Back to Journals » Journal of Pain Research » Volume 13
Wnt3a Inhibitor Attenuates Remifentanil-Induced Hyperalgesia via Downregulating Spinal NMDA Receptor in Rats
Authors Gao Y , Zhou S, Pan Y, Gu L, He Y, Sun J
Received 21 February 2020
Accepted for publication 30 April 2020
Published 19 May 2020 Volume 2020:13 Pages 1049—1058
DOI https://doi.org/10.2147/JPR.S250663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Yuan Gao,* Songyi Zhou,* Yizhao Pan, Lijun Gu, Yuting He, Jiehao Sun
Department of Anesthesiology, 1st Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiehao Sun Shangcai Cun 1#, Ouhai District, Wenzhou 325000, People’s Republic of China
Tel +86-13676721930
Fax +86-577-88513632
Email [email protected]
Purpose: The upregulation of spinal NMDA receptor is a crucial mechanism in remifentanil-induced hyperalgesia (RIH). Wnt3a/β-catenin pathway plays an important role in neuropathic pain. We hypothesized that wnt3a inhibitor (iwp-2) could downregulate the expression of NR2B subunit in NMDA receptor, in order to relieve RIH.
Materials and Methods: The study has 2 phases. The phase 1 study is designed by different doses of iwp-2 groups to create an appropriate iwp-2 dose used in RIH alleviation. The phase 2 study is designed to prove that the wnt3a inhibitor could downregulate the activation of the NR2B to inhibit RIH in rats. Thermal hyperalgesia (PWTL) and mechanical allodynia (PWMT) were evaluated after RIH. The area under the PWTL and PWMT curves (AUC) were calculated. The amount of activated NR2B subunit, c-fos, NF-κB, β-catenin, wnt3a and p-GSK-3β (Ser9) were detected in the lumbar spinal cord.
Results: Remifentanil infusion could induce overexpression of β-catenin and wnt3a in rats. Iwp-2 (60μM, 120μM, 180μM) could dose-dependently inhibit thermal hyperalgesia and mechanical allodynia in rats. In phase 2 study, both NR2B subunit antagonist Ro25-6981 and iwp-2 decreased the amount of activated NR2B, enhanced p-GSK-3β (Ser9), reduced β-catenin, c-fos and NF-κB in the lumbar spinal cord (p < 0.001). In comparison with the group iwp-2, the group of Ro25-6981 had more benefit in reversing hyperalgesia, including higher AUC value of PWTL (p = 0.022) and PWMT (p = 0.035).
Conclusion: Remifentanil exposure could induce overexpression of wnt3a and enhance the production of β-catenin in the spinal dorsal horn. Inhibition of wnt3a response was capable of attenuating RIH in alleviating hyperalgesia-related behavioral parameters, as well as reducing overexpression of c-fos, NF-κB, NR2B in spinal dorsal horn.
Keywords: remifentanil, hyperalgesia, iwp-2, NR2B, wnt3a/β-catenin
Introduction
Remifentanil, a kind of potential analgesic, is commonly used in the induction and maintenance in general anesthesia. Due to its ultra-short acting character, remifentanil could be used in high doses during the operations. However, nowadays remifentanil was found to have paradoxical nociceptive effect, characterized as hypersensitivity to allodynia and thermal stimulation, the effect of which could counteract its own antinociception.1 Consumption of postoperative analgesics would increase after the exposure of remifentanil.2–5 In animal research, intravenous remifentanil anesthesia could lead to remifentanil-induced hyperalgesia (RIH) and could dramatically exacerbate pre-existing pain after surgery. In most of the animal researches, RIH was noticed to begin at 2 h and peak at 48 h after remifentanil exposure.1,4
Recent reports have demonstrated that NMDA receptor-mediated synaptic plasticity was essential to chronic pain.6,7 RIH has confirmed to be attributed to the phosphorylation of NR2B subunit in NMDA receptor. NMDA antagonists could dose-dependently relieve RIH8 and reverse the phosphorylation of NR2B subunit.9 While the downstream molecular mechanism is still poorly understood.
The wnt3a/β-catenin pathway is a set of evolutionarily conserved signals which takes part in the development like dendrite morphogenesis and synapse formation. Recently, the effect of this pathway involved in chronic neuropathic pain10 has been elucidated clearly. Wnt3a/β-catenin pathway was found to be a key target to mediate sensory neuron excitability, especially in spinal dorsal neuron.10–14 The activation of NMDA receptor caused the upregulation of β-catenin proteins.15 Wnt3a/β-catenin antagonist could abolish the activation of NMDA receptor.15 Moreover, wnt antagonist can prevent neuronal apoptosis induced by NMDA receptor-mediated excitotoxicity in vivo and in vitro models.10
In this study, we speculated that the spinal inhibition of wnt3a/β-catenin signaling pathway could reverse the overexpression of NR2B subunit in NMDA receptor, hence provide theoretical basis of the treatment on RIH.
Materials and Methods
Animals
The experimental procedures were approved by the Animal Care Committee, Wenzhou Medical University. The animal procedures were performed in accordance with the Declaration of the National Institutes of Health Guide for Care and Use of Laboratory Animals, USA. Adult male SD rats (220 ± 20g) had an acclimation period for at least 10 days. All animals were maintained at controlled temperature (23 ± 0.5°C) and relative humidity (55 ± 10%) with 12 h light: 12 h dark cycle. They were housed in individually ventilated cages with food and water freely available.
Test Drugs
Remifentanil hydrochloride (Ultiva) (batch number: 90A05161, Ren Fu Co., Yichang, China)
GluN2B antagonist Ro25-6981 (R7150, Sigma-Aldrich Co., St. Louis, MO, USA)
Iwp-2 (681671, EMD Chemicals, Darmstadt, Germany).
Remifentanil was infused through caudal vein. Both Ro25-6981 and iwp-2 were injected intrathecally through PE-10 tube which had been implanted at spinal L3-4 intervertebral space in advance. All of the test drugs were given under sevoflurane anesthesia (induction, 3.0%, maintenance, 1.5%. Maruishi Pharmaceutical Co., Ltd., Japan) by a nose mask.
Experiment Related Procedures
Intrathecal catheters (PE-10 tube, ID0.28, OD0.61, Smiths Medical, UK) were implanted between L3-4 intervertebral space under sevoflurane anesthesia. 20μL of 2% lidocaine was administrated through this catheter 3 days later. Only the rats showing lower limb paralysis after lidocaine test were included for further experiments. All following experiments including RIH model establishment were carried out 7 days later after intrathecal catheter implantation.
Under sevoflurane anesthesia, remifentanil was infused intravenously at a rate of 1.2μg·kg−1·min−1 for 60 min. During infusion, a longitudinal incision was made in the plantar surface of the right hind paw to simulate surgical stress.9 After hemostasis, the incision was ligated and covered with antiseptic gauze. Ro25-6981 and iwp-2 were dissolved in 1% DMSO and injected intrathecally 30 min before remifentanil infusion. A schematic diagram of the experimental protocol is shown in Figure 1.
 |
Figure 1 (A, B) Schematic diagram of the experimental protocol. (A) The timeline of experiment. (B) Experimental intervention in each group. |
Both the intrathecal catheters implantation and surgical incision were performed by Gao independently.
Experiment Protocol
Phase 1: Iwp-2 Dose Determination in RIH
Thirty rats were randomly divided into five groups (n = 6 in each group): group C (controlled group); group R (rats with RIH model); group iwplow (intrathecal (i.t.) 60μM iwp-2 in rats with RIH model), group iwpmedium (i.t. 120μM iwp-2 in rats with RIH model) and group iwphigh (i.t. 180μM iwp-2 in rats with RIH model). 10μL different doses of iwp-2 were injected using a microinjection syringe 30 min before RIH model establishment. An additional 20μL normal saline was administered to flush the catheter.
PWMT and PWTL tests were performed at −24 h, 2 h, 6 h, 10h, 24 h and 48 h after remifentanil exposure. After behavior tests, the L4-6 segments of the spinal cord were quickly removed for Western blot analysis. Expression of NR2B, wnt3a, β-catenin, and production of c-fos, NF-kB were measured (n = 3 in each group).
Phase 2: The Effect of Test Drugs in RIH
Twenty-four rats were randomly divided into four groups (n = 6 in each group): group C; group R (rats with RIH model); group iwp (i.t. 180μM iwp-2 in rats with RIH model); group Ro (i.t. 1.5μg Ro25-6981 in rats with RIH model). 10μL both iwp-2 and Ro25-6981 were injected separately using a microinjection syringe 30 min before RIH model establishment.
Behavioral tests were performed at −24 h, 2 h, 6 h, 10h, 24 h, and 48 h after remifentanil exposure. After the behavioral test, the L4-6 segments of the spinal cord were removed for Western blot analysis. Expression of NR2B, wnt3a, β-catenin, c-fos, NF-kB, GSK-3β, p-GSK-3β (Ser9) was measured (n = 3 in each group). The amount of nuclear β-catenin was also detected (n = 3 in each group).
Behavior Test
Paw Withdrawal Mechanical Threshold (PWMT)
PWMT was assessed by electronic von Frey anesthesiometer (IITC INC, Life Science instrument, CA, USA) and recorded by transducer (ALMEMO 2450, Ahlborn, Germany). Before testing, each animal was acclimatized for 10 min. Through a mesh bottom (1×1 cm), a 0.8-mm diameter straight filament was applied vertically to right plantar surface adjacent to the wound (approximately 1 mm). Paw withdrawal or licking was defined as a positive response. The test was repeated 3 times with an interval of 5 min. The mean PWMT was defined as the average value from the 3 tests. A maximal cut-off value of 25 g was applied to prevent tissue damage.
Paw Withdrawal Thermal Latency (PWTL)
PWTL was evaluated by testing equipment (Model 336, Series 8, IITC INC, Life Science instrument, CA, USA). Rats were fixed in a clear plastic chamber with a glass floor and allowed to habituate for 10 min before testing. An infrared heat source under the glass was focused on the right plantar surface adjacent to the wound until withdrawal was observed. A cut-off time of 30 s was established to prevent tissue damage. The test was repeated 3 times with an interval of 5 min. The mean PWTL was defined as the average value from the 3 tests.
Western Blotting
After the last behavioral test at 48 h, the L4-6 segments of rats were quickly removed and frozen at −80°C. Tissue samples were homogenized in lysis buffer containing a cocktail of protease inhibitors (Sigma-Aldrich Co.). The lysate was centrifuged at 13,000 rpm for 20 min at 4°C and supernatant was collected as the total proteins. To extract nuclear protein, an NE-PER Nuclear Cytoplasmic Extraction Reagent kit (Pierce, Rockford, IL, USA) was used. Protein samples were separated by SDS-PAGE (8% or 12%) gel and transferred onto PVDF membrane (Millipore, MA). The following primary antibodies were used: anti-c-Fos (1:2000, ab190289, abcam, UK), anti-NF-kB p65 (1:1000, ab7970, abcam, UK), anti-Wnt3a (1:1000, ab219412, abcam, UK), anti-β-catenin (1:5000, ab32572, abcam, UK), anti-phospho-GSK-3β (Ser9) (1:1000, 5558, CST, USA), anti-GSK-3β (1:1000, 12456, CST, USA), anti-NMDAR2B (1:1000, 4212, CST, USA). β-actin (1:1000, 4970, CST, USA) or Histone H3 (1:2000, 4499, CST, USA) were used as endogenous control. The membrane was washed with TBST buffer and further incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:10,000, BL003A, Biosharp Co., China) and visualized in ECL system. The intensity of each band was assayed in Chemi-Doc XRS imaging system (Bio-Rad, CA) and analyzed by ImageJ software (NIH, Bethesda, MD). The results were expressed as the percentage of β-actin or Histone H3 immunoreactivity.
Statistical Analysis
Data were presented as the mean ± SD or mean ± SEM. We calculated the time–effect curves (AUC) data, depicting the PWMT and PWTL values over time. The AUC was measured during 48 h after RIH according to the trapezoidal rule by multiplying the time interval with PWMT or PWTL values. The statistical analyses of Western blot, AUC and behavioral tests were analyzed using one-way ANOVA with Bonferroni Correction. P < 0.05 was considered statistically significant. Statistical analysis was done using SPSS 17.0 software.
Results
Iwp-2 Doses in RIH Model
There were no significant differences among all groups in the baselines of PWMT and PWTL (p > 0.05).
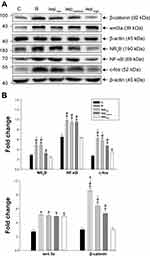
In the different doses of iwp-2, it was revealed that iwp-2 could dose-dependently inhibit RIH (Figure 2). Compared with the other two doses of iwp-2 (120μM and 60μM), the high dose group (180μM) could markedly decrease remifentanil evoked high levels in PWMT and PWTL from 2 h to 48 h after surgery (p < 0.001). Compared with group R, the medium dose of group iwp could effectively inhibit PWMT and PWTL from 2 h to 10 h. However, it failed to reverse the reduction of PWTL at 48h (p = 0.076) and PWMT at 24 h or 48 h (p = 1.0).
As shown in Figure 3, compared with group C, RIH model group markedly induced overexpression in c-fos, NR2B and NF-κB, as well as evoked an elevation in wnt3a, β-catenin production (p < 0.001). Iwp-2 dose-dependently inhibited remifentanil-induced activation of wnt3a/β-catenin signaling pathway and subsequently reverse overexpression of c-fos, NR2B and NF-κB (p < 0.001).
The Role of Iwp-2 to Reverse NR2B Activation in Order to Alleviate RIH
Behavioral Tests
Test drugs were administrated 30 min before remifentanil infusion and plantar incision. Compared with group R, pretreatment with iwp-2 could increase the thresholds of PMWT and PMWL in group iwp (p < 0.001). Compared with group iwp, pretreatment with i.t. Ro25-6981 was more effective in ameliorating remifentanil-induced thermal hyperalgesia at 24 h (p = 0.018) and 48 h (p = 0.013), as well as less mechanical allodynia at 24 h (p = 0.023) (Figure 4). Ro25-6981 had more excellence performance in higher AUC of the PMWT (p = 0.022) and PMWL (p = 0.035) curves in comparison with group iwp (Table 1).
 |
Table 1 Characteristics of AUC Values (0–48 h) |
Expression of Proteins in Spinal Dorsal Horn
Both iwp-2 and NMDA antagonist Ro25-6981 enhanced the RIH induced phosphorylation of GSK-3β, inhibited production and nuclear transport of β-catenin, hence inhibit the activity of wnt3a/β-catenin pathway (p < 0.001). Whereas neither iwp-2 (p = 0.566) nor Ro25-6981 (p = 1.000) was failed in the inhibition of wnt3a production in comparison with group C. Compared with the pretreatment of group Ro, iwp-2 had less effect on inhibiting the upregulation of NR2B subunit in NMDA receptor (p < 0.001) (Figure 5).
Discussion
Opioid used for analgesics during operation may potentially induce hyperalgesia, characterized as hypersensitive to pain and more consumption of postoperative analgesics. This study demonstrated that RIH decreased PWMT and PWTL to mechanical and thermal stimulation. The progression of RIH was associated with rapid-onset and long-lasting expression of wnt3a and c-fos in dorsal horn neurons. Via inhibiting the activation of NMDA receptor subtype NR2B, spinal wnt antagonist iwp-2 could decrease the amount of the c-fos and ameliorate the postoperative hyperalgesia. Until now, it is the first study to delineate the modulation of Wnt3a/β-catenin pathway to reverse RIH through inhibiting activation of NR2B subunit.
C-fos, which is an immediate early gene and the third message in the message transmission, is generally taken as a marker for activation status of neurons. Pain, resulting from nociceptive neuron proliferation and activation, can cause overexpression of c-fos.16
NF-κB is a nuclear transcription factor which could reflect the degree of inflammation and pain. The release of glutamate in the dorsal horn could initiate NMDA activation and subsequently enhance the expression of NF-κB. NMDA antagonist could provide antinociceptive effect by inhibiting NF-κB nuclear translocation and activation.17 The upregulation of NMDA and NF-κB was detected after remifentanil infusion.18 Wnt antagonist could prevent the RIH to reverse hypersensitivity and NF-κB activation after operations.
The mechanism of RIH is still unclear. RIH is usually attributed to the activation of NMDA receptor. The activation of NMDA receptor in spinal dorsal horn has been investigated extensively for the pain processing pathways in different models of pain.19 Remifentanil was previously proved to excite NMDA receptor subunits directly in vivo study.20 Overexpression and phosphorylation of NR2B subunit in spinal dorsal horn are closely correlated with central nociceptive hypersensitivity. Author’s earlier studies have demonstrated that various antagonists of NR2B subunit could dose-dependently alleviate the thermal and mechanical hyperalgesia and reverse the process of RIH.8 Through enhancing NR2B subtype activity, the increased amount of glutamate could result in calcium influx and trigger more propagation of the stimulus to the central nervous system.21
Wnt3a/β-catenin signaling pathway is crucial in the mechanism of neuropathic pain.10–13 Increasing evidence also suggests that wnt-signaling pathway is critically involved in chronic constriction injury, diabetic peripheral neuropathy, chemotherapy-induced neuropathic pain and other nerve injury models.10–12,22–24 Extensively studies have found that wnt pathway was indispensable to maintain synaptic plasticity in neurons.25 Via wnt-signaling pathway, NMDAergic neuron was stimulated through negatively modulate central inhibitory synaptic transmission.26,27 β-catenin, the dominant downstream activator, could reflect the activity of the wnt3a/β-catenin pathway. Once the pathway initiated, β-catenin would be promoted to accumulate and translocate into nucleus. Upregulated β-catenin in the spinal cord dorsal horn was observed in various neuropathic pain rodents.28,29 Besides, β-catenin is found to modulate synaptic plasticity and neuronal remodeling in vivo research.12 Stimulation of wnt-signaling pathway could promote the release of excitatory NMDA substance.30 So, we looked into the effect of wnt inhibitor on alterations of NMDA receptors expression when RIH occurs. In the trial, the overexpression of wnt3a and β-catenin in spinal dorsal horn after remifentanil exposure reveals that the pathway participated in the initiation of RIH.
The nuclear protein of β-catenin was involved in the development of the neuropathic pain.10 When the wnt canonical pathway is activated, the increased amount of β-catenin would migrate into the nucleus and initiate the transcription of the wnt target genes. To distinguish the nuclear β-catenin level from the overall protein, we detected the level of nuclear β-catenin in the spinal sample. The amount of nuclear β-catenin, like the overall β-catenin presented, was also elevated in the study.
NMDA receptor-dependent synaptic transmission is modulated by activity of GSK-3β.31 Interestingly, NMDA antagonist could also present antinociceptive and anti-inflammatory effects through inhibition of GSK-3β activity.32 However, the amount of the GSK-3β was not changed after remifentanil exposure in this study. GSK-3β (Ser9) phosphorylation activation was reported to decrease the NR2B activity and alleviate RIH after remifentanil infusion in animals.33 In this research, both wnt antagonist iwp-2 and NMDA antagonist were found to enhance the phosphorylation of GSK-3β (Ser9) and subsequently lessen the expression of c-fos, NR2B and NF-κB after remifentanil exposure.
Iwp-2 in this study was failed in reducing the overexpression of wnt3a which was otherwise slightly upregulated when exposed to remifentanil, the result was consistent with the previous research.34 It means that the overall synthesis of wnt3a would not be altered by the inhibition of wnt3a response.
Compared with group iwp-2, NR2B antagonist had better performance in the improvement of the behavioral tests, including higher threshold of thermal hyperalgesia and mechanical allodynia. It meant that except wnt3a/β-catenin pathway, other unknown pathways and confounding factors were also involved in RIH and NR2B activation. Further research about wnt3a involvement in the RIH should be performed in the future.
The doses of i.t. iwp-2 in this study was based on Zhang’s trial.10 Unlike the repetitive i.t. administration of 60μM iwp-2 (20μM multiple 3 times) to ameliorate chronic neuropathic pain in that trial, i.t. iwp-2 in this study was given in a single dose. In clinic, remifentanil was only infused in operations. So it was unrealistic to repetitive i.t. iwp-2 before or after operations only to prevent RIH. 180μM of iwp-2, rather than 60μM, was proved to be effective in the study.
Limitation
Although wnt3a antagonist could inhibit the activation of NR2B subunit in NMDA receptor, iwp-2 could not have the same excellent antinociceptive effect as the NR2B antagonist Ro25-6981 did in the trial. Maybe more doses of iwp-2 could provide equal analgesic effect with the NMDA antagonist. Better understanding of the wnt3a/β-catenin pathway can result in much needed improved treatment strategies for RIH.
Conclusion
This research highlighted the involvement of wnt3a/β-catenin pathway in RIH. It was announced that spinal wnt3a level was elevated when exposure to remifentanil. Spinal blocking of wnt3a response has the potential to alleviate pain-associated behavioral parameters, inhibit the upregulation of β-catenin, c-fos, NF-κB and NR2B in spinal dorsal horn. The mechanism of the wnt3a inhibitor to reverse RIH might be attributed to downregulation of β-catenin and increase of GSK-3β phosphorylation. Effective blockage of wnt3a signaling pathway provided a basis for exploring the therapeutic potential in RIH.
Abbreviations
RIH, remifentanil-induced hyperalgesia; AUC, area under the curve; NMDA, N-Methyl-D-aspartic acid; PWMT, paw withdrawal mechanical threshold; PWTL, paw withdrawal thermal latency.
Data Sharing Statement
The raw data required to reproduce these findings can be shared: DOI:10.6084/m9.figshare.11876454 at http://figshare.com/account/home.
Acknowledgments
We would like to thank Fangfang Liu PhD from Peking University for statistical preparation. Prince Henry Asamoah and Husain Alsari from school of international studies of Wenzhou medical university provided substantive suggestions in writing and manuscript editing.
Author Contributions
Yuan Gao: Conceptualization, Methodology, Formal analysis, Writing and Reviewing. Songyi Zhou: Methodology, Formal analysis, Data curation, Writing. Yizhao Pan: Formal analysis, Data curation. Lijun Gu: Formal analysis, Investigation. Yuting He: Methodology, Supervision. Jiehao sun: Conceptualization, Methodology, Software, Writing, Reviewing, Editing, Funding acquisition. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All of the authors have seen the original study data, reviewed the analysis of the data, and approved the final manuscript. The authors report no conflicts of interest in this work.
References
1. Angst MS, Clark JD. Opioid-induced hyperalgesia - A qualitative systematic review. Anesthesiology. 2006;104(3):570–587. doi:10.1097/00000542-200603000-00025
2. Shin SW, Cho AR, Lee HJ, et al. Maintenance anaesthetics during remifentanil-based anaesthesia might affect postoperative pain control after breast cancer surgery. Br J Anaesth. 2010;105(5):661–667. doi:10.1093/bja/aeq257
3. Chu LF, Dairmont J, Zamora AK, Young CA, Angst MS. The endogenous opioid system is not involved in modulation of opioid-induced hyperalgesia. J Pain. 2011;12(1):108–115. doi:10.1016/j.jpain.2010.05.006
4. Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 2008;109(2):308–317. doi:10.1097/ALN.0b013e31817f4c5d
5. Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance - intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93(2):409–417. doi:10.1097/00000542-200008000-00019
6. Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16(11):1258–1266. doi:10.1038/nm.2231
7. Xu ZL, Chen Y, Yu J, et al. TCF4 mediates the maintenance of neuropathic pain through Wnt/beta-Catenin signaling following peripheral nerve injury in rats. J Mol Neurosci. 2015;56(2):397–408. doi:10.1007/s12031-015-0565-y
8. Sun J, Lin H, Feng X, Dong J, Ansong E, Xu X. A comparison of intrathecal magnesium and ketamine in attenuating remifentanil-induced hyperalgesia in rats. BMC Anesthesiol. 2016;16:74–80. doi:10.1186/s12871-016-0235-9
9. Sun J, Lin H, He G, Lin W, Yang J. Magnesium sulphate attenuate remifentanil-induced postoperative hyperalgesia via regulating tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord. BMC Anesthesiol. 2017;17:30–38. doi:10.1186/s12871-017-0325-3
10. Zhang YK, Huang ZJ, Liu S, Liu YP, Song AA, Song XJ. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123(5):2268–2286. doi:10.1172/JCI65364
11. Itokazu T, Hayano Y, Takahashi R, Yamashita T. Involvement of Wnt/beta-catenin signaling in the development of neuropathic pain. Neurosci Res. 2014;79:34–40. doi:10.1016/j.neures.2013.12.002
12. Liu S, Liu YP, Huang ZJ, et al. Wnt/Ryk signaling contributes to neuropathic pain by regulating sensory neuron excitability and spinal synaptic plasticity in rats. Pain. 2015;156(12):2572–2584. doi:10.1097/j.pain.0000000000000366
13. Li YS, Xi Y, Li XJ, et al. Up-regulation of the biosynthesis and release of substance P through wnt/beta-catenin signaling pathway in rat dorsal root ganglion cells. PLoS One. 2015;10(6):e0129701. doi:10.1371/journal.pone.0129701
14. Balut CM, Gao YJ, Murray SA, Thibodeau PH, Devor DC. ESCRT-dependent targeting of plasma membrane localized KCa3.1 to the lysosomes. Am J Physiol Cell Ph. 2010;299(5):C1015–C1027. doi:10.1152/ajpcell.00120.2010
15. Wan XZ, Li B, Li YC, et al. Activation of NMDA receptors upregulates a disintegrin and metalloproteinase 10 via a Wnt/MAPK signaling pathway. J Neurosci. 2012;32(11):3910–3916. doi:10.1523/JNEUROSCI.3916-11.2012
16. Won L, Kraig RP. Insulin-Like Growth Factor-1 inhibits spreading depression-induced trigeminal calcitonin gene related peptide, oxidative stress & neuronal activation in rat. Brain Res. 2020;1732:146673. doi:10.1016/j.brainres.2020.146673
17. Yamaguchi K, Kumakura S, Murakami T, Someya A, Inada E, Nagaoka I. Ketamine suppresses the substance P-induced production of IL-6 and IL-8 by human U373MG glioblastoma/astrocytoma cells. Int J Mol Med. 2017;39(3):687–692. doi:10.3892/ijmm.2017.2875
18. Kaur A, Singh L, Singh N, Bhatti MS, Bhatti R. Ameliorative effect of imperatorin in chemically induced fibromyalgia: role of NMDA/NFkB mediated downstream signaling. Biochem Pharmacol. 2019;166:56–69. doi:10.1016/j.bcp.2019.05.012
19. Deng MC, Chen SR, Pan HL. Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell Mol Life Sci. 2019;76(10):1889–1899. doi:10.1007/s00018-019-03047-y
20. Hahnenkamp K, Nollet J, Van Aken HK, et al. Remifentanil directly activates human N-methyl-D-aspartate receptors expressed in xenopus laevis oocytes. Anesthesiology. 2004;100(6):1531–1537. doi:10.1097/00000542-200406000-00028
21. Guntz E, Dumont H, Roussel C, et al. Effects of remifentanil on N-methyl-D-aspartate receptor - an electrophysiologic study in rat spinal cord. Anesthesiology. 2005;102(6):1235–1241. doi:10.1097/00000542-200506000-00025
22. Tang J, Ji Q, Jin L, Tian M, Zhang LD, Liu XY. Secreted frizzled-related protein 1 regulates the progression of neuropathic pain in mice following spinal nerve ligation. J Cell Physiol. 2018;233(8):5815–5822. doi:10.1002/jcp.26358
23. Zhao Y, Yang Z. Effect of Wnt signaling pathway on pathogenesis and intervention of neuropathic pain. Exp Ther Med. 2018;16(4):3082–3088. doi:10.3892/etm.2018.6512
24. Resham K, Sharma SS. Pharmacologic inhibition of porcupine, disheveled, and β-Catenin in Wnt signaling pathway ameliorates diabetic peripheral neuropathy in rats. J Pain. 2019;20(11):1338–1352. doi:10.1016/j.jpain.2019.04.010
25. McLeod F, Salinas PC. Wnt proteins as modulators of synaptic plasticity. Curr Opin Neurobiol. 2018;53:90–95. doi:10.1016/j.conb.2018.06.003
26. Cerpa W, Latorre-Esteves E, Barria A. RoR2 functions as a noncanonical wnt receptor that regulates NMDAR-mediated synaptic transmission. P Natl Acad Sci USA. 2015;112:4797–4802. doi:10.1073/pnas.1417053112
27. Cerpa W, Gambrill A, Inestrosa NC, et al. Regulation of NMDA-receptor synaptic transmission by Wnt signaling. J Neurosci. 2011;31:9466–9471. doi:10.1523/JNEUROSCI.6311-10.2011
28. Bamji SX, Shimazu K, Kimes N, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40(4):719–731. doi:10.1016/S0896-6273(03)00718-9
29. Maguschak KA, Ressler KJ. A role for WNT/beta-catenin signaling in the neural mechanisms of behavior. J Neuroimmune Pharm. 2012;7(4):763–773. doi:10.1007/s11481-012-9350-7
30. Cerpa W, Ramos-Fernandez E, Inestrosa NC. Modulation of the NMDA receptor through secreted soluble factors. Mol Neurobiol. 2016;53(1):299–309. doi:10.1007/s12035-014-9009-x
31. Stephane P, Changiz T, Clarrisa B, et al. LTP Inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53:703–717. doi:10.1016/j.neuron.2007.01.029
32. Eduardo M, CecIlia CX, Brenda GN, et al. Antinociceptive and anti-inflammatory effects of ketamine and the relationship to its antidepressant action and GSK3 inhibition. Basic Clin Pharmacol. 2016;119(6):562–573. doi:10.1111/bcpt.12637
33. Li Y, Wang H, Xie K, et al. Inhibition of glycogen synthase kinase-3β prevents remifentanil- induced hyperalgesia via regulating the expression and function of spinal N-Methyl-D-Aspartate receptors in vivo and vitro. PLoS One. 2013;10:e77790. doi:10.1371/journal.pone.0077790
34. Resham K, Sharma SS. Pharmacological interventions targeting Wnt/beta-catenin signaling pathway attenuate paclitaxel-induced peripheral neuropathy. Eur J Pharmacol. 2019;864:172714. doi:10.1016/j.ejphar.2019.172714
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.