Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Willis Covered Stent for Treating Intracranial Pseudoaneurysms of the Internal Carotid Artery: A Multi-Institutional Study
Authors Lu D, Ma T, Zhu G, Zhang T, Wang N, Lei H, Sui J, Wang Z, He S , Chen L, Deng J
Received 10 November 2021
Accepted for publication 16 January 2022
Published 29 January 2022 Volume 2022:18 Pages 125—135
DOI https://doi.org/10.2147/NDT.S345163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Dan Lu,1,* Tao Ma,1,* Gemin Zhu,2,* Tao Zhang,3 Naibing Wang,1 Hui Lei,2 Jing Sui,1 Zhiguo Wang,1 Shiming He,1 Lei Chen,1 Jianping Deng3
1Department of Neurosurgery, Xi’an International Medical Center Hospital, Xi’an, People’s Republic of China; 2Department of Neurology, Xi’an Central Hospital, Xi’an, People’s Republic of China; 3Department of Neurosurgery, Tangdu Hospital, Air Force Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianping Deng
Department of Neurosurgery, Tangdu Hospital, Air Force Medical University, Xi’an, People’s Republic of China
, Email [email protected]; Lei Chen
Department of Neurosurgery, Xi’an International Medical Center Hospital, Xi’an, People’s Republic of China
, Email [email protected]
Objective: This work aimed to retrospectively analyze Willis covered stent (WCS)’s therapeutic efficacy in intracranial pseudoaneurysms (PSAs) of the internal carotid artery (ICA).
Methods: Between June 2018 and July 2021, 56 individuals with intracranial PSAs of the ICA treated with WCS in three centers were included to analyze information regarding medical records, operative parameters, imaging findings and follow-up data.
Results: All WCSs were successfully targeted to the ICA lesions. Total exclusion of PSA was found in 53 cases (94.6%) right upon surgery, and mild endoleak into the aneurysm remained in 3 cases (5.4%). Intraoperative thrombosis occurred in 1 case (1.8%), and tirofiban was utilized for recanalization. Follow-up by angiography showed total aneurysm occlusion in the total number of individuals, including in the 3 above cases with residual endoleak. In-stent stenosis occurred in 7 (12.5%) patients. No stent-related ischemic event was encountered. Predictive factors of late in-stent stenosis following WCS implantation in this patient group were irregular post-operative antiplatelet treatment (p = 0.015) and C4-C5 segment of the ICA (p = 0.043).
Conclusion: WCSs are effective in treating intracranial PSAs of the ICA.
Keywords: Willis covered stent, intracranial pseudoaneurysms, internal carotid artery, endoleak, in-stent stenosis
Introduction
Intracranial pseudoaneurysm (PSA) of the internal carotid artery (ICA) represents a rarely diagnosed but serious cerebrovascular problem, causing death in 20% or more cases.1 The commonest factor causing PSA is blunt or penetrating traumatic injury. In addition, iatrogenic parameters, infections, radiation, connective tissue pathologies and unknown reasons have been implicated.1 PSA formation is due to completely disrupted arterial wall, causing extravascular hematoma in communication with the ruptured artery.2 Therefore, PSA is difficult to manage by surgical clipping, mainly due to the friable nature, the fusiform morphology, and the lack of true neck.3 Microsurgical treatment has transitioned to endovascular therapy for intracranial PSAs recently, improving parent artery preservation and decreasing morbidity and death rate.4 In recent years, Willis covered stents (WCSs; MicroPort, China) have been considered a promising tool for treating intracranial PSAs. WCSs are balloon-expandable stents specially made for intracranial vessels, and comprise 3 parts: bare stent, expandable polytetrafluoroethylene (ePTFE) membrane and balloon catheter.5 Theoretically, WCSs are ideal for the treatment of intracranial PSA to immediately exclude aneurysm from the circulation. However, currently available data stemmed from small-sized case reports and single-center experiences because such cases are extremely scarce. In this large case series, our multi-center experience with endoluminal reconstruction of intracranial PSAs with WCS is presented.
Materials and Methods
Study Design and Patients
The present retrospective, observational, single-arm trial was carried out at three participating institutions in China (Air Force Medical University Affiliated Tangdu Hospital, Xi’an; Northwest University Affiliated Xi’an International Medical Center Hospital, Xi’an; and Xi’an Jiaotong University Affiliated Xi’an Central Hospital, Xi’an). The study followed the recommendations of the Declaration of Helsinki, and was approved by the ethics committees in the Tangdu Hospital, the Xi’an International Medical Center Hospital, and the Xi’an Central Hospital. We obtained informed consent from all the patients to have their records retrospectively examined. All the patients in the illustrative cases gave informed consent to have their case details and any accompanying images published. The databases of these three institutions in computerized patient record (CPR) systems were retrospectively examined. Only patients with a definite diagnosis of PSAs with intracranial internal carotid artery (ICA; Bouthillier’s C2-C7 segment) involvement were included. In the screening stage, a total of 67 patients treated via implantation of WCS were assessed. Of these, 11 were excluded for loss to follow-up (8 cases), incomplete patient information and/or surgical report (2 cases) and lost imaging data (1 case). Finally, 56 patients between June 2018 and July 2021 were included in the analysis. Patient demographic data, radiological images, endovascular procedure data and post-procedural data, as well as follow-up procedures and angiographic findings were reviewed.
Endovascular Technique
According to the condition of the aneurysm, single- or double-covered stent implantation was applied to treat intracranial PSAs. Single-covered stents are generally utilized first; double-covered ones are applied in case the parent artery is extremely tortuous or for aneurysms with >10-mm wide necks, for reducing endoleak incidence. In this study, no patient was implanted a covered stent plus coiling. The totality of cases received WCS implantation under general anesthesia. In brief, a 6-French long sheath (Neuron MAX, Cook, USA) or 8-French guiding catheter (MP 90 cm, Boston Scientific, USA) was first placed into the targeted ICA through the right femoral approach. In case the pathway was too tortuous, the use of a 5-French intracranial support catheter (Navien, EV3, USA; Catalyst, Stryker, USA) would enable stent delivery by providing enough support. Sometimes, the support catheter underwent navigation throughout the lesion segment to guarantee a safe navigation of the covered stent more effectively. Under roadmap guidance, a 300-cm long (0.014 inch in diameter) microguidewire (Synchro-14, Stryker, USA; Transcend, Boston Scientific, USA) underwent navigation into the distal segment of the parent artery in the presence or absence of a microcatheter. Then, a WCS (MicroPort, China) was delivered using the microguidewire to bridge the aneurysm orifice on the basis of the roadmap. Many reference angiograms were acquired perioperatively to confirm the stent position and avoid covering a critical side branch. Next, stent deployment via the aneurysm orifice was performed at 5–6 atm.6–8 The diameter of the stent utilized in a given individual needs to be ≤0.5 mm wider than that of the parent artery, and the stent length needs to be ≥4 mm greater than that of the aneurysm neck.9 In case of residual endoleak detection by immediate angiography, balloon re-inflation was carried out in stent’s proximal and distal parts at 8 atm for ensuring maximal stent expansion, thus ameliorating its stability and preventing endoleak. In the event of persistent endoleak following 2–3 balloon re-inflation attempts, the following approach was applied. For an endoleak at the stent’s distal end, the procedure would be ceased, and angiographic follow-up would be carried out after 3 months or more. In case of an endoleak at the stent’s proximal end, a second covered stent was considered to be used. Both covered stents must achieve at least 3 mm overlap, and the second stent must be at least 0.5 mm larger than the first. The patient was monitored for signs and symptoms post-procedurally until discharge.
Antithrombotic Regimens
For unruptured aneurysms, the individuals received 100 mg aspirin and 75 mg clopidogrel per day for 3 days or more before the covered stent undergoes implantation. For ruptured aneurysms, 300 mg clopidogrel and 300 mg aspirin were provided via a nasogastric tube 2 h prior to emergency surgery. Systemic intravenous heparin was administered in all patients during the procedure. After introducing the femoral sheath, a bolus of 4000–5000 IU of heparin was administered transvenously, with subsequent continuous infusion of 1000–2000 IU/h for maintaining an activated clotting time of 250–300s. Tirofiban was intra-arterially applied in individuals developing acute thrombosis or thromboembolism. Post-procedure, all patients continued with the dual antiplatelet regimen (75 mg clopidogrel and 100 mg aspirin daily) for 3 months or more. Oral administration of aspirin (100 mg/day) was generally applied for 2 years or more if no side effects occurred.
Outcomes and Definitions
In the 56 patients of this study, angiographic and clinical follow-ups were carried out approximately 3–12 months post-procedure. Aneurysm localization and size, neck width and dome-to-neck (D/N) ratio were obtained on presurgical DSA scans. Depending on the maximum diameter, aneurysms were categorized as small, large and giant (<10, 10–25 and >25 mm, respectively). A wide-necked aneurysm was considered with neck width ≥4 mm and/or D/N ratio <2. The modified Rankin scale (mRS) was utilized for assessing functional outcome at follow-up by certified neurosurgeons or neurologists, with mRS scores of 0–2 and >2 indicating favorable and unfavorable outcomes, respectively. Digital subtraction angiography (DSA) was performed to evaluate aneurysms in terms of residual endoleak, aneurysm regrowth and in-stent stenosis.
The primary outcome of this study on the basis of cerebral angiography encompassed technical success, endoleak and in-stent thrombosis/stenosis. Endoleak was reflected by any aneurysmal sac detected on the angiogram acquired right after stent implantation (immediate endoleak) or at angiographic follow-up (persistent endoleak). Late in-stent stenosis was considered with stent segment decreasing by >20% in diameter (encompassing the stent’s area and 5-mm segments proximal and distal to the stent edges) at follow-up on angiograms. The secondary outcome was procedure-associated morbidity or death rate. Neurological deficiency or death was considered procedure-associated if resulting from vessel perforation, endoleak, acute thrombosis in the stent, vasospasm or perioperative side branch coverage.
Statistical Analysis
SPSS 23 (SPSS, USA) was utilized for data analysis. Continuous data are mean ± SD. Categorical variables were presented as frequency or percentage. Multivariate logistic regression analysis of parameters showing p < 0.20 in univariate analysis was performed to determine independent predictive factors of late in-stent stenosis. P < 0.05 reflected statistical significance. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained in the multivariate model.
Results
Patient Demographics
The demographic information of the 56 patients are summarized in Table 1. The patients were 46.7 ± 13.0 years old, including 58.9% men. In terms of etiology, 35 cases (62.5%) had direct craniofacial trauma and 10 (17.9%) had iatrogenic injuries after transsphenoidal surgery. In the present series, 35 patients (62.5%) manifested hemorrhagic symptoms, including intracranial hemorrhage, epistaxis and hematocele of the paranasal sinuses. A total of 21 patients (37.5%) manifested non-emergency clinical symptoms, including headaches, dizziness, diplopia, seizures, neurological deficits and compressive effect. Totally 5 individuals (5/56, 8.9%) had concurrent carotid-cavernous fistula (CCF) and pseudoaneurysm. Skull base bone fractures were detected in 58.9% (33/56) of the patients.
 |
Table 1 Baseline Characteristics of Patients |
Aneurysm and Treatment Characteristics
The features of WCS-treated pseudoaneurysms are depicted in Table 2. Aneurysms were found in the cavernous segment (C4) in 20 cases (35.7%), the clinoid segment (C5) in 5 cases (8.9%), the ophthalmic segment (C6) in 21 cases (37.5%) and the petrous segment (C2) in 10 cases (17.9%). Totally 59 covered stents underwent implantation into 56 target arteries, encompassing 1 and 2 stents per aneurysm in 53 and 3 patients, respectively.
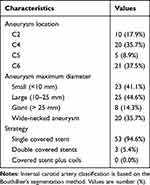 |
Table 2 Characteristics of the Study Pseudoaneurysms and Treatment |
Primary and Second Outcomes
Details of the endovascular procedure and follow-up are listed in Table 3. Technical success was achieved in all cases, with no ruptured aneurysm, artery perforation or stent migration/collapse. Total PSA alleviation without endoleak was obtained in 40 cases right upon the initial stent deployment. A mild endoleak was observed immediately in 16 (28.6%) patients upon the initial covered stent implantation. The endoleak was readily resolved by balloon re-inflation or a second covered stent in 13 patients. The remaining 3 patients had a minimal endoleak at the stent’s distal end and were followed-up. Acute thrombosis was encountered in one patient (1.8%, 1/56) during the procedure. Totally, 10 mL of tirofiban hydrochloride (Grandpharma, China) was injected immediately through the arterial pathway, and the affected artery was then recanalized without lasting sequelae. In addition, angiography performed right after the procedure revealed occluded ophthalmic artery in 9 individuals. Totally 6 cases initially presenting with vision loss had no changes indicating direct optic nerve injury after the procedure. In addition, the remaining 3 patients manifested no abnormal vision.
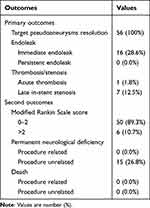 |
Table 3 Primary and Secondary Outcomes of the Patients |
All cases underwent follow-up DSA. Final angiographic follow-up times were 8.3 ± 2.8 months, ranging between 3 and 12 months. Angiographic follow-up revealed the absence of aneurysms in all individuals. Persistent endoleak was not observed. Totally 49 cases had good parent artery patency, and the remaining 7 (12.5%) had mild-to-moderate asymptomatic in-stent stenosis. No procedure-related morbidity or mortality was reported during follow-up. In this study, mRS scores at follow-up were 0–2 and >2 in 50 and 6 cases, respectively.
Predictive Factors of Late in-Stent Stenosis
Table 4 shows the predictive factors of late in-stent stenosis post-WCS implantation as determined by multivariable logistic regression analysis. Three variables (cerebral arteriosclerosis, irregular post-surgical antiplatelet treatment and C4-C5 segment of the ICA) with P < 0.20 in univariable analysis were further examined by multivariable analysis. In multivariate logistic regression analysis, only irregular post-surgical antiplatelet treatment (OR = 12.040, 95% CI 1.631–88.863; p = 0.015) and C4-C5 segment of the ICA (OR = 11.394; 95% CI 1.080–120.246; p = 0.043) independently predicted in-stent stenosis in the current patient group.
 |
Table 4 Univariate and Multivariate Analyses of Late in-Stent Stenosis |
Illustrative Cases
Case 1
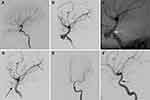
This 40-year-old patient had left vision loss following a severe fall injury. After the wound on the head had healed 1 month later, he was transferred to our hospital, complaining of massive epistaxis. A DSA examination detected a pseudoaneurysm on the C5 segment of the left ICA, with the parent artery showing stenosis (Figure 1A and B). A microguidewire was navigated into the left middle cerebral artery for the implantation of a covered stent. Perioperative angiography indicated successful navigation of the covered stent to the aneurysm orifice (Figure 1C). After Willis stent implantation, the pseudoaneurysm also disappeared. The ophthalmic artery was occluded by the covered stent, and the siphon curve was straighter (Figure 1D). Follow-up angiograms obtained at 8 months post-procedure showed total aneurysm disappearance, with in-stent stenosis without symptoms (Figure 1E and F). During 8 months of follow-up, no further epistaxis was detected, and left vision showed no improvement.
Case 2
A 68-year-old woman with epistaxis following a car accident for 6 days was transferred to our hospital. She underwent nasal packing for epistaxis in a local hospital. An emergency DSA examination was performed, confirming left internal carotid artery pseudoaneurysm of the C5 segment (Figure 2A and B). The Willis covered stent successfully traversed the support catheter and allowed a precise bridging of the aneurysm (Figure 2C). After the balloon was dilated, the covered stent was deployed at the parent artery’s aneurysm orifice (Figure 2D). Cerebral angiography right after stent placement showed total aneurysm disappearance with the parent artery having good patency (Figure 2E). Follow-up angiography at 10 months showed total aneurysm resolution and a patent parent artery (Figure 2F).
Case 3
A 56-year-old man was injured in a car accident. After the head injury had healed 5 months later, he was hospitalized in our institution, complaining of episodic headache and right pulsatile tinnitus. On examination, vascular bruit can be easily heard on the right tempus of the patient with a stethoscope. DSA examination revealed a giant pseudoaneurysm in the C2 segment of the right internal carotid artery (Figure 3A). A Willis covered stent (WCS) underwent navigation to the C2 segment, followed by balloon dilation and stent implantation (Figure 3B). After the initial deployment, an endoleak occurred at WCS’ proximal end (Figure 3C). Another balloon dilation was carried out for endoleak prevention (Figure 3D). Then, cerebral angiography immediately performed showed the endoleak and the pseudoaneurysm disappeared (Figure 3E). Follow-up angiography at 12 months revealed total aneurysm resolution and a patent parent artery (Figure 3F).
Discussion
WCS Advantages
Pseudoaneurysms are composed of only a friable layer of connective tissue, with no normal vessel wall elements. Such aneurysms are often devoid of a true neck, making complete clip occlusion difficult to achieve.10 Recently, endovascular intervention has been considered an effective strategy for the treatment of pseudoaneurysms. Endovascular treatments include a “destructive strategy” (sacrifice of the parent artery) and a “reconstructive strategy” (preservation of the parent artery).11 With the growing popularity of special intracranial stents, reconstruction approaches have been increasingly critical for the treatment of pseudoaneurysms. Aneurysm sac packing utilizing coils, balloons or fluids has been applied to preserve parent artery patency, but pseudoaneurysm rupture likely occurs during the procedure due to the lack of true vessel wall.12–14 The recently expanded use of flow diverting stents, such as Pipeline, has provided a potential solution.15,16 Multiple case reports and series demonstrated the efficacy and safety of flow diverting stents in treating pseudoaneurysms.17–19 Although flow diverting devices constitute striking advances in endovascular treatment of intracranial pseudoaneurysms, some associated limitations do exist. First, use of flow diverting stents cannot provide immediate exclusion of aneurysm from circulation, with total occlusion potentially requiring weeks. In the meantime, the patient is at high risk of rehemorrhage upon subsequent antiplatelet treatment.20 Secondly, following flow diverting stent implantation, the destabilization of the aneurysmal wall may promote aneurysm rupture, mostly because of the redirected aneurysm inflow jet and altered intra-aneurysmal shear forces.21,22 Compared with a flow diverting stent, the WCS seems safer and more efficient in treating pseudoaneurysms, and has several advantages: (1) WCSs allow instant exclusion of the aneurysm sac from the circulation; (2) WCSs have no procedural manipulations in the aneurysm sac, reducing the odds of surgery-associated rupture; (3) the absence of coil embolization in the aneurysm sac results in no mass effects.23 In this study, the WCS had good efficacy in occluding ICA pseudoaneurysms.
WCS Navigation
Despite these advantages, there are still many concerns over the use of WCSs. The WCS was specially engineered for utilization in intracranial vessels. It has advantages in flexibility, with an elevated odds of successful navigation.24 However, WCS navigation requires further attention, notably in the deep curves of the intracranial vasculature. Both stent and parent artery might be injured as the stent laboriously navigates to pass the tortuous vasculature.25,26 The delivery channel generated via the intracranial support catheter helps the WCS easily advance through the tortuous artery. In this study, successful delivery to target vessels was achieved for all stents with no overt resistance, indicating the highly effective delivery for this coaxial technique.
Limitations of the WCS
Occlusion of Side Branches
The coverage of side branches and/or perforating arteries accompanying WCS use are also a major consideration. Important branches originating from the ICA are ophthalmic (OA), anterior choroidal (AchA) and posterior communicating (PcomA) arteries. It is rare to observe PcomA or AChA occlusion by the WCS, since pseudoaneurysm cases occurring in the C7 segment are rare. In addition, the abundance of WCS versions with various diameters and lengths can guarantee total aneurysm orifice coverage and vital perforator preservation. Prior to deploying the stent, the surgeon must carefully evaluate the angiogram from several angles so that AChA and PcomA ostia are not covered. For a pseudoaneurysm occurring in the C5 or C6 segment of the ICA close to the OA, it is difficult to avoid OA occlusion using the WCS. However, deep and superficial anastomotic OA networks guarantee blood supply of the central retinal artery, which is crucial for vision.27,28 Hence, some authors have proposed the sacrifice of OA if needed.29 In this study, 9 cases showed OA occlusion after WCS implantation. Totally 6 patients with vision loss after severe trauma had no changes after the procedure. The remaining 3 had no blindness post-procedure because of the compensatory effects of anastomotic branches from the ipsilateral external carotid artery. However, in our opinion, the surgeon must carefully select a WCS of adequate length to prevent OA occlusion during surgery. Indeed, it is difficult to evaluate whether OA’s blood supply could be compensated by the anastomotic network during surgery.
Endoleak
Endoleak after WCS placement remains a non-negligible issue featuring continuous blood flow in the aneurysm sac. Persistent endoleak is a major factor contributing to continuous aneurysm dilation or rupture, notably in case of acute aneurysm rupture.30 WCS deployment probably causes endoleak formation because of mismatched sizes of stent and parent vessel, incompatible sectional shape, possible PTFE membrane rupture and potential stent shortening.31 In previous reports assessing WCS, the rates of immediate endoleak ranged from 16% to 32%.32 As shown above, endoleak was found in 16 (16/56, 28.6%) cases right after the initial balloon inflation. In general, most of immediate endoleak events can be suppressed by balloon re-inflation at the stent’s proximal and distal parts. However, stent and lesion mismatching represents the main reason for endoleak occurrence; therefore, confirming the accurate position using several reference angiograms is indispensable prior to stent deployment, as well as selecting an indicated stent size, as described in Methods. For ultra-wide necked aneurysms, double covered stents may be employed, using the “end-to-end” technique. In this series, balloon re-inflation and double covered stents sealed 81.3% (13/16) of immediate endoleaks. Minor endoleaks might be readily resolved during follow-up. Ma et al found that endoleaks had a high spontaneous healing rate (7/9, 77.8%) during follow-up.25 In this study, minimal endoleak at the stent’s distal end in 3 patients healed spontaneously at follow-up, and such cases might just undergo observation.
In-Stent Stenosis
In this study, a perioperative thrombogenic event was found in one case (1/56, 1.8%). In the latter patient, intra-arterial injection of tirofiban hydrochloride successfully recanalized the affected artery. Compared with other stents, WCSs have relatively higher thrombogenicity.33 Hence, late in-stent stenosis represents an additional complication potentially occurring during follow-up. In-stent stenosis rate for WCS has been reported to range from 8.4% to 29.0%.34–36 In this study, 7 patients (7/56, 12.5%) showed asymptomatic in-stent stenosis at angiographic follow-up. Multivariate analysis showed that C4-C5 segment of the ICA and irregular post-procedure antiplatelet therapy were powerful predictors of late in-stent stenosis. As shown above, in-stent stenosis was found frequently in the C4-C5 segment of the ICA. The possible explanations are as follows. First, the C4-C5 segment of the ICA generated in the skull base is tortuous. Deploying a balloon-expanded stent would surely disrupt the endothelium of this vascular segment. Secondly, this curved vascular segment causes obvious stent angulations after placement, and may limit stent expansion and apposition. In addition, insufficient post-procedure dual antiplatelet therapy is another powerful predictor of late in-stent stenosis. In-stent stenosis mostly results from neointimal hyperplasia.37 Dual antiplatelet treatment is considered a critical factor in inhibiting in-stent neointimal hyperplasia.38
Conclusion
This retrospective, observational, single-arm study revealed that the WCS provides a safe and effective solution in treating intracranial PSAs by reconstruction and preservation of the ICA. Irregular post-operative antiplatelet treatment and C4-C5 segment of the ICA were predictors of long-term late in-stent stenosis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zheng Y, Lu Z, Shen J, Xu F. Intracranial pseudoaneurysms: evaluation and management. Front Neurol. 2020;11:582. doi:10.3389/fneur.2020.00582
2. Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000;8(1):e4. doi:10.3171/foc.2000.8.1.1829
3. Aljuboori Z, Meyer K, Ding D, James R. Endovascular treatment of a traumatic middle cerebral artery pseudoaneurysm with the pipeline flex embolization device. World Neurosurg. 2020;133:201–204. doi:10.1016/j.wneu.2019.10.008
4. Wang W, Liang X, Chen G, et al. Treatment of intracranial pseudoaneurysms with a novel covered stent: a series of 19 patients with midterm follow-up. Front Neurol. 2020;11:580877. doi:10.3389/fneur.2020.580877
5. Li MH, Li YD, Gao BL, et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. AJNR Am J Neuroradiol. 2007;28(8):1579–1585. doi:10.3174/ajnr.A0668
6. Li MH, Zhu YQ, Fang C, et al. The feasibility and efficacy of treatment with a Willis covered stent in recurrent intracranial aneurysms after coiling. AJNR Am J Neuroradiol. 2008;29(7):1395–1400. doi:10.3174/ajnr.A1096
7. Zhu YQ, Li MH, Fang C, et al. Application of the Willis covered stent in the treatment of aneurysm in the cisternal segment of the internal carotid artery: a pilot comparative study with midterm follow-up. J Endovasc Ther. 2010;17(1):55–65. doi:10.1583/09-2688.1
8. Li MH, Li YD, Tan HQ, Luo QY, Cheng YS. Treatment of distal internal carotid artery aneurysm with the Willis covered stent: a prospective pilot study. Radiology. 2009;253(2):470–477. doi:10.1148/radiol.2532090037
9. Lai XB, Li MH, Tan HQ, et al. Predictors of in-stent stenosis and occlusion after endovascular treatment of intracranial vascular disease with the Willis covered stent. J Clin Neurosci. 2013;20(1):122–127. doi:10.1016/j.jocn.2012.01.051
10. Charbel FT, Gonzales-Portillo G, Hoffman W, Cochran E. Distal internal carotid artery pseudoaneurysms: technique and pitfalls of surgical management: two technical case reports. Neurosurgery. 1999;45(3):
11. Wang K, Peng XX, Liu AF, et al. Covered stenting is an effective option for traumatic carotid pseudoaneurysm with promising long-term outcome. J Korean Neurosurg Soc. 2020;63(5):590–597. doi:10.3340/jkns.2019.0202
12. Ogilvy CS, Tawk RG, Mokin M, et al. Stent-assisted coiling treatment of pediatric traumatic pseudoaneurysm resulting from tumor surgery. Pediatr Neurosurg. 2011;47(6):442–448. doi:10.1159/000339353
13. Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59(2):291–300. doi:10.1227/01.NEU.0000223650.11954.6C
14. Bush RL, Lin PH, Dodson TF, Dion JE, Lumsden AB. Endoluminal stent placement and coil embolization for the management of carotid artery pseudoaneurysms. J Endovasc Ther. 2001;8(1):53–61. doi:10.1177/152660280100800109
15. Chen SH, McCarthy DJ, Sheinberg D, et al. Pipeline embolization device for the treatment of intracranial pseudoaneurysms. World Neurosurg. 2019;127:e86–e93. doi:10.1016/j.wneu.2019.02.135
16. Sami MT, Gattozzi DA, Soliman HM, et al. Use of Pipeline™ embolization device for the treatment of traumatic intracranial pseudoaneurysms: case series and review of cases from literature. Clin Neurol Neurosurg. 2018;169:154–160. doi:10.1016/j.clineuro.2018.04.012
17. Kim JD, Barber SM, Diaz OM, et al. Post-traumatic amaurosis secondary to paraophthalmic internal carotid artery pseudoaneurysm treated with pipeline embolization device. J Neuroophthalmol. 2013;33(4):359–362. doi:10.1097/WNO.0b013e3182a30427
18. Nerva JD, Morton RP, Levitt MR, et al. Pipeline Embolization Device as primary treatment for blister aneurysms and iatrogenic pseudoaneurysms of the internal carotid artery. J Neurointerv Surg. 2015;7(3):210–216. doi:10.1136/neurintsurg-2013-011047
19. Griauzde J, Ravindra VM, Chaudhary N, et al. Use of the Pipeline embolization device in the treatment of iatrogenic intracranial vascular injuries: a bi-institutional experience. Neurosurg Focus. 2017;42(6):E9. doi:10.3171/2017.3.FOCUS1735
20. McAuliffe W, Wenderoth JD. Immediate and midterm results following treatment of recently ruptured intracranial aneurysms with the Pipeline embolization device. AJNR Am J Neuroradiol. 2012;33(3):487–493. doi:10.3174/ajnr.A2797
21. Kulcsár Z, Houdart E, Bonafé A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol. 2011;32(1):20–25. doi:10.3174/ajnr.A2370
22. Hampton T, Walsh D, Tolias C, Fiorella D. Mural destabilization after aneurysm treatment with a flow-diverting device: a report of two cases. J Neurointerv Surg. 2011;3(2):167–171. doi:10.1136/jnis.2010.002873
23. Wan-Yin S, Ming-Hua L, Lei Y, Yue-Qi Z, Jian-Ping G. Application of dual Willis covered stents in the management of large fusiform carotid aneurysms in a canine model. Vascular. 2014;22(6):432–438. doi:10.1177/1708538113519443
24. Tang C, Efficacy QS. Safety of Willis covered stent for treatment of internal carotid artery aneurysms. J Craniofac Surg. 2017;28(3):e263–e265. doi:10.1097/SCS.0000000000003565
25. Ma L, Xu JC, Yan S, et al. A single-center experience in the endovascular treatment of carotid siphon aneurysms using the Willis covered stent: a retrospective analysis. J Neurointerv Surg. 2018;10(12):1197–1202. doi:10.1136/neurintsurg-2017-013695
26. Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009;110(3):431–436. doi:10.3171/2008.7.JNS08257
27. Geibprasert S, Pongpech S, Armstrong D, Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009;30(8):1459–1468. doi:10.3174/ajnr.A1500
28. Perrini P, Cardia A, Fraser K, Lanzino G. A microsurgical study of the anatomy and course of the ophthalmic artery and its possibly dangerous anastomoses. J Neurosurg. 2007;106(1):142–150. doi:10.3171/jns.2007.106.1.142
29. Liu LX, Zhang CW, Lin S, et al. Application of the Willis covered stent in the treatment of ophthalmic artery segment aneurysms: a single-center experience. World Neurosurg. 2019;122:e546–e552. doi:10.1016/j.wneu.2018.10.098
30. Hoit DA, Schirmer CM, Malek AM. Stent graft treatment of cerebrovascular wall defects: intermediate-term clinical and angiographic results. Neurosurgery. 2008;62(5Suppl 2):
31. Wang W, Li MH, Li YD, et al. Treatment of traumatic internal carotid artery pseudoaneurysms with the Willis covered stent: a prospective study. J Trauma. 2011;70(4):816–822. doi:10.1097/TA.0b013e3181f892af
32. Yan P, Zhang Y, Ma C, Liang F, Zhu H, Jiang C. Application of the Willis Covered Stent in the treatment of intracranial unruptured aneurysms in internal carotid artery: a retrospective single-center experience. J Clin Neurosci. 2020;78:222–227. doi:10.1016/j.jocn.2020.04.045
33. Liu LX, Song MY, Xie XD. In-stent stenosis in the patient with internal carotid aneurysm after treated by the Willis covered stent: two case reports and literature review. Medicine (Baltimore). 2017;96(7):e6101. doi:10.1097/MD.0000000000006101
34. Wang W, Li MH, Li YD, Gu BX, Lu HT. Reconstruction of the internal carotid artery after treatment of complex traumatic direct carotid-cavernous fistulas with the Willis covered stent: a retrospective study with long-term follow-up. Neurosurgery. 2016;79(6):794–805. doi:10.1227/NEU.0000000000001266
35. Zhu YQ, Li MH, Lin F, et al. Frequency and predictors of endoleaks and long-term patency after covered stent placement for the treatment of intracranial aneurysms: a prospective, non-randomised multicentre experience. Eur Radiol. 2013;23(1):287–297. doi:10.1007/s00330-012-2581-4
36. Tan HQ, Li MH, Zhang PL, et al. Reconstructive endovascular treatment of intracranial aneurysms with the Willis covered stent: medium-term clinical and angiographic follow-up. J Neurosurg. 2011;114(4):1014–1020. doi:10.3171/2010.9.JNS10373
37. Hoffmann R, Mintz GS, Mehran R, et al. Intravascular ultrasound predictors of angiographic restenosis in lesions treated with Palmaz-Schatz stents. J Am Coll Cardiol. 1998;31(1):43–49. doi:10.1016/S0735-1097(97)00438-5
38. Hemetsberger R, Farhan S, Strehblow C, et al. Association between the efficacy of dual antiplatelet therapy and the development of in-stent neointimal hyperplasia in porcine coronary arteries. Coron Artery Dis. 2008;19(8):635–643. doi:10.1097/MCA.0b013e32831425ed
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.