Back to Journals » Drug Design, Development and Therapy » Volume 14
Wogonin Ameliorates Renal Inflammation and Fibrosis by Inhibiting NF-κB and TGF-β1/Smad3 Signaling Pathways in Diabetic Nephropathy
Authors Zheng Z , Zhu W , Lei L , Liu X , Wu Y
Received 1 August 2020
Accepted for publication 10 September 2020
Published 8 October 2020 Volume 2020:14 Pages 4135—4148
DOI https://doi.org/10.2147/DDDT.S274256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Zhi-chao Zheng, Wei Zhu, Lei Lei, Xue-qi Liu, Yong-gui Wu
Department of Nephrology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China
Correspondence: Yong-gui Wu
Department of Nephrology, The First Affiliated Hospital of Anhui Medical University, No. 218, Jixi Road, Hefei, Anhui 230032, People’s Republic of China
Tel +86 0551 6292 2111
Email [email protected]
Introduction: Diabetic nephropathy (DN) has become an increasing threat to health, and inflammation and fibrosis play important roles in its progression. Wogonin, a flavonoid, has been proven to suppress inflammation and fibrosis in various diseases, including acute kidney injury. This study aimed at investigating the effect of wogonin on diabetes-induced renal inflammation and fibrosis.
Materials and Methods: Streptozotocin (STZ)-induced diabetic mouse models received gavage doses of wogonin (10, 20, and 40 mg/kg) for 12 weeks. Metabolic indices from blood and urine and pathological damage of glomerulus in the diabetic model were assessed. Glomerular mesangial cells SV40 were cultured in high glucose (HG) medium containing wogonin at concentrations of 1.5825, 3.125, and 6.25 μg/mL for 24 h. Inflammation and fibrosis indices were evaluated by histopathological, Western blotting, and PCR analyses.
Results: Wogonin treatment ameliorated albuminuria and histopathological lesions in diabetic mice. Inflammatory cytokines, such as monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and related signaling pathway NF-κB were downregulated after the administration of wogonin in vivo and in vitro. Furthermore, wogonin reduced the expression of extracellular matrix (ECM), including fibronectin (FN), collagen IV (Col-IV), α-smooth muscle actin (α-SMA), and transforming growth factor-β 1 (TGF-β 1) in the kidneys of diabetic mice and HG-induced mesangial cells. Moreover, the inhibition of TGF-β 1/Smad3 pathway might be responsible for these changes.
Conclusion: Wogonin may ameliorate renal inflammation and fibrosis in diabetic nephropathy by inhibiting the NF-κB and TGF-β 1/Smad3 signaling pathways.
Keywords: diabetic nephropathy, wogonin, inflammation, fibrosis
Introduction
Diabetic nephropathy (DN), a major complication of diabetes mellitus (DM), has become an accelerator of chronic renal failure.1 About 25% of diabetic patients develop DN, out of which approximately 50% of patients may eventually end up with end-stage renal disease (ESRD).2 Thus, DN has become the main cause of ESRD, and there is an urgent need to develop effective drugs for DN.
Inflammation plays a critical role in the pathogenesis of DN, and the activation of various inflammatory factors is involved in the renal reaction to inflammation.3 The expression of interleukin-1β (IL-1β) increases in the serum and kidney of DN patients, and closely takes part in the progression of DN.4 Excessive levels of tumor necrosis factor-α (TNF-α) can also be detected in the serum of diabetic patients, enhancing glomerular vasoconstriction and filtration rate.5 The up-regulation of mononuclear chemotactic protein-1 (MCP-1), which participates in renal injury, is also correlated with the mechanism of DN.6,7 The activation of NF-κB by several stimuli promotes transcription and expression of inflammatory factors in DN.8,9 With the development of DN, the continuous effects of inflammation result in the recruitment of fibroblasts, which finally lead to renal fibrosis.10
The pathological characteristics of DN, such as glomerular basement membrane (GBM) thickening, mesangial expansion, glomerulosclerosis, and tubulointerstitial fibrosis, are closely related to disease progression.11,12 Mesangial cells and matrix, the major components of the glomerulus, support its structure, function, and regulation.13 Hyperglycemia can stimulate the mesangial cells to produce cytokines and growth factors, promote the expression of extracellular matrix (ECM), such as collagen IV (Col-IV) and fibronectin (FN), and then gradually changes the glomerular structure until DN ensues.14 Besides, mesangial cells can induce secretion of α-smooth muscle actin (α-SMA) during the progression of DN, which aggravates the fibrosis. Activation of the transforming growth factor-β1 (TGF-β1) and the TGF-β1/Smad signaling pathway in renal fibrosis can lead to the accumulation of ECM.15–17 Studies have shown that TGF-β1 increased in diabetic models, and the inhibition of TGF-β1 could effectively prevent glomerular enlargement and reduce fibrosis.18,19 Another research found that knockout of Smad3 suppressed inflammation and fibrosis in the kidney of db/db mice.20 These previous findings indicate that the TGF-β1/Smad3 pathway is a potential target for the treatment of renal fibrosis in DN.
Wogonin (5,7-dihydroxy-8-methoxyflavone, Figure 1), classed as a flavonoid, is isolated from Scutellaria baicalensis Georgi, a plant that has proved to be therapeutic in DN by the prevention of oxidative stress and inflammatory process to reduce the occurrence of complications.21 Wogonin exhibits a variety of biological functions, including anticancer, anti-inflammatory, antioxidant, anti-allergic, and antiapoptotic properties.22,23 Previous studies have substantiated that wogonin can improve insulin sensitivity and lipid metabolism in db/db mice, suggesting a protective effect in diabetes.24 Additionally, wogonin can alleviate the fibrosis of renal tubular epithelial cells, and the TGF-β1/Smad pathway may be a potential target.25 Wogonin inhibits inflammatory factors in acute kidney injury (AKI) models, which indicates that it may play an important role in renal inflammation.26 The studies of wogonin provide theoretical basis, then diabetic models and HG-induced glomerular mesangial cells were used to explore the effects of Wogonin on DN. In our results, we demonstrated that wogonin can ameliorate renal inflammation and fibrosis in DN by inhibiting the NF-κB and TGF-β1/Smad3 signaling pathways.
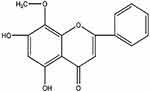 |
Figure 1 Molecular structure of wogonin. |
Methods and Materials
Antibodies and Reagents
Antibodies were purchased from commercial sources, including rabbit anti-collagen IV from Proteintech (Wuhan, China), mouse anti-glyceraldehyde-3phosphate dehydrogenase (GAPDH) from Novus Biological (Littleton, CO, USA), rabbit anti-fibronectin and rabbit anti-TGF-β1 from Abcam (Cambridge, MA, USA), and rabbit anti-NF-κB-p65, rabbit anti-NF-κB-p-p65, rabbit anti-p-Smad3, and rabbit anti-Smad3 from Cell Signaling Technology (Beverly, MA, USA). Fetal bovine serum (FBS) was procured from Wisent Bioproducts (Saint-Bruno, QC, Canada), D-glucose, D-mannitol and Streptozotocin (STZ) from Sigma-Aldrich (Saint Louis, MO, USA), and Trizol from Life Technologies (Carlsbad, CA, USA), dulbecco’s modified eagle medium (DMEM) from Thermo Fisher Scientific (Waltham, MA, USA). Albumin ELISA kit was purchased from Abcam (Cambridge, USA), and creatinine assay kit was from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Wogonin was obtained from Topscience (Shanghai, China); Horseradish-peroxidase (HRP)-conjugated goat anti-rabbit/mouse IgG and 3.3-diaminobenzidine (DAB) was from Beijing Zhongshan Biotechnology (Beijing, China).
Animals and Experimental Design
Weight-matched C57/BL male mice, 6–8 weeks of age, purchased from the Model Animal Research Center of Nanjing University (Nanjing, China) were employed in our study. Mice were defined as diabetic models after daily administration of 50 mg/kg streptozotocin (STZ) via intraperitoneal injection for 5 days until the blood glucose levels of mice were higher than 16.7 mmol/L. All mice received normal diet and were randomly arranged into six groups: normal control (NC), normal control+wogonin (Wogonin), diabetic group (DM), diabetic group+wogonin (DM+Wogonin 10 mg/kg, DM+Wogonin 20 mg/kg, DM+Wogonin 40 mg/kg). Mice in DM+Wogonin groups were gavaged with wogonin every other day for 12 weeks, and the wogonin group received 40 mg/kg wogonin. NC and DM groups were administered with an appropriate amount of saline. The doses of wogonin in vivo were screened in pre-experiment. Animal experiments were performed in compliance with “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health Publication No. 85–23, revised 1996). The study has been approved by the Animal Ethical Committee of Anhui Medical University.
Sample Collection and Biochemical Analysis
A 24-hour urine sample was collected using metabolism cages after 12 weeks of gavage, in which mice had free access to water and food. The level of 24-hour urinary albumin excretion was measured using a mouse albumin ELISA kit. The blood glucose levels were measured by extracting blood from the tip of the tail. All mice were given laparotomy under anesthesia to separate kidneys for histological and protein analyses. Hearts were exposed by cutting the rib cage bilaterally, followed by cardiac punctures to collect 200–300 µL of blood, and serum was obtained for estimating serum creatinine (SCr) levels using the creatinine assay kit. Meanwhile, the kidney/body weight was calculated.
Histological Analysis
The kidneys of mice were sectioned and fixed in 4% paraformaldehyde, embedded in paraffin after dehydration, and then the tissue was sliced for pathological staining by periodic acid-Schiff (PAS) and MASSON staining methods. The structure of kidneys was observed by a microscope (Leica, Bensheim, Germany). Glomerular mesangial expansion index was assessed using a score of 0–4, based on the ratio of mesangial matrix expansion area to glomerular area: 0, no expansion; 1, expansion area <25%; 2, 25%-50%; 3, 50%-75%; 4, >75%. Tubular injury score was graded from 0–3 based on the tubular vacuolization and dilation region: 0, no tubular damage; 1, <25%; 2, 25%-50%; 3, >50%. The fibrotic areas showed by MASSON staining in each photograph were measured using the ImageJ program.
Transmission Electron Microscope
The renal cortex was cut into 1 mm3 cubes for processing specimens that can be observed under a transmission electron microscope. Tissues were fixed in 2.5% glutaraldehyde, followed by soaking in 1% osmic acid. After the process of dehydration and embedding, the sections were stained with uranyl acetate and lead citrate in copper grids. Transmission electron microscope (Hitachi Limited, Tokyo, Japan) was used to explore the alterations in the kidney among different groups.
Immunohistochemical Analysis
The paraffin-embedded kidney was sectioned at 4 μm and soaked in xylene and graded ethanol. Heat-induced epitope retrieval was used to expose the antigen. The 4-μm thick tissue was blocked with goat serum for 30 min, followed by incubation with antibodies against FN (1:150), Col-IV (1:200), TGF-β1 (1:200), and α-SMA (1:150) at 4 °C overnight. After rinsing thrice in phosphate-buffered saline (PBS) for 5 min each, the sections were incubated with HRP-conjugated rabbit IgG for 45 min. Next, the slides rinsed with PBS were stained after incubation with DAB. Nuclear staining was performed with hematoxylin. The relative area of positive signals in each photograph was measured using the ImageJ program.
Cell Culture and Experimental Design
The glomerular mesangial cells SV40 purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China) were cultured until exponential growth was observed in low glucose (5.5 mM glucose) DMEM with 5% FBS and cultivation conditions maintained at 37 °C, 5% CO2. Next, the cells were incubated in mannitol (25 mM), low glucose (LG), LG with wogonin, high glucose (HG) (25 mM glucose), and HG with Wogonin mediums for 24 h; mannitol and LG mediums were used as controls. In brief, cells were grouped as ⑴ mannitol (M); ⑵ low glucose (LG); ⑶ low glucose+wogonin (LG+6.25 μg/mL); ⑷ high glucose (HG); ⑸ high glucose+wogonin (HG+1.5825, HG+3.125, HG+6.25 μg/mL). In vitro, we used MTT experiment to screen optimal concentrations in SV40.
Western Blotting
Cell lysates and the fragments of kidney tissue were lysed in a compound containing radio-Immunoprecipitation Assay (RIPA) and phenylmethanesulfonyl fluoride (PMSF) (Beyotime, Jiangsu, China). BCA reagent (Beyotime, Jiangsu, China) was used to quantify the protein concentration in supernatants after centrifugation for 20 min. The 10% PAGE Gel Fast Preparation Kit (Epizyme, Shanghai, China) enabled the separation of proteins which was followed by a transfer of proteins from gels to the nitrocellulose filter membranes. Next, the membranes were incubated with primary antibodies overnight at 4 °C, including FN (1:1000), Col-IV (1:500), TGF-β1 (1:1000), α-SMA (1:1000), p65 (1:1000), p-p65 (1:1000), Smad3 (1:1000), and p-Smad3 (1:1000). After incubation with horseradish peroxidase-conjugated antibodies for 45 min, the membranes were exposed in a chemiluminescence system. ImageJ program was used to measure the density of the bands.
RNA Extraction and qRT-PCR
The mRNA extracted from cells and tissues was reverse-transcribed to cDNA by using HiScript®II Q RT SuperMix (Vazyme, Nanjing, China), the mixture was reacted at 50°C for 15 min, 80°C for 5 sec. Then generated cDNA, primers, Ace Q qPCR SYBR®Green Master Mix (Vazyme, Nanjing, China) mainly composed an amplified reaction system in PCR-Cycler (Bio-Rad, California, USA) for real-time PCR detection. It was performed at 95°C for 5 min, then 40 cycles were carried out in the condition that 95°C for 10 sec and 60°C for 30 sec. The primer sequences: IL-1β: F 5ʹ-GCCTCGTGCTGTCGGACCCATAT-3ʹ, R 5ʹ-TCCTTTGAGGCCCAAGGCCACA-3ʹ; TNF-α: F 5ʹ-CCCTCCTGGCCAACGGCATG-3ʹ, R 5ʹ-TCGGGGCAGCCTTGTCCCTT-3ʹ; MCP-1: F 5ʹ-TTGACCCGTAAATCTGAAGCTAAT-3ʹ, R 5ʹ-TCACAGTCCGAGTCACACTAGTTCAC-3ʹ; GAPDH: F 5ʹ-ACCCCAGCAAGGACACTGAGCAAG-3ʹ, R 5ʹ-GGCCCCTCCTGTTATTATGGGGGT-3ʹ.
GAPDH was used as normalization and the mRNA levels were analysed with the aid of 2−∆∆Ct method.
Statistical Analyses
The diagrams and graphs were analyzed using GraphPad Prism 7.0 software and ImageJ. All data were in normal distribution, which allowed for statistics management using one-way ANOVA. Values were displayed as Mean ± SEM. p <0.05 was considered to be statistically significant.
Results
Effects of Wogonin on the Levels of Primary Metabolic Parameters in Diabetic Mice
The general indicators in mice were measured to test the effect of wogonin. In our data, the levels of blood glucose, urinary albumin, kidney/body weight, and SCr increased in the DM group compared with the NC group. Furthermore, the 24 h urine albumin levels also increased to more than ten times in diabetic models, which suggested dysfunction in the kidneys. After treatment with wogonin, the blood glucose showed no difference in values among DM+Wogonin and DM groups. However, the other three indexes showed notable results as their levels decreased significantly in DM+Wogonin groups, indicating that the kidney damage in diabetic mice was mitigated by wogonin. There was no difference between NC and Wogonin groups among all indexes (Figure 2).
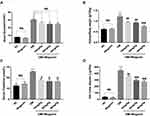 |
Figure 2 Primary metabolic parameters in mice. |
Effects of Wogonin on Renal Histopathological Changes in Diabetic Mice
Light and electron microscopy were used to evaluate histopathological changes intuitively. In the PAS staining, we observed an increased deposition of glycogen in the mesangium of diabetic mice, and the renal injury covered glomerular hypertrophy, GBM thickening, mesangial expansion, tubular dilation, which were in agreement with the typical renal alteration of DN. In contrast, the histopathological lesion in the kidney of diabetic mice lightened with the wogonin application (Figure 3A and D–E). Furthermore, overproduction of collagen and fibrous protein along with pathological damage was observed in kidney tissues of diabetic mice with MASSON staining, while DM+Wogonin groups displayed ameliorating effects compared to the DM group. (Figure 3B and F).
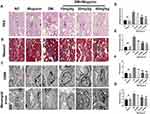 |
Figure 3 Pathology results in the kidney of mice. |
The renal injury was observed at the micro-scale by a transmission electron microscope. Results of the group containing diabetic mice without treatment showed an increasing mesangial matrix diffused in the glomerular mesangial region, thicker irregular GBM, and more fusion of the foot processes when compared to the NC group. However, treatment with wogonin lessened pathological impairments in the kidney of diabetic mice (Figure 3C and G).
Expression of Inflammatory Cytokines and NF-κB Signaling Pathway in the Kidney of Diabetic Mice
We detected the cytokines that were implicated in renal inflammation to assess the inflammatory process correlated with kidney disorder. After treatment with wogonin, diabetic mice presented a reduction in inflammatory response compared to that of the NC group, including the mRNA levels of inflammatory cytokines TNF-α, IL-1β, and MCP-1 (Figure 4A–C).
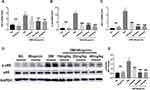 |
Figure 4 Expression of renal inflammatory cytokines and NF-κB signaling pathway in the kidney of mice. |
The mechanisms of wogonin in down-regulating inflammation were investigated by detecting the protein levels in NF-κB signaling pathway. The Western blot results showed that p-p65 was over-activated in the DM group; on the contrary, we observed that wogonin treatment induced significant decreases in different sets of doses (10, 20, and 40 mg/kg). Therefore, it could be assumed that wogonin reacted against inflammation (Figure 4D–E).
Effects of Wogonin on Renal Fibrosis in Diabetic Mice
Renal fibrosis was measured to further determine the influence of wogonin on the antifibrotic activity. In the immunohistochemical sections (Figure 5A–D), positive staining areas of FN, Col-IV, and α-SMA were confined to a small scale in response to wogonin in the treatment groups, while the expression is relatively high in the DM group. Apart from this observation, a similar trend was displayed in the Western blot (Figure 5E–H); the fibrotic protein levels of FN, Col-IV, and α-SMA increased to some extent in diabetic mice, while in the DM+Wogonin groups, the expression was significantly reduced.
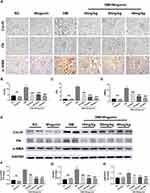 |
Figure 5 Immunohistochemistry and Western blot results of ECM in the kidney of mice. |
Expression of TGF-β1/Smad3 Signaling Pathway in the Kidney of Diabetic Mice
Next, the evaluation of the TGF-β1/Smad3 signaling pathway was performed using Western blotting and immunohistochemistry to explore the presumed mechanism of the antifibrotic activity of wogonin. Increasing levels of TGF-β1/Smad3 signaling pathway was seen during renal fibrosis in diabetic mice, while wogonin effectively decreased the levels of TGF-β1 and p-Smad3 in treatment groups, and wogonin in 40 mg/kg dose did not affect the pathway expression in the kidney of C57 mice. In addition, the inhibition of TGF-β1 was also observed in the immunohistochemical sections in DM+Wogonin groups (Figure 6).
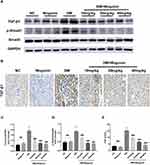 |
Figure 6 Expression of TGF-β1/Smad3 signaling pathway in the kidney of mice. |
Effective Concentrations of Wogonin by MTT Analysis
Results from our study showed that there was no inhibition of the cell viability when SV40 was stimulated by wogonin at concentrations less than 12.5 μg/mL in LG condition (Figure 7A). A range lower than 12.5 μg/mL was then chosen to screen the suitable concentrations in HG condition. Furthermore, HG promoted proliferation of SV40 compared to LG condition; however, it was notably suppressed by wogonin at concentrations of 1.5825, 3.125, and 6.25 μg/mL (Figure 7B). The results provided reference access to the final concentration of wogonin in vitro experiment.
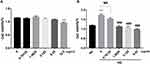 |
Figure 7 Cell viability of SV40 treated with different concentrations of wogonin. |
Expression of Inflammatory Cytokines and NF-κB Signaling in HG-Treated SV40
High concentrations of glucose, known as an inducing factor for inflammation, enhanced the inflammatory reaction in SV40. It was manifested as elevated mRNA expression of TNF-α, MCP-1, and IL-1β in the HG group (Figure 8A–C). Additionally, the phosphorylation of NF-κBp65 in the HG group was also higher than that of the LG groups, while the administration of wogonin markedly inhibited the mRNA expression of those inflammatory factors and protein levels of p-p65 in HG condition (Figure 8D–E).
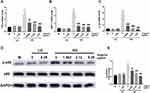 |
Figure 8 Analysis of inflammatory cytokines and NF-κB signaling pathway in SV40. |
Expression of the Fibrotic Protein in HG-Induced SV40
The ECM expression of SV40 cultured in HG medium delivered a similar increasing trend in the results as the in vivo experiments. After treatment with wogonin, all the three concentrations showed marked decreases in the protein levels of Col-IV, FN, and α-SMA in HG condition. There was no statistical difference in these protein levels among the control groups (M, LG, LG+6.25) (Figure 9A–D).
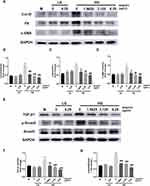 |
Figure 9 The expression of ECM and TGF-β1/Smad3 signaling pathway in SV40. |
Expression of TGF-β1/Smad3 Signaling in HG-Induced SV40
After 24 h stimulation with HG, increased expression of TGF-β1 and p-Smad3 was seen in the HG group compared to the LG groups, which resulted in a significant expansion in ECM. However, cells incubated with wogonin in the HG group showed a declining tendency of TGF-β1 expression and lowered phosphorylation of Smad3. The expression levels between M, LG, and LG+6.25 groups failed to show any statistical difference in their values (Figure 9E–G).
Discussion
Traditional Chinese medicines have been widely used in kidney diseases. Our previous study showed several principal components of traditional Chinese medicines, such as protocatechuic aldehyde and Rutaecarpine officinalis sub alkaloid, exhibiting protective effects in attenuating AKI.27 Therefore, focusing on finding active ingredients from herbal medicine for DN, such as wogonin, appear to have merit, especially since wogonin has been proved to be effective among various diseases, including AKI, tubulointerstitial fibrosis, diabetes, and diabetic cardiomyopathy. Moreover, this is the first study exploring the therapeutic potential of wogonin in DN.
Results showed that the levels of urine albumin in the DM group rose tenfold compared with the C57 mice; meanwhile, the renal structural and pathological hallmarks of DN could be observed, including hyperplastic mesangial cells, overproduction of ECM, and basement membrane thickness. These alterations were considered as proof that the renal damage model of diabetes was established. Albuminuria serves as the key marker for diagnosing DN and is associated with inflammatory response, expansion of mesangial matrix, GBM thickening, and glomerulosclerosis.28,29 Notably, wogonin reduced urinary albumin, alleviated the pathological renal lesion in mice, and ameliorated serum creatinine, suggesting the protective role of wogonin in the kidney of diabetic mice. It is noteworthy that wogonin exerted no hypoglycemic action on DN mice in our study, which indicated that wogonin ameliorated DN potentially without affecting the blood glucose level.
Inflammatory cytokines were detected in our study to evaluate the biological effects of wogonin. We found that wogonin could reduce the expression of IL-1β, TNF-α, and MCP-1 in the kidney of diabetic mice; the same effect was seen in mesangial cells stimulated by HG. Previously reported research had demonstrated that wogonin could down-regulate lipopolysaccharide (LPS)-induced TNF-α and IL-1β expression, diminish TNF-α and IL-1β release to lower cytotoxicity in nerve cells, and inhibit MCP-1 expression in human endothelial cells.30 Our results are further supported by an analogous study, wherein wogonin inhibited the expression of IL-1β, IL-6, and TNF-α in diabetic cardiomyopathy.23 Nevertheless, the specific mechanism of action of wogonin against these inflammatory factors remains unclear. In terms of DN, the inflammatory factors are linked to eventual detrimental effects in pathogenesis. In particular, IL-1β facilitates the proliferation of mesangial cells and matrix accumulation,31,32 which may account for the intrinsic interaction that wogonin affects inflammation and fibrosis. The synthesis and release of TNF-α influence the pathology of DN at an early stage.33 In addition, wogonin may control the progress of DN by regulating MCP-1, as an increase in MCP-1 levels can expedite the progressive tubulointerstitial lesions and renal inflammation in DN.6,7 These results further confirm the anti-inflammatory effect of wogonin in diabetes and show the potential benefit of modulating inflammatory molecules in the treatment of DN.
To support the underlying mechanisms of wogonin that could suppress inflammatory factors, the NF-κB signaling pathway was evaluated. There appears to be a consensus that NF-κB is an important transcription factor in the progression of DN. It exists in an inactive state in the cells until the cells are stimulated by specific conditions, and then the downstream pathway is activated.34 Enhanced activation of NF-κB has been found in both DN patients and diabetic models, which is consistent with our results in the experiments. Furthermore, its interaction with the inflammatory response may aggravate renal injury in diabetic patients.35–37 NF-κB inhibitors can inhibit the inflammation and oxidative stress to alleviate renal injury.38 The activation of NF-κB is related with the release of inflammatory cytokines in DN, including IL-1β, TNF-α, and MCP-1.8 Wogonin, which display similar effects of down-regulating inflammatory cytokines, possibly combines with NF-κB to suppress the inflammation. The results are consistent with another study wherein wogonin inhibited HG-induced vascular inflammation by decreasing the expression of p-p65 in primary human endothelial cells.22 The mechanisms of action of wogonin against inflammation are not limited to the NF-κB suppression. Some studies have mentioned that the anti-inflammatory property of wogonin may be attributed to the inhibition of catalase, LPS, diacylglycerol (DAG), and protein kinase C (PKC) pathway.30 This suggests some future research directions for us.
Another vital finding of our results suggested that wogonin exerted antifibrotic effects on DN. It is widely accepted that high glucose expedites the clustering of ECM in glomerular mesangial cells.39 The over-accumulation of ECM subsequently leads to glomerulosclerosis and tubulointerstitial fibrosis. During the evolution of fibrosis, the myofibroblasts are activated with α-SMA, which is present in the interstitial space under pathological conditions.16 Our study showed an increasing accumulation of ECM in the kidney of diabetic mice and HG-induced mesangial cells, which implied the occurrence of renal fibrosis, while the administration of wogonin retarded the tendency of ongoing fibrosis. This is in consensus with the results provided by Meng et al25 that wogonin may serve as a potential antifibrotic agent in treating renal fibrosis.
Several factors have been taken into account regarding the mechanisms used by wogonin to attenuate fibrosis. In previous research, the TGF-β1/Smad pathway was regarded as a possible and effective target in the course that wogonin reacted to renal tubule fibrosis.25 More importantly, our results suggesting that wogonin can not only decrease the expression of TGF-β1 but also inhibit the phosphorylation of Smad3 in DN has approved of the possibility. Targeting the TGF-β1/Smad pathway has become a feasible approach to control the progression of DN. TGF-β1 is considered a major pathogenic factor in DN, leading to the progression of renal fibrosis.40 The activated TGF-β1 receptor interacts with Smad2 and Smad3 to form a heterodimer complex with co-Smad4, which stimulates the nucleus to initiate transcriptional signals and increases the expression of collagen and fibronectin.17 Smad3 is highly relevant to renal fibrosis. Studies have shown that conditional knockout of Smad3 can inhibit fibrosis on the kidney of STZ-induced diabetic models.41 These reports provide a basis that a reduction in TGF-β1/Smad3 activation may be responsible for the restraint of renal fibrosis in the therapeutic action of wogonin.
Given the results that both inflammation and fibrosis were inhibited by wogonin, the internal connection between the two ameliorative parts is worth investigating. Accumulating evidence has stated the relation between inflammation and fibrosis. Fibrosis may be the end result of ongoing inflammation. The inflammatory process aggravated by elevated TGF-β1 contributes to the gathering and activating fibroblasts.42 Furthermore, the invasion of pro-inflammatory cytokines in the kidney is implicated in the accretion of ECM, which finally results in the progression of renal fibrosis.43 Another review elucidates that the activation of NF-κB can aggravate renal fibrosis by promoting the expression of Col-IV and FN.44 Therefore, the mechanism by which wogonin affects renal inflammation and fibrosis by regulating these molecules is an open question.
In summary, wogonin has shown anti-inflammatory and antifibrotic effects against DN by inhibiting the activation of NF-κB and TGF-β1/Smad3 signaling pathway. Although the exact mechanisms of inflammation and fibrosis are still complex and there may be more than one effective point of wogonin, these results are promising and suggest the therapeutic properties of wogonin in DN.
Acknowledgments
We acknowledge the Scientific Research Foundation of the Institute for Translational Medicine of Anhui Province (number: 2017zhyx01) for supporting the whole research. We thank Chunxu Li for providing technical assistance in pathology and Guanjun Chen for experiment management. We would like to thank Editage for providing advanced English language editing service.
Author Contributions
Yong-gui Wu designed the experiments; Zhi-chao Zheng and Wei Zhu performed the experiments; Lei Lei and Xue-qi Liu analyzed the data and prepared figures; Zhi-chao Zheng drafted the manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546. doi:10.4103/2230-8210.183480
2. Gheith O, Farouk N, Nampoory N, Halim MA, Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5:49.
3. Moreno JA, Gomez-Guerrero C, Mas S, et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs. 2018;27:917–930.doi:10.1080/13543784.2018.1538352
4. Garrido W, Jara C, Torres A, et al. Blockade of the adenosine A3 receptor attenuates caspase 1 activation in renal tubule epithelial cells and decreases interleukins IL-1β and IL-18 in diabetic rats. Int J Mol Sci. 2019;20(18):4531. doi:10.3390/ijms20184531
5. Niewczas MA, Ficociello LH, Johnson AC, et al. Serum concentrations of markers of TNF alpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009;4(1):62–70. doi:10.2215/CJN.03010608
6. Wada T, Furuichi K, Sakai N, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58(4):1492–1499. doi:10.1046/j.1523-1755.2000.00311.x
7. Tanifuji C, Suzuki Y, Geot WM, et al. Reactive oxygen species-mediated signaling pathways in angiotensin II-induced MCP-1 expression of proximal tubular cells. Antioxid Redox Signal. 2005;7(9–10):1261–1268.doi:10.1089/ars.2005.7.1261
8. Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44(11):1957–1972.doi:10.1007/s001250100000
9. Luo C, Yang H, Tang C, et al. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol. 2015;28(1):744–750. doi:10.1016/j.intimp.2015.07.018
10. Liu Y. Renal fibrosis, new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. doi:10.1038/sj.ki.5000054
11. Wolf G. New insights into the pathophysiology of diabetic nephropathy: from hemodynamics to molecular pathology. Eur J Clin Investig. 2004;34:785–796.doi:10.1111/j.1365-2362.2004.01429.x
12. Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14(5):1358–1373. doi:10.1097/01.ASN.0000065640.77499.D7
13. Schlöndorff D, Banas B. The mesangial cell revisited: no cell is an island. J Am Soc Nephrol. 2009;20(6):1179–1187. doi:10.1681/ASN.2008050549
14. Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16(Suppl 1):S30. doi:10.1681/ASN.2004110970
15. Abbound HE. Mesangial cell biology. Exp Cell Res. 2012;318:979–985. doi:10.1016/j.yexcr.2012.02.025
16. Loeffler I, Wolf G. Epithelial-to-mesenchymal transition in diabetic nephropathy: fact or fiction? Cells. 2015;4(4):631–652. doi:10.3390/cells4040631
17. Tang PM, Zhang YY, Mak TS, Tang PC, Huang XR, Lan HY. Transforming growth factor-β signaling in renal fibrosis: from Smads to non-coding RNAs. J Physiol. 2018;596(16):3493–3503.doi:10.1113/JP274492
18. Benigni A, Zoja C, Campana M, et al. Beneficial effect of TGF-β antagonism in treating diabetic nephropathy depends on when treatment is started. Nephron Exp Nephrol. 2006;104:e158.doi:10.1159/000094967
19. Gagliardini E, Benigni A. Role of anti-TGF-β antibodies in the treatment of renal injury. Cytokine Growth Factor Rev. 2006;17(1–2):89–96. doi:10.1016/j.cytogfr.2005.09.005
20. Xu BH, Sheng J, You YK, et al. Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism. 2020;103:154013. doi:10.1016/j.metabol.2019.154013
21. Guo MF, Dai YJ, Gao JR, Chen PJ. Uncovering the mechanism of astragalus membranaceus in the treatment of diabetic nephropathy based on network pharmacology. J Diabetes Res. 2020;2020:5947304. doi:10.1155/2020/5947304
22. Ku SK, Bae JS. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015;48(9):519–524. doi:10.5483/BMBRep.2015.48.9.017
23. Khan S, Zhang D, Zhang Y, Li M, Wang C. Wogonin attenuates diabetic cardiomyopathy through its anti-inflammatory and anti-oxidative properties. Mol Cell Endocrinol. 2016;428(C):101–108. doi:10.1016/j.mce.2016.03.025
24. Bak EJ, Kim J, Choi YH, et al. Wogonin ameliorates hyperglycemia and dyslipidemia via PPARα activation in db/db mice. Clin Nutr. 2014;33(1):156–163. doi:10.1016/j.clnu.2013.03.013
25. Meng XM, Ren GL, Gao L, et al. Anti-fibrotic effect of wogonin in renal tubular epithelial cells via Smad3-dependent mechanisms. Eur J Pharmacol. 2016;789:134–143. doi:10.1016/j.ejphar.2016.07.014
26. Meng XM, Li HD, Wu WF, et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab Invest. 2018;98:79–94. doi:10.1038/labinvest.2017.115
27. Liu XQ, Jin J, Li Z, et al. Rutaecarpine derivative Cpd-6c alleviates acute kidney injury by targeting PDE4B, a key enzyme mediating inflammation in cisplatin nephropathy. Biochem Pharmacol. 2020;180:114132. doi:10.1016/j.bcp.2020.114132
28. Papadopoulou-Marketou N, Kanaka-Gantenbein C, Marketos N, Chrousos GP, Papassotiriou I. Biomarkers of diabetic nephropathy: A 2017 update. Crit Rev Clin Lab Sci. 2017;54(5):326–342. doi:10.1080/10408363.2017.1377682
29. Campbell RC, Ruggenenti P, Remuzzi G. Proteinuria in diabetic nephropathy: treatment and evolution. Curr Diab Rep. 2003;3(6):497–504.doi:10.1007/s11892-003-0014-0
30. Khan S, Kamal MA. Can wogonin be used in controlling diabetic cardiomyopathy? Curr Pharm Des. 2019;25(19):2171–2177. doi:10.2174/1381612825666190708173108
31. Rivero A, Mora C, Muros M, García J, Herrera H, Navarro-González JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond). 2009;116:479–492.doi:10.1042/CS20080394
32. Sedor JR, Konieczkowski M, Huang S, et al. Cytokines, mesangial cell activation and glomerular injury. Kidney Int Suppl. 1993;39:S65.
33. Navarro JF, Mora-Fernández C. The role of TNF-α in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17(6):441–450. doi:10.1016/j.cytogfr.2006.09.011
34. Navarro-González JF, Mora-Fernández C, de Fuentes MM, García-Pérez J. Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7(6):327–340. doi:10.1038/nrneph.2011.51
35. Mezzano S, Aros C, Droguett A, et al. NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19(10):2505–2512.doi:10.1093/ndt/gfh207
36. Starkey JM, Haidacher SJ, LeJeune WS, et al. Diabetes-induced activation of canonical and noncanonical nuclear factor-κB pathways in renal cortex. Diabetes. 2006;55(5):1252–1259. doi:10.2337/db05-1554
37. Schmid H, Boucherot A, Yasuda Y, et al. European Renal cDNA Bank (ERCB) Consortium. Modular activation of nuclear factor-κB transcriptional programs in human diabetic nephropathy. Diabetes. 2006;55(11):2993–3003. doi:10.2337/db06-0477
38. Kolati SR, Kasala ER, Bodduluru LN, et al. BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-κB pathway. Environ Toxicol Pharmacol. 2015;39(2):690–699. doi:10.1016/j.etap.2015.01.019
39. Li XQ, Tian W, Liu XX, et al. Corosolic acid inhibits the proliferation of glomerular mesangial cells and protects against diabetic renal damage. Sci Rep. 2016;6:26854.doi:10.1038/srep26854
40. Voelker J, Berg PH, Sheetz M, et al. Anti-TGF-β1 antibody therapy in patients with diabetic nephropathy. J Am Soc Nephrol. 2017;28:953–962.doi:10.1681/ASN.2015111230
41. Li J, Qu X, Yao J, et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:2612–2624.
42. Kim TW, Kim YJ, Seo CS, et al. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-ß and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine. 2016;23(4):331–339. doi:10.1016/j.phymed.2016.01.013
43. Wang Y, Nie M, Lu Y, et al. Fucoidan exerts protective effects against diabetic nephropathy related to spontaneous diabetes through the NF-κB signaling pathway in vivo and in vitro. Int J Mol Med. 2015;35:1067–1073.doi:10.3892/ijmm.2015.2095
44. Zheng HX, Qi SS, He J, et al. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J Agric Food Chem. 2020;68(15):4399–4410. doi:10.1021/acs.jafc.0c00680
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
