Back to Journals » International Journal of Women's Health » Volume 14
Why Syphilis Infection is High Among Pregnant Women in Refugee Camps? A Case in Ethiopia
Received 21 December 2021
Accepted for publication 18 March 2022
Published 1 April 2022 Volume 2022:14 Pages 481—489
DOI https://doi.org/10.2147/IJWH.S354045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Amare Tadesse,1 Abdi Geda2
1Medicine Sans Frontiers (MSF), Gambella, Gambella Region, Ethiopia; 2Public Health Department, College of Health Science, Mettu University, Mettu, Oromia Region, Ethiopia
Correspondence: Abdi Geda, Public Health Department, College of Health Science, Mettu University, PO Box: 318, Mettu, Oromia Region, Ethiopia, Email [email protected]
Background: Almost 1 million pregnant women were estimated to be infected with syphilis in 2016, resulting in over 350,000 adverse birth outcomes including 200,000 stillbirths and new-born deaths (7– 9). More than half of infected pregnant women transmit the infection to their babies, resulting in adverse pregnancy outcomes, including foetal death, stillbirth, preterm birth, low birth weight, neonatal death, and congenital infection in infants.
Objective: The objective of the study was to assess the syphilis status and associated factors among pregnant women attending antenatal care in Kule refugee camp health facilities, Gambella regional state, Southwest Ethiopia, in 2021.
Methods: Facility based cross-sectional study design was conducted among 374 pregnant women in a Kule refugee camp health facility from March 1, to July 15, 2021. A systematic random sampling technique was used to select the study participants. After the interview, 2mL of venous blood sample was drawn from each of the study participants. Then, RPR test was done. When RPR tests were positive, the study subjects were considered as syphilis positive.
Results: The overall syphilis positivity rate was 11.8%. Education status (unable to read and write) [AOR 6.6; 95% CI (1.5, 29.3)], presence of other STDs [AOR 3.6; 95% CI (1.4, 8.8)], having polygamy husband [AOR 3.3; 95% CI (1.6, 6.9)] and being HIV positive [AOR=5.5; 95% CI (1.1, 27.5), P=0.04] were among factors associated with syphilis infection.
Conclusions and recommendation: This study showed that there is very high syphilis prevalence and it is still a very important public health problem the study area. Therefore, syphilis screening and treatment of pregnant women towards the first ANC points and health education towards the mode of transmission and prevention of syphilis have to be strengthened.
Keywords: syphilis prevalence, Kule refugee camp, Gambella, Ethiopia, pregnant women, ANC
Introduction
Syphilis is a systemic disease caused by a type of spirochete bacteria known as Treponema pallidum.1,2 It is transmitted through sexual contact, but can also be spread from an infected mother to the fetus during pregnancy or to the baby at the time of birth.3,4 The disease has four stages: primary, secondary, latent and tertiary stages. The signs and symptoms of syphilis vary depending on the four stages it presents. It is most infectious during the primary and secondary stages.2,5,6
Almost 1 million pregnant women were estimated to be infected with syphilis in 2016, resulting in over 350,000 adverse birth outcomes including 200,000 stillbirths and new-born deaths.7–8 More than half of infected pregnant women transmit the infection to their babies, resulting in adverse pregnancy outcomes, including foetal death, stillbirth, preterm birth, low birth weight, neonatal death, and congenital infection in infants.3,9,10 The annual direct medical costs of addressing the adverse outcomes related to syphilis among pregnant women is calculated to be US $309 million globally.11
Syphilis is the second most common infectious cause of stillbirth worldwide. In sub-Saharan Africa, it is estimated that nearly 1 million pregnancies are at risk of syphilis infection annually. It contributes to approximately 20% of neonatal deaths.12–14
A study conducted on global prevalence and incidence estimates in 2016 indicated that global syphilis prevalence was 0.5%, (amounting to a total 6.3 million syphilis case).15,16 On the other hand, a systematic review and meta-analysis study conducted in SSA showed that the pooled prevalence of syphilis among pregnant women in Sub-Saharan Africa was 2.9%%). According to this study, East and Southern African regions had higher syphilis prevalence among pregnant women (3.2%, and 3.6%) respectively) than even the Sub-Saharan African pooled prevalence.12
A recent systematic review and meta-analysis study conducted in Ethiopia showed that the pooled prevalence of syphilis among pregnant women in Ethiopia was 2.32%.17 Even though antenatal screening for syphilis for all pregnant women is being implemented, more than 23,000 syphilis-related adverse pregnancy outcomes are estimated to occur annually in Ethiopia.17–19 Variations in socio-demographic characteristics, having multiple sexual partners, lack of access to treatment of sexually transmitted diseases (STDs including syphilis), a history of miscarriage and stillbirth, comorbidities such as HIV/AIDS and cultural practices are amongst the risk factors associated with syphilis infection.9,12,17,20–22
Even if limited studies have been conducted on the prevalence of syphilis infection and associated factors among pregnant women in Ethiopia, it is still insufficient in the Gambella region, specifically at refugees set up, where there are no advanced health facilities and lack of study information, polygamy and levirate marriages are very common, as well as male circumcision is rare and high HIV prevalence (6%).23,24
Thus, this study assessed the magnitude of syphilis infection and factors associated with it among pregnant women, in Kule refugee camp of Gambella Region, southwest Ethiopia.
Materials and Methods
Study Area and Period
A facility-based cross-sectional study design was conducted in Kule refugee camp from March 1 to July 15, 2021. Kule Refugee camp is located at about 50km from Gambella town, capital city of the Gambella regional state, and 760km away from capital Addis Ababa. The Kule refugee camp was established in May 2014 in response to the major refugee influx from South Sudan. According to the May 2020 UNHCR report, it is home for 45,397 South Sudanese refugees. Among the total population, 10,441 (23%) are females in reproductive age (15–49 years) and around 2388 are pregnant women living in the camp.25 The camp is divided into seven zones (A-G). Each zone has zonal leaders who are selected among the refugees. There are four health centers in the zones and all provide ANC services for pregnant women. The camp is administered by Administration for Refugee and Returnee Affairs (ARRA). Different humanitarian organizations (NGOs) are acting in the areas proving humanitarian assistance.25
Study Population
All pregnant women attending ANC follow up in the health facilities of Kule refugee camp were source population and study participants were selected from the source population by systematic random sampling method.
Eligibility Criteria
Inclusion Criteria
Pregnant women who visited health facilities in Kule refugee camp at any gestational age during the data collection period were included.
Sample Size and Sampling Technique
The sample size was calculated for both objectives. For the first objective sample size was calculated using a single population proportion formula:- with the assumptions of: Z= 1.96 at 95% confidence interval, d= Margin of error assumed to be (0.02), P – Proportion of syphilis among pregnant women from previous study =2.6%9 and 10% non-response rate; 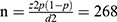
As shown below in Table 1, the sample size for the second objective (for factors) was calculated by using double population proportion formula using open Epi version 3.03 Statistical Calculated by considering the following assumptions after reviewing previous literatures: Zα/2: 1.96 at 95% confidence level, Zβ: power = statistical power of 80%, P1: the probability of outcome in the unexposed, P2: the probability of outcome in the exposed and r: ratio of unexposed to exposed (Table 1).
 |
Table 1 Sample Size Calculation for Different Significant Factors Associated with Syphilis Infection Among Pregnant Women Attending Antenatal Care Visits from Previous Studies, 2021 |
A systematic random sampling technique was used to select study participants. Sample size was allocated proportionally to all health centers based on their respective client load/flow.
Data Collection Procedure
A structured questionnaire adapted from different literature and a structured checklist adopted from the integrated ANC card of the Federal Ministry of Health was used. The tools were prepared in English and translated into the local language (Nuer) and then translated back to English by different language experts to check for consistency.
The data collectors and supervisors were trained for two days before commencement of actual data collection. A pretest was done on 37 pregnant women at other than the study area. The data collected by the pretest study was entered into SPSS and was analyzed. The finding was checked for consistency and reliability. Interviewer administered face-to-face interview was used to collect data. After the interview, 2mL of venous blood sample was drawn from each of the study participants by trained laboratory technician. Then, the sample was transferred into labeled EDTA tubes. Then the sample was centrifuged to identify serum from the whole blood. Then, RPR test was done by adding 2 drops of buffer to the serum and the test result was interpreted within 15 minutes. When RPR tests were positive, the study subjects were considered as syphilis positive. Then the result of the test was declared to clients and linked to health care providers for further counseling and treatment.
Data Quality Management and Data Analysis
A structured pre-tested questionnaire and checklist were used for collection of information to assure data quality. The tools were prepared in English and translated into the local language (Nuer) and then translated back to English by different language experts. Training was given to data collectors and the lab technician on the importance and objective of the study, ethical issues and how to use the tool. The authors and supervisors were following the data collectors on daily basis. The filled questionnaire and checklist were checked for completeness and consistency by the supervisors and authors daily.
Data was coded and entered into EPI DATA version 3.1, statistical software and then exported to SPSS version 25.0 for further analysis.
Descriptive analysis was done on socio-demographic, obstetric, behavioral, and sero-status of syphilis and presented using tables and graphs.
Bivariate and multivariate logistic regression analysis was done to identify factors associated with the outcome variable. Variables with a p-value < 0.25 during bi-variable logistic regression were considered as candidates for multivariate analysis. The goodness of fit for the final regression model was checked by the Hosmer-Lemeshow goodness of fit test and a p-value of > 0.05 considered a good fit. The final model Hosmer-Lemeshow goodness of fit test for syphilis infection was 0.89. Variables with p value less than 0.05 at 95% confidence level was declared statistically significant. The adjusted odds ratio is used to measure the strength of the association.
Results
Socio-Demographic Characteristics
A total of 374 pregnant women participated in this study, making a response rate 100%. The age of participants ranged from 16–40 years. Above half, 194 (51.9%) of study participants were in the age group of 20 to 29 years old. Almost all, 360 (96.3%) of the study participants were married, while 8 (2.1%) and 6 (1.6%) were widowed and divorced respectively. More than half, 210 (56.1%) of the study participants were unable to read and write, while 92 (24.6%) were able to read and write, 54 (14.4%) attended high school and 18 (4.8%) attended college and above. Occupationally, 310 (82.9%) of the participants were housewives, while 54 (14.4%) and 10 (2.7%) were students and employee respectively. The detail is available below in Table 2.
 |
Table 2 Socio-Demographic Characteristics of Pregnant Women Attending ANC in Kule Refugee Health Facilities, March 15 to July 15, 2021 (N=374) |
Obstetric History
Slightly less than half, 175 (46.8%) of study participants were multi gravidae. Two thirds, 248 (66.3%) of the participants were in the first trimester of pregnancy. Moreover, 30 (8%) of the participants had a previous history of neonatal loss or still birth, 18 (4.8%) had a history of spontaneous abortion, and 35 (9.4%) encountered current STI symptoms, whereas about 8 (2.1%) of the study participants who attended ANC were positive for HIV (Table 3).
 |
Table 3 Obstetric History of Pregnant Women Attended ANC in Kule Refugee Health Facilities, March 15 to July 15, 2021 (n=374) |
Behavioral Characters of the Study Participants
Regarding behavioral patterns of the study participants, 59 (15.8%) of the study participants had polygyny husband, whereas about 12 (3.2%) and 4 (1.1%) had history of multiple sexual partners and condom use, respectively.
Syphilis Sero-Prevalence
An overall magnitude of syphilis sero-positivity was 11.8% [95% CI: 8.6, 15.2]. Almost two third, 28 (63.6%) of syphilis sero-positivity was observed among the pregnant women in the age interval of 20 and 29 years.
Factors Associated with Syphilis Sero-Positivity Infection Among the Study Participants
During bivariate logistic regression analysis, educational status, previous history of still birth, current symptoms of other STIs/STDs, HIV status, history of multiple sexual partners, polygyny and experience of abortion were associated with syphilis sero-positivity and candidates for multivariate analysis.
On multivariate logistic regression analysis, variables such as presence of other STDs, polygyny, educational status, HIV status and were identified as factors significantly associated with syphilis sero-positivity.
Pregnant women who had co-morbidity with other STDs were 3.6 times more likely to have syphilis sero-positivity when compared to their counterparts [AOR=3.6; 95% CI (1.4, 8.8), p<0.006]. Likewise, pregnant women who had a polygyny husband were 3.3 times more likely to be syphilis sero-positive when compared to their counterparts [AOR=3.3; 95% CI (1.6, 6.9), P<0.002]. Furthermore, pregnant women having co-morbidity with HIV/AIDS were 5.5 times more likely to be syphilis sero-positive when compared to their counterparts [AOR=5.5; 95% CI (1.1, 27.5), P=0.04]. Pregnant who were unable to read and write were 6.6 times more likely to be syphilis sero-positive than those who attended high school and above [AOR=6.6; 95% CI (1.5, 29.3), P<0.01]. The detail is presented by table as follow (Table 4).
 |
Table 4 Factors Associated with Syphilis Sero-Positivity Among Pregnant Women Attending ANC Follow Up at Health Facilities in Kule Refugee Camp, Gambella from March 15 to July 15, 2021 (n=374) |
Discussion
This study assessed the magnitude of syphilis and associated factors among pregnant women in Kule Refugee Camp, Gambella Regional State, Ethiopia. The prevalence of syphilis was 11.8% [95% CI: 8.6, 15.2].
This finding is highest among in the studies conducted in Yirgalem hospital (5.1%),26 Sede Muja district, South Gondar (1.9%),22 Bahir Dar at Felege Hiwot Referral Hospital (2.6%),12 Jimma University Hospital (1.1%),21 Wolemera district public health centers (1.2%)27 and Debre Berhan public health institutions (1.8%).20 The possible reasons for this variation might be attributed to the difference in sexual practice and behavior of the communities, awareness of syphilis, cultural practices and differences in study setting (refugee setup).
It is also higher than the studies done in Ethiopia (2.32%),17 Sub-Saharan Africa (2.9%),12 Khartoum, Sudan (3%)28 and Zimbabwe (1.2%).29 This might be due to the difference in study setting, type of population being studied, access to health care service including information education communication (IEC) on reproductive health and health seeking behavior to treatment of STDs (syphilis), sexual behavior, cultural practices and awareness of syphilis.
This study revealed that pregnant women who had symptoms of other sexually transmitted diseases (STDs) other than HIV were more likely to have syphilis sero-positivity when compared to their counterparts. This is in line with studies conducted at Debre Berhan and Bahir Dar at Felege Hiwot Referral Hospital.9,30 This finding may show that syphilis sero-positivity and other STDs have the same risk factors, mode of transmission and one increases/facilitates the risk of acquisition of the other.
This result also showed that pregnant women who had polygyny were more likely to have syphilis sero-positivity when compared to their counterparts. This fits with the study conducted in Harare, Zimbabwe.29 This might be attributable to the fact that having multiple sexual partners is a risk for syphilis sero-positivity or increases the chance of infection.
The pregnant women having co-morbidity with HIV/AIDS were more likely to have infection with syphilis compared with their counterparts. This finding is supported by the studies done in Debre Berhan, Bahir Dar at Felege Hiwot Referral Hospital, Wolemera District, and Yirgalem.9,26,27,30 This shows that these infections share the same risk factor (risky sexual behaviors), mode of transmission and the presence one facilitates for the occurrences of the other.
Pregnant women who were unable to read and write were more likely to have syphilis sero-positivity when compared to their counterparts. This fits with the study done in Bahir Dar at Felege Hiwot Referral Hospital Ethiopia,9 which shows syphilis sero positivity was high among unable to read and write study participants. This may show study subjects with no education or less educational attainment are at high risk of getting syphilis since they may have less awareness of prevention methods and the etiology of syphilis. Women with a history of miscarriage and stillbirth were more likely to be infected by syphilis and were significantly associated with syphilis sero-positivity in many previous studies,9,27,30,31 but not significantly associated with syphilis sero-positivity in this study.
This finding has significant practical implications. High syphilis sero-positivity rates among pregnant women in this humanitarian setting indicates the presence of active syphilis.32,33 This – high syphilis sero-positivity rate- has immediate consequences like high still birth rate, high neonatal mortality rate, high prevalence of low birth weight, preterm delivery and others.
Ethiopia has implanted the Million Development Goals related to reproductive health (MDG 1990–2015; particularly goal 3, 4, 5) in line with Health Sector Transformation Plan (HSTP-I) and National Reproductive Strategies, guided by WHO intervention guidelines in the last three decades. However, the fact that still there is very high syphilis positivity rate indicates the necessity to revise the implementation strategy both in general population and humanitarian settings like this one.
Nowadays, Sustainable Development Goals, in which reproductive health Problems including STIs are targeted, is under implementation in Ethiopia through HSTP-II (2021–2025) in general and National Reproductive Health Strategy (2021–2025) in particular. The National Reproductive Health Strategy, set an objective to increase syphilis testing coverage for pregnant women from its current status of 55% to 85% in the next five year.34 The health care coverage for syphilis (screening and treatment) in Ethiopia is very low while the positivity rate (among those who are screened) is very high. This, - the discrepancy between high positivity rate and very low health Care (including treatment) - may enforce Ethiopia to fall short of its plan of Triple Elimination of MTCT of HIV, HBV and Syphilis.
Strength and Limitation of the Study
Strength
Using primary data by collecting blood samples directly from study participants, including all the health facilities in the refugee camp is among the strength.
Limitation of the Study
Since this is a facility-based cross-sectional study, the result of the study might not reflect the actual burden of the community.
Conclusion and Recommendation
Conclusion
High syphilis prevalence was identified in these pregnant women. Implementing universal syphilis screening and treatment during the first antenatal care visit and prompt treatment of pregnant women and their sexual partners can reduce mother-to-child transmission and negative birth outcomes associated with syphilis in this setting.
Recommendation
Health Care Provider
- Giving regular health education to pregnant women in antenatal clinics to inform them about their health, mode of transmission, avoidance of risky behaviors (like polygyny) syphilis sero-positivity and other STDs, and the risk of syphilis to both born and unborn children.
- Strengthen partner testing and treating for syphilis.
Humanitarian Organizations (MS, ARRA, UNHCR, etc.)
- Amplify antenatal services by increasing access to and implementing a strong program to deal with STIs and syphilis, in particular, at the first antenatal visit.
- Plan and implement an effective health education program to target females at child bearing age and the community regarding syphilis as a disease and risky behaviors (like polygyny) through community outreach workers.
Regional Health Bureaus, Zonal and Other Health Offices
- Monitoring and evaluation to see if the necessary screening service is undertaken.
- Support the necessary logistics for screening and treating syphilis sero-positivity.
Researchers
- Qualitative and prospective studies should be conducted that include more at the community level to generate more and deeper information to improve and strengthen the program.
Abbreviations
ARRA, Administrative of Refugee and Return Affaires; CI, confidence interval; COR, crude odds ratio; ETB, Ethiopian Birr; FANC, focused antenatal care; HIV, human immunodeficiency virus; IRB, Institutional Review Board; MSF, Medicine Sans Frontiers; MTCT, mother to child transmission; PMTCT, prevention of mother to child transmission; RPR, rapid plasma reagin; STDs, sexually transmitted diseases; STI, sexually transmitted infection; UNHCR, United Nations High Commissioners for Refugees; VDRL, Venereal Diseases Research Laboratory; WHO, World Health Organization.
Data Sharing Statement
All the data used or mentioned in this research are available.
Ethics Approval and Consent to Participate
This research was done according to Declaration of Helsinki. The ethical approval for this study was obtained from the Research Ethical Committee of Mettu University. Then, necessary explanation was given to participants about the purpose and benefits of the study and their right on decision to participate in the study.
Informed consent was obtained from respondents who were 18 years and above. Interviews and blood sample withdrawal was made under strict privacy. But regarding respondents who were below 18 years of age, only assent was taken from participants. Informed consent was obtained from their representatives. This is in line with Article 25 of Declaration of Helsinki which says ”When a subject deemed legally incompetent, such as a minor child, is able to give assent to decisions about participation in research, the investigator must obtain that assent in addition to the consent of the legally authorized representative”35.
Acknowledgment
We would like to acknowledge Mettu University for financial support. We are also grateful to the data collectors and respondents who took part in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no financial support in the research, authorship, and publication of this article was received.
Disclosure
The authors declare that they have no competing interests.
References
1. Dnp KT, Cdc TG, Aids HIV, Awareness STD, April M. Sexually Transmitted Diseases 2021.
2. The New York City Department of Health and Mental Hygiene Bureau of Sexually Transmitted Infections. The Diagnosis, Management and Prevention of Syphilis an Update and Review.
3. World Health Organization. Syphilis in pregnancy; 2021. Available from: https://www.who.int/data/gho/data/themes/topics/indicator-groups/indicator-group-details/GHO/antenatal-care-anc-attendees-tested-for-syphilis.
4. Korenromp EL, Rowley J, Alonso M, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes—Estimates for 2016 and progress since 2012. PLoS One. 2019;14(2):e0211720. doi:10.1371/journal.pone.0211720
5. Public Health STI Enhanced Response Unit. Syphilis CDNA National Guidelines for Public Health Units.
6. Guideine Development Group. Maternity and Neonatal Clinical Guideline Syphilis in Pregnancy.
7. World Health Organization. Sexually transmitted infections (STIs). 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-stis.
8. World Health Organization. Sexually transmitted infections [Internet]. [cited November 15, 2021]. Available from: https://www.who.int/data/gho/data/themes/sexually-transmitted-infections.
9. Tareke K, Munshea A, Nibret E. Seroprevalence of syphilis and its risk factors among pregnant women attending antenatal care at Felege Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia: a cross ‑ sectional study. BMC Res Notes. 2019;12(1):34–35. doi:10.1186/s13104-019-4072-z
10. European Centre for Disease Prevention and Control. Syphilis and congenital syphilis in Europe A review of epidemiological trends and options for response; 2018:4.
11. Abenezer C. Magnitude of HIV and syphilis and associated factors among pregnant women attending antenatal care in wolemera district; 2017.
12. Hussen S, Tadesse BT. Prevalence of syphilis among pregnant women in Sub-Saharan Africa: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:3–4. doi:10.1155/2019/4562385
13. Maronezzi G, Brichi Pesce G, Martins DC, Do Prado CM, Molena Fernandes CA. Syphilis in pregnant and congenital: epidemiological profile and prevalence. Enfermería Glob. 2020;19(1):138.
14. da Silva, K. A. G., do Nascimento Oliveira, K. C. P., de Almeida, D. M. Outcomes in fetuses and newborns exposed to infections during pregnancy. Eev Bras Enfrem. 2021;74(3):1–7.
15. Korenromp IEL, Rowley J, Alonso M, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes-Estimates for 2016 and progress since 2012. PLoS One. 2019;14(2):e0211720. doi:10.1371/journal.pone.0211720
16. Kachingwe ©edith. Global Accelerated Action for the Health of Adolescents (AA-HA!) Guidance to Support Country Implementation Annexes 1–6 and Appendices I–IV Global Accelerated Action for the Health of Adolescents (AA-HA!) Guidance to Support Country Implementation. Geneva: World Health Organization; 2017.
17. Geremew H, Geremew D. Sero ‑ prevalence of syphilis and associated factors among pregnant women in Ethiopia: a systematic review and meta ‑ analysis. Syst Rev. 2021;10(1):1–9. doi:10.1186/s13643-020-01552-x
18. Kebede KM, Abateneh DD, Belay AS, Manaye GA. The epidemiology of syphilis in Ethiopia: a protocol for systematic review. Syst Rev. 2019;210:4–9.
19. Scho A, Mesfun MG, Nordmann T, Breuer M. Prevalence and impact of sexually transmitted infections in pregnant women in central Ethiopia. Int J STD AIDS. 2018;29(3):2.
20. Alene TY. Sero-prevalence of Syphilis and HIV and associated factors in pregnant women attending ANC Clinics in Debre Berhan Public Health Institutions, North Shewa, Ethiopia: retrospective cross- sectional study. J HIV Retro Virus. 2020;6(5):2–3.
21. Fikadu B, Gebrish S, Asfaw T. Sero-Prevalence of Syphilis Among Pregnant Women Attending Antenatal Care clinic at Jimma University Specialized Hospital. J Med Sci. 2019;5(1):3–4.
22. Yitbarek GY. Prevalence of syphilis among pregnant women attending antenatal care clinic, Sede Muja District, South Gondar, Northwest Ethiopia. J Pregnancy. 2019;2019:4–5.
23. Kibret GD, Ferede A, Leshargie CT, Wagnew F, Ketema DB, Alebel A. Trends and spatial distributions of HIV prevalence in Ethiopia. Infect Dis Poverty. 2019;8(1):4. doi:10.1186/s40249-019-0594-9
24. Ali S, Sewunet T, Sahlemariam Z, Kibru G, Guerra MR, Chaoubah A. Neisseria gonorrhoeae among suspects of sexually transmitted infection in Gambella hospital, Ethiopia: risk factors and drug resistance. BMC Res Notes. 2016;9:1–8. doi:10.1186/s13104-015-1837-x
25. UNHCR. Kule refugee camp; 2020:1.
26. Amsalu A, Ferede G, Assegu D. High seroprevalence of syphilis infection among pregnant women in Yiregalem hospital southern Ethiopia. BMC Infect Dis. 2018;4:4–6.
27. Chegan A. Assessment of magnitude of HIV and Syphilis and associated factors among pregnant women attending antenatal care in Wolemera District; 2017:2–5.
28. Abdelmola AO. Prevalence and factors associated with syphilis among pregnant women attending antenatal care, Khartoum State, Sudan. Int J Adv Med. 2018;5(2):2–6. doi:10.18203/2349-3933.ijam20181062
29. Kurewa NE, Mapingure MP, Munjoma MW, Chirenje MZ, Rusakaniko S, Stray-Pedersen B. The burden and risk factors of sexually transmitted infections and reproductive tract infections among pregnant women in Zimbabwe. BMC Infect Dis. 2016;10:8.
30. Zinabie S. Sero-prevalence of syphilis and HIV and associated factors in pregnant women attending antenatal care clinics in debre berhan public health institutions, Ethiopia. Am J Biomed Life Sci. 2018;6(3):60.
31. Endris M, Deressa T, Belyhun Y, Moges F. Seroprevalence of syphilis and human immunodeficiency virus infections among pregnant women who attend the University of Gondar teaching hospital, Northwest Ethiopia: a cross sectional study. BMC Infect Dis. 2016;15(1):1–7.
32. Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatology Venereol. 2021;35(3):574–588. doi:10.1111/jdv.16946
33. Wi TEC, Ndowa FJ, Ferreyra C, et al. Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. J Int AIDS Soc. 2019;22(S6):8–18. doi:10.1002/jia2.25343
34. Ethiopian Ministry of Health. Health Sector Transformation Plan II 2020/2021–2024/2025. Ethiop Minist Heal. 2021;25:1–128.
35. World Health Association. Declaration of Helsinki World medical association declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
