Back to Journals » Journal of Inflammation Research » Volume 16
WKYMVm Works by Targeting Immune Cells
Authors Yang Y , Zhao J, Jiang C, Zhang Y, Han M, Liu H
Received 21 September 2022
Accepted for publication 24 December 2022
Published 6 January 2023 Volume 2023:16 Pages 45—55
DOI https://doi.org/10.2147/JIR.S390394
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Yuting Yang,1,* Jin Zhao,2,* Chunmeng Jiang,1 Yue Zhang,1 Mei Han,1 Hui Liu1
1Department of Gastroenterology, Second Hospital of Dalian Medical University, Dalian, Liaoning, 116000, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Air Force Medical Center, PLA, Beijing, 100000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Liu; Mei Han, Department of Gastroenterology, Second Hospital of Dalian Medical University, 467 Zhongshan Road, Shahekou Region, Dalian, Liaoning, 116000, People’s Republic of China, Email [email protected]; [email protected]
Abstract: WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met) is a synthetic hexapeptide identified as a potent agonist of FPRs. FPRs are widely expressed on the cell membrane of immune cells. Therefore, WKYMVm participates in the regulation of immune cells by activating FPRs, and plays a therapeutic role in infections, tumors, autoimmune diseases and so on. WKYMVm can promote the chemotactic migration, increase the bactericidal activity of neutrophils and monocytes. WKYMVm also regulates the number and polarization of macrophages, affects the maturation of DCs and the differentiation of T cells, and promotes the activation and chemotaxis of NK cells. These functions make WKYMVm a candidate drug for immunotherapy. In this paper, we summarize the regulatory effects and underlying mechanisms of WKYMVm on six immune cells (neutrophils, monocytes, macrophages, DCs, T cells and NK cells) to increase comprehensive understanding and promote further research on WKYMVm.
Keywords: WKYMVm, FPRs, immune cells, chemotaxis, polarization
Introduction
WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met), a synthetic hexapeptide identified from a combinatorial library of peptides by functional screening, is a ligand of formyl peptide receptors (FPRs).1 FPRs are members of the G protein-coupled receptor family and have three subtypes in humans, including FPR1, FPR2 and FPR3. Fpr1 and Fpr2 in mice are homologous sequences of human FPR1 and FPR2, but homologous sequences of FPR3 have not been found in mice.2 At first, FPRs are mainly found in myeloid cells. With the increase of research, members of the FPRs gene family are also found in non-myeloid cells such as hepatocytes, glial cells, lung epithelial cells and intestinal epithelial cells,3–5 which suggests that FPRs have complex functions in different cells. For example, previous study have found that FPR2 promotes the progression of gastric cancer by promoting the invasion and metastasis of gastric cancer cells.6 Cpd43, a mixed agonist of FPR1 and FPR2, has also been reported to significantly reduce the progression of rheumatoid arthritis.7 Different subtypes of FPRs have different cell distributions, functions, and ligands. FPR1 has a tissue distribution pattern similar to that of FPR2, which is widely expressed in monocytes, neutrophils, macrophages, dendritic cells (DCs) and natural killer (NK) cells.1 FPR3 transcripts have individual differential expression in NK cells and have not been found in neutrophils.1,8 The expression pattern of FPRs is closely related to cell subtypes and the degree of cell differentiation. For example, the expression level of FPR2 is downregulated in the process of human monocytes differentiating into myeloid DCs.9 Immature DCs mainly express FPR1 and FPR3, while mature DCs only express FPR3.10,11 The reason for different expression profiles of FPRs is still unclear, which may be related to different functions of cells. The agonists of FPRs mainly include: N-formyl peptide, microbial-derived non-formyl peptide, host-derived peptide, host-derived non-peptide, ligands from peptide library and non-peptide library.1 However, different ligands have different affinities for FPRs. For example, E. coli-derived tripeptide fMLF has the highest affinity for FPR1, but has a lower affinity for FPR2. Two peptide domains in the HIV-1 envelope protein gp120 are potent agonists of FPR2, but not FPR1.1,12 According to the involved cell types and receptor subtypes, the activation of FPRs can induce a variety of pro- and anti-inflammatory responses.
WKYMVm is an exogenous agonist of FPRs, with the highest affinity for FPR2 and weaker affinity for FPR1 and FPR3.13 WKYMVm only needs the amount of picomolar (pM) concentration to trigger chemotaxis of phagocytes and mobilize calcium when acting on FPR2. However, nanomolar (nM) concentration is required when WKYMVm is used to induce chemotactic migration of cells through FPR1.14 The half-maximal effective concentrations of WKYMVm for calcium mobilization through FPR2 and FPR3 were 75 pM and 3 nM, respectively.15 The effects of WKYMVm on sepsis,16,17 lung injury,18,19 tumors,20,21 bone defect repair,22 insulin resistance,23 neurodegenerative diseases24 and other diseases have been frequently reported. For example, WKYMVm binds to FPR2 and activates the interleukin-6/gp130/signal transducer and activator of transcription 3 (IL-6/gp130/STAT3) signaling pathway to promote hepatocyte regeneration and alleviate liver fibrosis.25 In CALU-6 lung cancer cells, WKYMVm can promote lung cancer cell proliferation.26 After activating FPR2, WKYMVm can inhibit extracellular-signal-regulated kinase (ERK) and nuclear factor-κB (NF-κB) signaling pathways, and inhibit M1 microglial polarization, thereby alleviating tissue damage and decreased exercise capacity in rat models with spinal cord injury.27 WKYMVm have a short half-life in the body because of its small size.28 Lee et al developed a liquid chromatographic-electrospray ionization-time-of-flight/mass spectrometric (LC-ESI-TOF/MS) method to evaluate the pharmacokinetics of WKYMVm in rat plasma. After injecting WKYMVm into rats, they got the relevant pharmacokinetic parameters. The data obtained by intraperitoneal injection (2.5 mg/kg) were T1/2 4.9 ± 2.1 min, Tmax 5 min, Cmax 87.6 ± 20.3 ng/mL, AUClast 895.7 ± 86.6 min*ng/mL. The data acquired by intravenous injection (2.5 mg/kg) showed that T1/2 15.7 ± 8.1 min, Tmax 2 min, Cmax 312.5 ± 307.5 ng/mL, AUClast 2677.9 ± 3492.4 min*ng/mL.29 Due to the short half-life and rapid degradation, a high dosage of WKYMVm and repeated injections (4 mg/kg, twice daily for 2 days) are required to achieve the therapeutic effect in the treatment of multiple microorganism-induced sepsis models.16 It has been reported that conjugate of anti-cotinine antibody with WKYMVm can significantly improve half-life while retaining the therapeutic efficacy of WKYMVm.30 In addition, wrapping WKYMVm with injectable poly (lactide-co-glycolide) (PLGA) microspheres and injecting it into the injury site can also significantly prolong the action time of WKYMVm and increase the therapeutic effect of WKYMVm.31 The receptors of WKYMVm were first found in immune cells, so its regulatory effect on immune cells have been studied most extensively. This article will focus on the regulation of WKYMVm on six immune cells, including neutrophils, monocytes, macrophages, DCs, T cells and NK cells, to provide a reference for comprehensive understanding and promote in-depth research on WKYMVm.
WKYMVm and Immune Cells
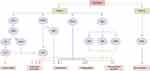
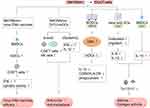
Studies have found that WKYMVm can participate in innate and acquired immune responses by acting on immune cells.32 By activating FPR1 and FPR2, WKYMVm is able to activate phospholipase C (PLC) and phosphatidylinositol 3-kinase γ (PI3Kγ) signaling pathways in immune cells.33–35 The activation of PLC can hydrolyze membrane-bound phosphoinositol-4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), and then activate protein kinase C (PKC) to induce superoxide production. IP3 mediates the release of stored Ca2+ in cells to promote immune cell degranulation.34 After PI3Kγ is activated by WKYMVm through FPR1 and FPR2, on the one hand, it can activate PKC to promote the production of superoxide, and on the other hand, it can participate in the superoxide production, chemotaxis, phagocytosis and transcriptional regulation of immune cells by activating protein kinase B (AKT).34,35 Binding to FPR1 and FPR2, WKYMVm can also activate Rho, which is involved in regulating chemotaxis and phagocytosis, and Ras, which activates the mitogen-activated protein kinase (MAPK) signaling pathway, thereby participating in the regulation of superoxide production, chemotaxis, phagocytosis, and transcriptional regulation.34 Unlike the functional diversity of FPR1 and FPR2, the functions of FPR3 are currently less studied. Kang et al found that WKYMVm stimulated ERK activity and inhibited DCs maturation by acting on FPR1 and FPR3 (Figure 1).36 Due to the different expression patterns of FPRs in different cell types and cell differentiation stages, and the binding of FPRs to WKYMVm is also affected by some proteins, such as cytoskeletal proteins, which can affect the dissociation rate of FPRs after binding with ligands.1 The signaling pathways activated by WKYMVm in different immune cells are not the same. Of course, other reasons except for these two still need to be further investigated. Next, we will introduce the regulatory mechanism of WKYMVm on six kinds of immune cells in detail.
WKYMVm and Neutrophils
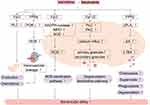
Neutrophils express a variety of chemokine receptors, pattern recognition receptors and regulatory receptors.37 Neutrophils are usually in an inactive state under normal circumstances and activated when an inflammatory response occurs in the body. The activated neutrophils migrate towards the site of inflammation under the action of inflammatory mediators, and this complex process can be roughly divided into capturing, rolling, adhesion, crawling and transmigration.38 Neutrophils play roles mainly through phagocytosis, reactive oxygen species (ROS) sterilization pathways, degranulation sterilization pathways and neutrophil extracellular traps (NETs) sterilization pathways.39 It has been found that WKYMVm prefers combining with FPR2 than FPR1 despite the existence of both FPR1 and FPR2 in neutrophils, and it activates neutrophils via FPR1 only when signaling via FPR2 is blocked.40 WKYMVm increases the bactericidal activity of neutrophils through multiple mechanisms.
Firstly, WKYMVm fights against bacterial infections by promoting the production and chemotaxis of neutrophils. By acting on Fpr2 to activate PLC, WKYMVm can induce an increase in neutrophil precursors and neutrophils in the bone marrow of septic mice and promote the chemotaxis of neutrophils, resulting in increased numbers of neutrophils in the peripheral blood and inflammatory sites.41 In addition, during the chemotaxis of neutrophils, WKYMVm enhances the leakage of microvessels by promoting the production of PI3Kγ dependent ROS, making it easier for neutrophils to pass through microvessels and reach the inflammatory sites.35
Secondly, WKYMVm enhances bacterial clearance by promoting neutrophil ROS sterilization pathways. By combining with Fpr2, WKYMVm activates NADPH-oxidase, myeloperoxidase (MPO) and superoxide dismutase (SOD) in a concentration-dependent manner, promotes neutrophils to produce ROS (such as H2O2, NO, OCl−, etc.), which can react with bacterial substrates and kill bacteria.19,42,43 Chemotherapy for cancer patients may lead to neutropenia, which makes patients prone to secondary infections. WKYMVm treatment has been reported to increase the production of ROS and bactericidal activity of neutrophils in patients receiving chemotherapy, but this effect needs to be confirmed by a large sample of clinical research.20,44
Next, WKYMVm promotes neutrophil degranulation sterilization pathways to remove bacteria. Acting on the bone marrow-derived neutrophils, WKYMVm activates PLC and PKC, increases calcium influx, and then promotes the production of neutrophil primary granules and secondary granules.45
And then, WKYMVm is also able to promote the production of neutrophil leukotriene B4 (LTB4). By binding to FPR2 on the cell surface of neutrophils isolated from the peripheral blood of healthy people, WKYMVm activates cytosolic phospholipase A2 (cPLA2), increases the production of arachidonic acid (AA) which is the precursor of LTB4, and converts AA to LTB4 under the action of perinuclear membrane 5-lipoxygenase (5-LO). Increased LTB4 can stimulate neutrophil chemotaxis, superoxide production, phagocytosis, and degranulation.46,47 The effect of WKYMVm on neutrophils is shown in Figure 2.
WKYMVm and Monocytes
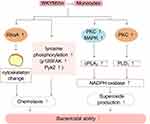
Monocytes derived from progenitor cells in the bone marrow play an important role in defending against microbial pathogens. When inflammation occurs in the body, monocytes enter tissues from the blood and differentiate into macrophages and DCs, thereby promoting the body’s defense function.48,49
Previous studies have demonstrated that FPRs are expressed in monocytes.50 WKYMVm induces chemotactic migration of monocytes by acting on FPRs. WKYMVm activates RhoA to regulate the cytoskeleton, and also can stimulate tyrosine phosphorylation of proteins such as focal adhesion kinase (p125FAK) and proline-rich tyrosine kinase 2 (Pyk2), thereby facilitating the chemotactic migration of monocytes.32 In addition, WKYMVm induces calcium influx in monocytes and stimulates cPLA2 and phospholipase D1 (PLD1), thereby activating NADPH oxidase and promoting superoxide production and bactericidal ability of monocytes. Although the activation of both cPLA2 and PLD1 requires calcium influx, the upstream signaling pathways involved are not identical and the processes are relatively independent. Stimulating cPLA2 requires the activation of PKC and MAPK, while PLD1 requires only PKC.51 The effect of WKYMVm on monocytes is shown in Figure 3.
WKYMVm and Macrophages
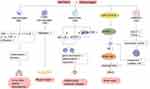
Macrophages play an indispensable role in a variety of physiological and pathological processes, such as organ development, tissue homeostasis, host defense, acute and chronic inflammation. Macrophage polarization is the differentiation of macrophages into subtypes with different phenotypes and functions according to the changes in the surrounding microenvironment. Macrophages are usually divided into two main phenotypes based on distinct activation pathways: classically activated macrophages (M1) and alternatively activated macrophages (M2).52,53 M1 macrophages can produce a large number of pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β) and IL-6, thereby aggravating the inflammatory response and promoting the development of the diseases, while M2 macrophages exert anti-inflammatory effects by secreting anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor β (TGF-β), thus participating in angiogenesis, anti-fibrosis and so on.54
WKYMVm regulates the number of macrophages and the production of inflammatory cytokines. WKYMVm treatment can significantly reduce the infiltration of macrophages at the lesion sites,18,55 downregulate the expression of pro-inflammatory cytokines (such as TNF-α, IL-1β, interleukin-1α (IL-1α) and IL-6) and upregulate the production of anti-inflammatory cytokines (such as IL-10 and TGF-β),16,55 thus playing a role in inhibiting inflammation and alleviating fibrosis in hyperoxia-induced lung injury, sepsis and scleroderma. In addition, WKYMVm promotes the infiltration of CD68+ macrophages into wounds at the early stage of skin wound recovery in streptozotocin-induced diabetic mice to speedup wound healing.56 Osteoclasts are macrophages present in bone tissue. By activating Fpr2, WKYMVm can downregulate the expression of osteoclast marker genes, inhibit the differentiation and maturation of osteoclasts, and play a protective role in inflammatory osteolytic disease.22 On the one hand, WKYMVm can directly inhibit the production of osteoclasts by reducing the phosphorylation of STAT3 and NF-κB. On the other hand, it can also reduce the binding of CD9 and gp130 by inhibiting the expression of CD9, decrease the phosphorylation of STAT3, and indirectly inhibit the production of osteoclasts.22
WKYMVm promotes M2 macrophage polarization. Janus kinase 1 (JAK1)/STAT6 signaling pathway is a key signaling pathway for polarization of M2 macrophages. WKYMVm can effectively stimulate JAK1 and STAT6 phosphorylation, thereby increasing peroxisome proliferator-activated receptor γ (PPARγ) expression and inducing macrophage polarization to M2 type.57 Moreover, WKYMVm can downregulate the expression of interferon-stimulating gene 15 (ISG15) and transcription factor EB (TFEB) by activating Fpr2 in mouse bone marrow-derived mesenchymal stem cells (mBMSCs), resulting in decreased lysosomal activity and increased exosomes secretion in mBMSCs, and promote polarization of M2 type macrophages through miRNA-146 in exosomes.58 Moreover, WKYMVm promotes M2 macrophages to secrete platelet-derived growth factor-BB (PDGF-BB), thus enhancing the pro-angiogenesis effect of M2 macrophages.57 Taken together, WKYMVm can play a therapeutic role in bone repair by promoting the polarization of M2 macrophages and angiogenesis. The effect of WKYMVm on macrophages is shown in Figure 4.
WKYMVm with DCs and T Cells
DCs are the most powerful antigen-presenting cells with the role of capturing antigens and presenting them to T cells for immune response.59 Immature DCs lack the expression of co-stimulating molecules and have strong phagocytosis and antigen processing capabilities. When DCs mature, the expression of adhesion molecules, co-stimulating molecules (such as CD40, CD80, and CD86) and antigen-presenting molecules is upregulated, and the ability of mature DCs (mDCs) to present antigens to T cells is greatly enhanced. The mDCs also can secrete a variety of cytokines and chemokines to regulate the functions of other immune cells.60–62 When the naive T cells specifically recognize the antigens, they will be activated, and then proliferate and differentiate into effector T cells which including cytotoxic T lymphocytes (CTLs), helper T cells and regulatory cells.
WKYMVm promotes the maturation of DCs and the production of CTLs. CTLs play an important role in immune defense against tumors and intracellular pathogens such as viruses and bacteria. When WKYMVm is co-injected with HIV, HBV and influenza DNA vaccines, WKYMVm can raise the expression level of CD80 on the surface of bone marrow-derived dendritic cells (BMDCs), promote the maturation of DCs, and significantly enhance the vaccine-induced CD8+ T cell responses, increase the production of interferferon γ (IFN-γ) and cytolytic activity.63 The combination treatment of WKYMVm, 5-fluorouracil (5-FU) and mDCs significantly promotes the recruitment of local CD8+ T cells and NK cells in tumors and the production of systemic IFN-γ and interleukin-12 (IL-12), thereby inhibiting primary tumors and improving anti-metastasis activity.21
However, some studies have found that WKYMVm can also inhibit the maturation of DCs. WKYMVm activates ERK signaling pathway through acting on FPR1 and FPR3, and inhibits the maturation of human monocyte-derived dendritic cells (MODCs) induced by lipopolysaccharide (LPS), which is characterized by decreased IL-12 secretion, downregulated CD86/human leukocyte antigen DR (HLA-DR) expression, and enhanced phagocytosis.36 Another function of WKYMVm is to affect the polarization of helper T cell 1 (Th1) and helper T cell 17 (Th17). Th1 and Th17 are important factors in inducing and aggravating the occurrence and development of various autoimmune diseases, and mDCs can induce helper T cells to differentiate into multiple subtypes such as Th1 and Th17. Therefore, inhibiting the maturation of DCs and overactivation of Th1 and Th17 immune responses may be effective in alleviating autoimmune diseases. In non-eosinophilic asthma mouse model, WKYMVm acts on Fpr2 and inhibits the maturation and migration of mice lung DCs, downregulates the expression of cytokines IL-12 and IL-6 produced by DCs, thereby inhibiting the polarization of Th1 and Th17 and allergen specific Th1 and Th17 immune responses.64 Another study found that WKYMVm inhibited the polarization of Th1 and Th17 to reduce joint damage in mice with collagen arthritis by binding to Fpr1 on the surface of BMDCs and promoting cytokine IL-10 secretion.65 As mentioned above, WKYMVm affects the maturation of DCs and the differentiation of T cells by activating different FPRs in different disease models. In conclusion, WKYMVm may play an active immunomodulatory role in viral infections, tumors, autoimmune diseases, and allergic diseases by affecting DCs maturation and T cells polarization. The effect of WKYMVm on DCs and T cells is shown in Figure 5.
WKYMVm and NK Cells
NK cells play a key role in the first line of defense against cancer cells and virus infections.66,67 Typically, resting NK cells are transformed into activated NK cells with higher killing activity under the action of various cytokines such as interleukin-2 (IL-2), TNF-α, IL-12 and so on. Most human NK cells typically express FPR1 and FPR2, while only a minority of human can express FPR3 in their NK cells, which suggesting individual differences.8 By activating FPRs, WKYMVm can enhance immune function and chemotaxis of NK cells. Binding to FPR1 and FPR2, WKYMVm elicits the cytolytic activity of resting NK cells by activating the c-Jun N-terminal kinase (JNK) signaling pathway as well as upregulating CD107a.8 In addition, WKYMVm induces IFN-γ production by stimulating resting NK cells. When acting on the surface of IL-2-activated NK cells, WKYMVm promotes the chemotactic migration of NK cells by stimulating the ERK signaling pathway.8 Similarly, these effects of WKYMVm are also found in the mouse melanoma model. WKYMVm treatment can increase the levels of IFN-γ and IL-2, which are key cytokines for maintaining normal function and promoting activation of NK cells in tumor tissues, and promote the chemotactic migration of NK cells to tumor tissues by activating ERK signaling pathway, thus inhibiting tumor growth. The suppressing effect of WKYMVm on tumor development in mouse melanoma is abrogated with NK cell depletion.68 Therefore, WKYMVm may play an active role in anti-tumor by regulating NK cells to assist NK cells-based immunotherapy in the future. The effect of WKYMVm on NK cells is shown in Figure 6.
Conclusions
This article summarizes the research status of WKYMVm in the treatment of related diseases by regulating immune cells through different mechanisms. Neutrophils, monocytes, macrophages, DCs, T cells and NK cells perform different functions in the immune response, and WKYMVm regulates different signaling pathways in these six cells to produce different effects. The signaling pathways and specific functions triggered by WKYMVm in cells after binding to FPRs are very complex, and the regulation of WKYMVm on immune cells is affected by the expression pattern of FPRs, skeleton proteins and other factors. In this article, we try to summarize the relevant studies that have been carried out in order to clearly understand the effect of WKYMm on immune cells. Of course, the current research also has some flaws. For example, whether WKYMVm influences NETs; what factors affect the different regulatory effects of WKYMVm on macrophages in different disease microenvironments; how the bidirectional effect of WKYMVm on DCs maturity is regulated. In addition, the effect of WKYMVm on NK cells is less studied. A lot of effects and mechanisms of WKYMVm on immune cells have been demonstrated in animal models, but the data about its effects in humans is still lacking. The above views are expected to enlighten more readers and promote further in-depth research on WKYMVm.
Although the research of WKYMVm has the above-mentioned shortcomings, it is obvious that WKYMVm has great potential as a candidate drug based on its regulatory effect on immune cells. Therefore, how to apply the potential therapeutic effect of WKYMVm to the treatment of clinical diseases has become a goal that we need to further explore. Studying the roles of WKYMVm in disease development and the regulatory mechanisms on immune cells are of great significance to promote its practical application in clinic. Because of the limitation of short half-life, no drug with WKYMVm as the main component has been successfully applied in clinic. However, with the successful determination of the crystal structure of FPR2-WKYMVm complex by researchers and the development of chemical modification and microspheres encapsulation technology, we believe that WKYMVm related drugs will soon be applied to the clinic.
Abbreviations
WKYMVm, Trp-Lys-Tyr-Met-Val-D-Met; FPRs, formyl peptide receptors; DCs, dendritic cells; NK, natural killer; pM, picomolar; nM, nanomolar; IL-6, interleukin-6; STAT3, signal transducer and activator of transcription 3; ERK, extracellular-signal-regulated kinase; NF-κB, nuclear factor κB; PLC, phospholipase C; PI3Kγ, phosphatidylinositol 3-kinase γ; PIP2, phosphoinositol-4,5-bisphosphate; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; AKT, protein kinase B; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; ROS, reactive oxygen species; NETs, neutrophil extracellular traps; MPO, myeloperoxidase; SOD, superoxide dismutase; LTB4, leukotriene B4; cPLA2, cytosolic phospholipase A2; AA, arachidonic acid; 5-LO, 5-lipoxygenase; p125FAK, focal adhesion kinase; Pyk2, proline-rich tyrosine kinase 2; PLD1, phospholipase D1; TNF-α, tumor necrosis factor α; IL-1β, interleukin-1β; IL-10, interleukin-10; TGF-β, transforming growth factor β; IL-1α, interleukin-1α; JAK1, Janus kinase 1; PPARγ, peroxisome proliferator-activated receptor γ; ISG15, interferon-stimulating gene 15; TFEB, transcription factor EB; mBMSCs, mouse bone marrow-derived mesenchymal stem cells; PDGF-BB, platelet-derived growth factor-BB; CTLs, cytotoxic T lymphocytes; BMDCs, bone marrow-derived dendritic cells; IFN-γ, interferon-γ; 5-FU, 5-fluorouracil; mDCs, mature dendritic cells; IL-12, interleukin-12; Th1, helper T cell 1; Th17, helper T cell 17; MODCs, monocyte-derived dendritic cells; LPS, lipopolysaccharide; HLA-DR, human leukocyte antigen DR; IL-2, interleukin-2; JNK, c-Jun N-terminal kinase.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Medical Science Research Project of Dalian (No. 2112015), the National Natural Science Foundation of China (No. 82100620) and the Doctoral Startup Research Fund of Liaoning Province (2021-BS-218).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ye RD, Boulay F, Wang JM, et al. International union of basic and clinical pharmacology. LXXIII. nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61(2):119–161. doi:10.1124/pr.109.001578
2. Gao JL, Chen H, Filie JD, Kozak CA, Murphy PM. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51(2):270–276. doi:10.1006/geno.1998.5376
3. Rotrosen D, Malech HL, Gallin JI. Formyl peptide leukocyte chemoattractant uptake and release by cultured human umbilical vein endothelial cells. J Immunol. 1987;139(9):3034–3040.
4. Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin, interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 1995;61(1):71–78. doi:10.1016/0165-5728(95)00075-d
5. McCoy R, Haviland DL, Molmenti EP, Ziambaras T, Wetsel RA, Perlmutter DH. N-formylpeptide and complement C5a receptors are expressed in liver cells and mediate hepatic acute phase gene regulation. J Exp Med. 1995;182(1):207–217. doi:10.1084/jem.182.1.207
6. Hou XL, Ji CD, Tang J, et al. FPR2 promotes invasion and metastasis of gastric cancer cells and predicts the prognosis of patients. Sci Rep. 2017;7(1):3153. doi:10.1038/s41598-017-03368-7
7. Crocetti L, Vergelli C, Guerrini G, et al. Novel formyl peptide receptor (FPR) agonists with pyridinone and pyrimidindione scaffolds that are potentially useful for the treatment of rheumatoid arthritis. Bioorg Chem. 2020;100:103880. doi:10.1016/j.bioorg.2020.103880
8. Kim SD, Kim JM, Jo SH, et al. Functional expression of formyl peptide receptor family in human NK cells. J Immunol. 2009;183(9):5511–5517. doi:10.4049/jimmunol.0802986
9. Yang D, Chen Q, Le Y, Wang JM, Oppenheim JJ. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J Immunol. 2001;166(6):4092–4098. doi:10.4049/jimmunol.166.6.4092
10. Yang D, Chen Q, Gertz B, et al. Human dendritic cells express functional formyl peptide receptor-like-2 (FPRL2) throughout maturation. J Leukoc Biol. 2002;72(3):598–607. doi:10.1189/jlb.72.3.598
11. Migeotte I, Riboldi E, Franssen JD, et al. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med. 2005;201(1):83–93. doi:10.1084/jem.20041277
12. Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35(8):2486–2495. doi:10.1002/eji.200526338
13. Baek SH, Seo JK, Chae CB, Suh PG, Ryu SH. Identification of the peptides that stimulate the phosphoinositide hydrolysis in lymphocyte cell lines from peptide libraries. J Biol Chem. 1996;271(14):8170–8175. doi:10.1074/jbc.271.14.8170
14. Le Y, Gong W, Li B, et al. Utilization of two seven-transmembrane, G protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide WKYMVm for human phagocyte activation. J Immunol. 1999;163(12):6777–6784.
15. Christophe T, Karlsson A, Dugave C, Rabiet MJ, Boulay F, Dahlgren C. The synthetic peptide Trp-Lys-Tyr-Met-Val-Met-NH2 specifically activates neutrophils through FPRL1/lipoxin A4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor FPRL2. J Biol Chem. 2001;276(24):21585–21593. doi:10.1074/jbc.M007769200
16. Kim SD, Kim YK, Lee HY, et al. The agonists of formyl peptide receptors prevent development of severe sepsis after microbial infection. J Immunol. 2010;185(7):4302–4310. doi:10.4049/jimmunol.1001310
17. Horewicz VV, Crestani S, de Sordi R, Rezende E, Assreuy J. FPR2/ALX activation reverses LPS-induced vascular hyporeactivity in aorta and increases survival in a pneumosepsis model. Eur J Pharmacol. 2015;746:267–273. doi:10.1016/j.ejphar.2014.11.026
18. Kim YE, Park WS, Ahn SY, et al. WKYMVm hexapeptide, a strong formyl peptide receptor 2 agonist, attenuates hyperoxia-induced lung injuries in newborn mice. Sci Rep. 2019;9(1):6815. doi:10.1038/s41598-019-43321-4
19. Lee H, Lee J, Park Y, Kim JH, Eickelberg O, Yang SR. WKYMVm ameliorates acute lung injury via neutrophil antimicrobial peptide derived STAT1/IRF1 pathway. Biochem Biophys Res Commun. 2020;533(3):313–318. doi:10.1016/j.bbrc.2020.09.036
20. Kim H, Park JH, Lee EH, et al. Granulocyte function is stimulated by a novel hexapeptide, WKYMVm, in chemotherapy-treated cancer patients. Exp Hematol. 2006;34(4):407–413. doi:10.1016/j.exphem.2006.01.010
21. Kim SD, Lee HY, Shim JW, et al. A WKYMVm-containing combination elicits potent anti-tumor activity in heterotopic cancer animal model. PLoS One. 2012;7(1):e30522. doi:10.1371/journal.pone.0030522
22. Hu J, Li X, Chen Y, et al. The protective effect of WKYMVm peptide on inflammatory osteolysis through regulating NF-κB and CD9/gp130/STAT3 signalling pathway. J Cell Mol Med. 2020;24(2):1893–1905. doi:10.1111/jcmm.14885
23. Yoon JH, Kim D, Jang JH, et al. Proteomic analysis of the palmitate-induced myotube secretome reveals involvement of the annexin A1-formyl peptide receptor 2 (FPR2) pathway in insulin resistance. Mol Cell Proteomics. 2015;14(4):882–892. doi:10.1074/mcp.M114.039651
24. Kwon YW, Bae S, Jo YS, Seo Y, Yoon JH. Stimulation of the migration and expansion of adult mouse neural stem cells by the FPR2-specific peptide WKYMVm. Life. 2021;11:11. doi:10.3390/life11111248
25. Jun JH, Park SY, Park S, et al. Formyl peptide receptor 2 alleviates hepatic fibrosis in liver cirrhosis by vascular remodeling. Int J Mol Sci. 2021;22(4):2107. doi:10.3390/ijms22042107
26. Cattaneo F, Iaccio A, Guerra G, Montagnani S, Ammendola R. NADPH-oxidase-dependent reactive oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free Radic Biol Med. 2011;51(6):1126–1136. doi:10.1016/j.freeradbiomed.2011.05.040
27. Zhang W, Chen J, Guo W, et al. WKYMVm/FPR2 alleviates spinal cord injury by attenuating the inflammatory response of microglia. Mediators Inflamm. 2022;2022:4408099. doi:10.1155/2022/4408099
28. Sato AK, Viswanathan M, Kent RB, Wood CR. Therapeutic peptides: technological advances driving peptides into development. Curr Opin Biotechnol. 2006;17(6):638–642. doi:10.1016/j.copbio.2006.10.002
29. Lee BI, Park MH, Heo SC, et al. Quantification and application of a liquid chromatography-tandem mass spectrometric method for the determination of WKYMVm peptide in rat using solid-phase extraction. Biomed Chromatogr. 2018;32(3):5. doi:10.1002/bmc.4107
30. Park S, Kim SD, Lee HY, et al. A novel delivery platform for therapeutic peptides. Biochem Biophys Res Commun. 2014;450(1):13–18. doi:10.1016/j.bbrc.2014.05.049
31. Choi YH, Heo SC, Kwon YW, et al. Injectable PLGA microspheres encapsulating WKYMVM peptide for neovascularization. Acta Biomater. 2015;25:76–85. doi:10.1016/j.actbio.2015.07.033
32. Bae YS, Kim Y, Kim Y, Kim JH, Suh PG, Ryu SH. Trp-Lys-Tyr-Met-Val-D-Met is a chemoattractant for human phagocytic cells. J Leukoc Biol. 1999;66(6):915–922. doi:10.1002/jlb.66.6.915
33. O’Flaherty JT, Jacobson DP, Redman JF, Rossi AG. Translocation of protein kinase C in human polymorphonuclear neutrophils. Regulation by cytosolic Ca2(+)-independent and Ca2(+)-dependent mechanisms. J Biol Chem. 1990;265(16):9146–9152. doi:10.1016/S0021-9258(19)38823-4
34. Dorward DA, Lucas CD, Chapman GB, Haslett C, Dhaliwal K, Rossi AG. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am J Pathol. 2015;185(5):1172–1184. doi:10.1016/j.ajpath.2015.01.020
35. Hao L, Lei X, Zhou H, Marshall AJ, Liu L. Critical role for PI3Kγ-dependent neutrophil reactive oxygen species in WKYMVm-induced microvascular hyperpermeability. J Leukoc Biol. 2019;106(5):1117–1127. doi:10.1002/jlb.3a0518-184rr
36. Kang HK, Lee HY, Kim MK, et al. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met inhibits human monocyte-derived dendritic cell maturation via formyl peptide receptor and formyl peptide receptor-like 2. J Immunol. 2005;175(2):685–692. doi:10.4049/jimmunol.175.2.685
37. Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol. 2010;10(11):1325–1334. doi:10.1016/j.intimp.2010.08.012
38. Filippi MD. Neutrophil transendothelial migration: updates and new perspectives. Blood. 2019;133(20):2149–2158. doi:10.1182/blood-2018-12-844605
39. Vorobjeva NV, Chernyak BV. NETosis: molecular mechanisms, role in physiology and pathology. Biochemistry. 2020;85(10):1178–1190. doi:10.1134/s0006297920100065
40. Karlsson J, Fu H, Boulay F, Bylund J, Dahlgren C. The peptide Trp-Lys-Tyr-Met-Val-D-Met activates neutrophils through the formyl peptide receptor only when signaling through the formylpeptide receptor like 1 is blocked. A receptor switch with implications for signal transduction studies with inhibitors and receptor antagonists. Biochem Pharmacol. 2006;71(10):1488–1496. doi:10.1016/j.bcp.2006.02.010
41. Kim HS, Park MY, Lee SK, Park JS, Lee HY, Bae YS. Activation of formyl peptide receptor 2 by WKYMVm enhances emergency granulopoiesis through phospholipase C activity. BMB Rep. 2018;51(8):418–423. doi:10.5483/BMBRep.2018.51.8.080
42. Björkman L, Forsman H, Önnheim K. Data on the NADPH-oxidase activity induced by WKYMVm and galectin-3 in bone marrow derived and exudated neutrophils isolated from four different mouse strains. Data Brief. 2017;10:349–353. doi:10.1016/j.dib.2016.12.010
43. Onnheim K, Bylund J, Boulay F, Dahlgren C, Forsman H. Tumour necrosis factor (TNF)-alpha primes murine neutrophils when triggered via formyl peptide receptor-related sequence 2, the murine orthologue of human formyl peptide receptor-like 1, through a process involving the type I TNF receptor and subcellular granule mobilization. Immunology. 2008;125(4):591–600. doi:10.1111/j.1365-2567.2008.02873.x
44. Kim H, Noh EK, Lee EJ, et al. Enhanced bactericidal function by WKYMVm in patients with acute leukemia. Leuk Res. 2008;32(5):717–725. doi:10.1016/j.leukres.2007.09.006
45. Boxio R, Bossenmeyer-Pourié C, Vanderesse R, Dournon C, Nüsse O. The immunostimulatory peptide WKYMVm-NH activates bone marrow mouse neutrophils via multiple signal transduction pathways. Scand J Immunol. 2005;62(2):140–147. doi:10.1111/j.1365-3083.2005.01651.x
46. Lee HY, Jo SH, Lee C, Baek S-H, Bae Y-S. Differential production of leukotriene B4 or prostaglandin E2 by WKYMVm or serum amyloid A via formyl peptide receptor-like 1. Biochem Pharmacol. 2006;72(7):860–868. doi:10.1016/j.bcp.2006.06.022
47. Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi:10.1126/science.294.5548.1871
48. van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–435. doi:10.1084/jem.128.3.415
49. Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi:10.1146/annurev.immunol.26.021607.090326
50. Bae YS, Ju SA, Kim JY, et al. Trp-Lys-Tyr-Met-Val-D-Met stimulates superoxide generation and killing of Staphylococcus aureus via phospholipase D activation in human monocytes. J Leukoc Biol. 1999;65(2):241–248. doi:10.1002/jlb.65.2.241
51. Bae YS, Kim Y, Kim JH, Lee TG, Suh PG, Ryu SH. Independent functioning of cytosolic phospholipase A2 and phospholipase D1 in Trp-Lys-Tyr-Met-Val-D-Met-induced superoxide generation in human monocytes. J Immunol. 2000;164(8):4089–4096. doi:10.4049/jimmunol.164.8.4089
52. Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76(3):509–513. doi:10.1189/jlb.0504272
53. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/j.immuni.2010.05.007
54. Izquierdo E, Cuevas VD, Fernández-Arroyo S, et al. Reshaping of human macrophage polarization through modulation of glucose catabolic pathways. J Immunol. 2015;195(5):2442–2451. doi:10.4049/jimmunol.1403045
55. Park GT, Kwon YW, Lee TW, et al. Formyl peptide receptor 2 activation ameliorates dermal fibrosis and inflammation in bleomycin-induced scleroderma. Front Immunol. 2019;10:2095. doi:10.3389/fimmu.2019.02095
56. Kwon YW, Heo SC, Jang IH, et al. Stimulation of cutaneous wound healing by an FPR2-specific peptide agonist WKYMVm. Wound Repair Regen. 2015;23(4):575–582. doi:10.1111/wrr.12315
57. Han X, Hu J, Zhao W, Lu H, Dai J, He Q. Hexapeptide induces M2 macrophage polarization via the JAK1/STAT6 pathway to promote angiogenesis in bone repair. Exp Cell Res. 2022;413(1):113064. doi:10.1016/j.yexcr.2022.113064
58. Zhao W, Hu J, He Q. The effect of the WKYMVm peptide on promoting mBMSC secretion of exosomes to induce M2 macrophage polarization through the FPR2 pathway. J Orthop Surg Res. 2021;16(1):171. doi:10.1186/s13018-021-02321-9
59. Domínguez-Andrés J, Joosten LA, Netea MG. Induction of innate immune memory: the role of cellular metabolism. Curr Opin Immunol. 2019;56:10–16. doi:10.1016/j.coi.2018.09.001
60. Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154(1):3–20. doi:10.1111/imm.12888
61. Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37(12):855–865. doi:10.1016/j.it.2016.09.006
62. Tiberio L, Del Prete A, Schioppa T, Sozio F, Bosisio D, Sozzani S. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol Immunol. 2018;15(4):346–352. doi:10.1038/s41423-018-0005-3
63. Lee CG, Choi SY, Park SH, Park KS, Ryu SH, Sung YC. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met as a novel adjuvant for DNA vaccine. Vaccine. 2005;23(38):4703–4710. doi:10.1016/j.vaccine.2005.03.051
64. Tae YM, Park HT, Moon HG, et al. Airway activation of formyl peptide receptors inhibits Th1 and Th17 cell responses via inhibition of mediator release from immune and inflammatory cells and maturation of dendritic cells. J Immunol. 2012;188(4):1799–1808. doi:10.4049/jimmunol.1102481
65. Park B, Lee M, Kim SD, et al. Activation of formyl peptide receptor 1 elicits therapeutic effects against collagen-induced arthritis. J Cell Mol Med. 2021;25(18):8936–8946. doi:10.1111/jcmm.16854
66. French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15(1):45–51. doi:10.1016/s095279150200002x
67. Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi:10.1016/s0065-230x(03)90004-2
68. Liu J, Li J, Zeng X, et al. Formyl peptide receptor suppresses melanoma development and promotes NK cell migration. Inflammation. 2014;37(3):984–992. doi:10.1007/s10753-014-9819-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.