary chronic GVHD.
As with many other inflammatory dermatoses, immune dysregulation was considered the predominant mechanism for the development of lichen planus (LP). Activated T cells, principally cytotoxic CD8+ T cells, launch an immune attack on basal keratinocytes with the help of CD4+ helper T cells secreting Th1 cytokines, resulting in LP-like lesions.7 LP has also been associated with numerous viral infections, including hepatitis C virus, hepatitis B virus, and VZV.7,8 In addition, genetic and environmental factors may also play roles in pathogenesis of LP.7 The development of lichen sclerosus (LS) lesions may be associated with excess T lymphocyte clones, with increased expression of autoimmune-related inflammatory cytokines (interleukins IL-1, IL-12, IL-2, IL-5, IL-10, tumor necrosis factor-α, interferon-γ).9 Upregulation of microRNA-155 levels reduced the suppression of CD4+ T cells by regulatory T cells and possibly promoted the proliferation of fibroblasts, leading to the epidermal sclerosis.9 The previously unrecognized existence of resident memory CD8+ T cells may also contribute to the pathogenesis of lichenoid reactions in postherpetic WIR lesions.10 Thus, the ratio of CD4+ and CD8+ T lymphocytes may be associated with the type of skin lesions. Chronic GVHD caused by the WIR after herpes zoster infection manifests as lichenoid-like lesions, which may indicate that CD8+ T lymphocytes are mainly up-regulated after VZV infection, but the specific mechanisms require further research.
WIR after herpes zoster infection manifested by cutaneous GVHD is rarely reported. We reviewed a total of 9 cases with this diagnosis, according to the current report (lichenoid type in 6 cases, sclerotic type in 2 cases and unknown in 1 case). Specific clinical features, the presence or absence of acute GVHD, and the interval of the herpes zoster infection and the setting of chronic GVHD are shown in Table 1. In our case, the isotopic response manifested as a typical lichen planus-like lesion, a diagnostic manifestation of chronic GVHD, therefore no histopathological examination was performed. When cutaneous lesions appear as erythematous, papules, and blisters, the differential diagnosis of persistent herpes zoster should be considered. Persistent herpes zoster often occurs under long-term immunosuppression.11 Serum VZV-IgM, IgG detection, histopathological examination and skin tissue herpes zoster virus polymerase chain reaction (PCR) detection can be performed when the identification is difficult.
 |
Table 1 Reported Cases of Chronic Cutaneous GVHD After Herpes Zoster Infection as a Result of Wolf’s Isotopic Response |
Conclusion
We describe a patient with chronic GVHD with an LS-like primary lesion who developed a typical WIR at the site of herpes zoster infection, presenting as LP-like GVHD. After literature review, we found that the ratio of CD4+ and CD8+ T lymphocytes may be related to the type of skin lesions. Chronic GVHD with WIR after herpes zoster infection mostly manifests as lichenoid lesions, which may indicate an upregulated proportion of CD8+ T lymphocytes.
Data Sharing Statement
Data are available on request due to privacy/ethical restrictions. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Consent
The patient provided consent for publication of the manuscript and figures. The case details do not require institutional approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding received for this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ghorpade A. Wolf’s isotopic response – lichen planus at the site of healed herpes zoster in an Indian woman. Int J Dermatol. 2010;49:234–235. doi:10.1111/j.1365-4632.2008.03997.x
2. Happle R, Kluger N. Koebner’s sheep in Wolf’s clothing: does the isotopic response exist as a distinct phenomenon? J Eur Acad Dermatol Venereol. 2018;32:542–543. doi:10.1111/jdv.14664
3. Ruocco V, Ruocco E, Brunetti G, et al. Wolf’s post-herpetic isotopic response: infections, tumors, and immune disorders arising on the site of healed herpetic infection. Clin Dermatol. 2014;32(5):561–568. doi:10.1016/j.clindermatol.2014.04.003
4. Wang T, Zhang M, Zhang Y, et al. Wolf’s isotopic response after herpes zoster infection: a study of 24 new cases and literature review. Acta Derm Venereol. 2019;99:953–959. doi:10.2340/00015555-3269
5. Kawano N, Gondo H, Kamimura T, et al. Chronic graft-versus-host disease following varicella-zoster virus infection in allogeneic stem cell transplant recipients. Int J Hematol. 2003;78:370–373. doi:10.1007/BF02983564
6. Cotliar JA. Atlas of Graft-versus-Host Disease: Approaches to Diagnosis and Treatment. Springer; 2017.
7. Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789–804. doi:10.1016/j.jaad.2018.02.010
8. Mizukawa Y, Horie C, Yamazaki Y, et al. Detection of varicella-zoster virus antigens in lesional skin of zosteriform lichen planus but not in that of linear lichen planus. Dermatology. 2012;225(1):22–26. doi:10.1159/000339771
9. Fergus KB, Lee AW, Baradaran N, et al. Pathophysiology, clinical manifestations, and treatment of lichen sclerosus: a systematic review. Urology. 2020;135:11–19. doi:10.1016/j.urology.2019.09.034
10. Ise M, Tanese K, Adachi T, et al. Postherpetic Wolf’s isotopic response: possible contribution of resident memory T cells to the pathogenesis of lichenoid reaction. Br J Dermatol. 2015;173:1331–1334. doi:10.1111/bjd.13968
11. Gallagher JG, Merigan TC. Prolonged herpes-zoster infection associated with immunosuppressive therapy. Ann Intern Med. 1979;91:842–846. doi:10.7326/0003-4819-91-6-842
12. Palacios-Alvarez I, Santos-Briz A, Jorge-Finnigan C, et al. Chronic cutaneous lichenoid graft-versus-host disease at the area of herpes zoster infection and at a vaccination site. Br J Dermatol. 2015;173(4):1050–1053. doi:10.1111/bjd.13894
13. Mehra T, Metzler G, Bauer J, et al. Isotopic response of graft versus host disease following herpes zoster infection: case report and review of the literature. Acta Derm Venereol. 2012;92(4):383–384. doi:10.2340/00015555-1290
14. Martires KJ, Baird K, Citrin DE, et al. Localization of sclerotic-type chronic graft-vs-host disease to sites of skin injury: potential insight into the mechanism of isomorphic and isotopic responses. Arch Dermatol. 2011;147:1081–1086. doi:10.1001/archdermatol.2011.226
15. Raymond AK, Singletary HL, Nelson KC, et al. Dermatomal sclerodermoid graft-vs-host disease following varicella-zoster virus infection. Arch Dermatol. 2011;147:1121–1122. doi:10.1001/archdermatol.2011.256
16. Kroth J, Tischer J, Samtleben W, et al. Isotopic response, Köbner phenomenon and Renbök phenomenon following herpes zoster. J Dermatol. 2011;38(11):1058–1061. doi:10.1111/j.1346-8138.2011.01329.x
17. Sanli H, Anadolu R, Arat M, et al. Dermatomal lichenoid graft-versus-host disease within herpes zoster scars. Int J Dermatol. 2003;42(7):562–564. doi:10.1046/j.1365-4362.2003.01723_2.x
18. Lacour JP, Sirvent N, Monpoux F, et al. Dermatomal chronic cutaneous graft-versus-host disease at the site of prior herpes zoster. Br J Dermatol. 1999;141:587–589. doi:10.1046/j.1365-2133.1999.03074.x
19. Baselga E, Drolet BA, Segura AD, et al. Dermatomal lichenoid chronic graft-vs-host disease following varicella-zoster infection despite absence of viral genome. J Cutan Pathol. 1996;23:576–581. doi:10.1111/j.1600-0560.1996.tb01453.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.