Back to Journals » Patient Preference and Adherence » Volume 14
Why Do People Living with HIV Adhere to Antiretroviral Therapy and Not Comorbid Cardiovascular Disease Medications? A Qualitative Inquiry
Authors Muiruri C , Sico IP , Schexnayder J, Webel AR, Okeke NL, Longenecker CT, Gonzalez JM , Jones KA, Gonzales SE , Bosworth HB
Received 21 March 2020
Accepted for publication 19 May 2020
Published 16 June 2020 Volume 2020:14 Pages 985—994
DOI https://doi.org/10.2147/PPA.S254882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Charles Muiruri,1,2 Isabelle P Sico,1 Julie Schexnayder,3 Allison R Webel,3 Nwora Lance Okeke,4 Christopher T Longenecker,5 Juan Marcos Gonzalez,1 Kelley A Jones,1 Sarah E Gonzales,1 Hayden B Bosworth1
1Department of Population Health Sciences, Duke University School of Medicine, Durham, NC, USA; 2Duke Global Health Institute, Duke University, Durham, NC, USA; 3Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, OH, USA; 4Department of Medicine, Duke University Medical Center, NC, USA; 5Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, USA
Correspondence: Charles Muiruri Email [email protected]
Background: After achieving viral suppression, it is critical for persons living with HIV (PLWH) to focus on prevention of non-AIDS comorbidities such as cardiovascular disease (CVD) in order to enhance their quality of life and longevity of life. Despite PLWH elevated risk of developing CVD compared to individuals without HIV, PLWH do not often meet evidence-based treatment goals for CVD prevention; the reasons for PLWH not meeting guideline recommendations are poorly understood. The objective of this study was to identify the factors associated with adherence to CVD medications for PLWH who have achieved viral suppression.
Methods: Qualitative data were obtained from formative research conducted to inform the adaptation of a nurse-led intervention trial to improve cardiovascular health at three large academic medical centers in the United States. Transcripts were analyzed using content analysis guided by principles drawn from grounded theory.
Results: Fifty-one individuals who had achieved viral suppression (< 200 copies/mL) participated: 37 in 6 focus groups and 14 in individual semi-structured interviews. Mean age was 57 years (SD: 7.8); most were African Americans (n=31) and majority were male (n=34). Three main themes were observed. First, participants reported discordance between their healthcare providers’ recommendations and their own preferred strategies to reduce CVD risk. Second, participants intentionally modified frequency of CVD medication taking which appeared to be related to low CVD risk perception and perceived or experienced side effects with treatment. Finally, participants discussed the impact of long-term experience with HIV care on adherence to CVD medication and motivational factors that enhanced adherence to heart healthy behaviors.
Conclusion: Findings suggest that future research should focus on developing interventions to enhance patient–provider communication in order to elicit beliefs, concerns and preferences for CVD prevention strategies. Future research should seek to leverage and adapt established evidence-based practices in HIV care to support CVD medication adherence.
Keywords: medication adherence, persons living with HIV, viral suppression, cardiovascular disease, qualitative research
Background
The United States is on course to reach the targets of the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90–90-90 goals.1 90–90-90 targets stipulate that 90% of people living with HIV (PLWH) will know their status; 90% of those will be on antiretroviral therapy (ART); and 90% of those on ART will be virally suppressed.2 After achieving viral suppression, it is critical for PLWH to focus on prevention of non-AIDS comorbidities such as cardiovascular disease (CVD) in order to enhance quality of life and increase lifespan.3 PLWH have a 1.5 to 2 times higher risk of developing CVD compared to individuals without HIV, a risk that persists despite viral suppression.4,5 Unfortunately, PLWH do not often meet evidence-based treatment goals for CVD prevention and the reasons are poorly understood.6–10
A critical factor for achieving CVD treatment guidelines is optimal CVD medication adherence.11 To date, a majority of research in medication adherence for PLWH has focused on ART.12 For PLWH to achieve viral suppression, they must attain and maintain near perfect ART adherence (90–95%).13 For those who have achieved viral suppression, their experience in overcoming treatment barriers for ART may transfer to other non-AIDS comorbidities.14 Yet some evidence suggests disparate levels of medication adherence are found across conditions among PLWH. For example, to date, in two studies it was observed that PLWH had significantly higher medication adherence to ART compared to hypertension, mental health and type 2 diabetes medications.15,16 In two other studies significant differences between rates for ART and hypertension, chronic kidney disease, or diabetes medications were not observed.17,18
Barriers to CVD medication adherence for PLWH who are adherent to ART may be different than for PLWH who are nonadherent to ART. It is therefore important to study these groups independently. Since treatment barriers that affect PLWH who are highly adherent to ART, may remain unchanged across different comorbid conditions like CVD, there is a need to look beyond barriers in evaluating the observed CVD medication nonadherence. For PLWH who are adherent to ART and have achieved viral suppression, nonadherence to CVD medications may be influenced by a combination of healthcare providers and individual patient factors including the health delivery systems.19
On the healthcare provider level, HIV healthcare providers have an important role in encouraging and monitoring ART adherence as described in their practice guidelines.20,21 HIV providers’ role in monitoring and encouraging adherence to other non-AIDS conditions such as CVD for PLWH who have achieved viral suppression is understudied. At the individual patient level, Cioe et al found that in a study of PLWH, greater than 90% of the participants knew that smoking, being overweight, high cholesterol and lack of exercise contributed to an increased risk of heart disease, but this knowledge was not associated with perceived risk of CVD.22 Webel et al also found no differences in perceived susceptibility, severity of CVD, perceived benefits of engaging in CVD prevention behaviors and perceived barriers between PLWH and those without HIV.23 Finally, since medication-taking decision may be driven by individual valuation of the underlying attributes of medications such as perceived medication benefits, side effects, dosage frequency among others,24–26 it is critical to evaluate how these factors affect CVD medication adherence in a population that is highly adherent to HIV treatment.
In order to develop a better understanding of the factors that influence adherence to CVD medications for PLWH who have achieved viral suppression, we analyzed qualitative data from an ongoing multi-site nurse-led intervention trial to improve cardiovascular health.19 The objective of this current study was to identify factors associated with adherence to CVD medications for PLWH who have achieved viral suppression.
Methods
In order to address the prevention of CVD among PLWH, the National Heart Lung and Blood Institute (NHLBI) recently announced and funded projects under RFA-HL-18-007 “ImPlementation REsearCh to Develop interventions for People Living with HIV (PRECLuDE)” in 2017.27 The purpose of PRECluDE was to stimulate the use of late-stage T4 translation research and implementation science strategies that address barriers impeding the scale-up and application of guideline-based interventions in community and clinical settings for the prevention, treatment, and control of co-morbid heart lung blood and sleep diseases and disorders for PLWH.28 Qualitative data for this study were obtained from baseline formative research conducted to inform the adaptation of a one of the PRECLuDe funded trials – A nurse-led intervention trial to extend the HIV treatment cascade for cardiovascular disease prevention (EXTRA-CVD).19,28,29 EXTRA-CVD is an implementation trial of a multicomponent, nurse-led intervention to control hypertension and hypercholesterolemia in individuals with well-controlled HIV infection.
To be eligible for EXTRA-CVD trial, participants had to be ≥18 years old, received care at a participating medical centers’ HIV clinic, with confirmed HIV diagnosis and undetectable HIV viral load, defined as the most recent HIV viral load <200 copies/mL checked within the past year. We used undetectable HIV viral load as an indicator for high adherence to ART in this study.2 Participants had to have hypertension (defined as systolic BP >130 mmHg on ≥2 occasions in the past 12 months or on an antihypertensive medication) and hyperlipidemia (defined as a non-HDL cholesterol >130 mg/dL or on cholesterol-lowering medication), assessed via chart abstraction for both.
Recruitment Process
Eligible PLWH were identified from the electronic medical record at Duke University Medical Center (DUMC) Durham NC, MetroHealth (MH) and University Hospitals (UH), Cleveland OH based on the study criteria. To increase participation, PLWH were also informed of the study by their healthcare providers during routine clinic visits. Eligible participants were approached by study coordinators and were consented. Study participants were recruited by purposive sampling technique from the clinics in which they received care.30
Data Collection
Data were collected through a combination of focus group discussions (FGDs) and individual semi structured interviews with PLWH at the three study sites. FGDs were approximately 60 minutes in length and conducted face-to-face with four to nine PLWH. Duration of individual interviews was approximately 30 minutes. PLWH who declined to participate in FGDs had the option to complete a face-to-face or telephone interview. Authors ARW and JS conducted the FGDs at UH and MH sites while SG conducted one FGD and individual semi structured interviews at DUMC between October 2018 and January 2019. All interviewers did not have any affiliations or relationships with participants. ARW and JS have extensive experience and training in conducting qualitative health research with PLWH. SG has extensive experience and training in conducting qualitative health research. Participants signed a written informed consent, except for telephone interviews for which verbal consent was provided according to an IRB approved script. All participants completed a brief survey. In the survey questions included demographics; whether they had a first degree relative with a risk factor for CVD; ability to take all their medications as prescribed within the past 30 days; percent of time they took their medication exactly as prescribed on a slider scale from 0 to 100, with anchors at 0%, 50–100% as well as a 6-point scale from “very poor” to “excellent.” These items were inclusive of all medications the participant took, regardless of reason.
During the interviews, participants were asked about CVD risk reduction behavior, how their HIV providers engaged them in discussing and managing CVD, their perceptions of their CVD risk, CVD medication adherence, and barriers and facilitators of adhering to CVD prevention.19
Data Analysis
Since the factors associated with CVD medication adherence for PLWH who have achieved viral suppression had not been described previously, transcripts were analyzed using content analysis-inductive approach31,32 guided by principles drawn from grounded theory (ie, open coding and constant comparison) to identify factors associated with hypertension and hyperlipidemia medication adherence.33 Two authors (CM and IS) first reviewed 7 transcripts in their entirety. In open coding, they independently segmented text into as many codes as possible using Dedoose software.34 The two analysts met to compare codes generated and discussed any discrepancies. In axial coding, they developed categories by combining conceptually related codes. These categories were applied to remaining transcripts, allowing for new emergent categories. During this process, the coders routinely met to discuss their emerging categories in order to cross-check their initial interpretations of the data. Through constant comparison and peer discussion, theoretical properties of the categories emerged, such as the relationships between categories and the conditions in which existing categories explained the research question. Additionally, the two analysts wrote brief memos, updated the codebook and summarized the dimensions of the categories. Another author (HB) was consulted to review any discrepancies between the analysts. As the properties of the categories were further developed and the relationships between categories made clearer, inter-related categories were linked under unifying concepts or themes that explained the multi-level factors associated with medication adherence.
This study was covered under the EXTRA-CVD trial protocol that was IRB approved at UH Cleveland Medical Center (Protocol # 03–18-16), with reliant review at all participating sites in accordance with the NIH single IRB policy (Duke IRB Protocol #00092437; MetroHealth IRB Protocol #00000685).35 The study was also registered at clinicaltrials.gov (NCT03643705). This study was conducted in accordance with the principles of the Declaration of Helsinki.
Results
Fifty-one individuals participated in this study. Thirty-seven participated in 6 FGDs and 14 were in individual semi structured interviews. Mean age was 57 years (SD: 7.8); most were African American (n= 31) and majority were male (n=34). Forty-eight (94%) were on hypertension medications, cholesterol medications, or both. Thirty-seven had public health insurance and 31 rated their ability to take medications as prescribed as “excellent.” Table 1.
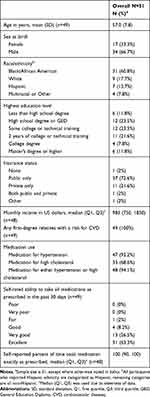 |
Table 1 Demographics of PWH at Duke, University Hospitals and Metro Health |
We identified three main themes: 1) CVD prevention preferences and practices; 2) Impact of long-term HIV care on adherence to CVD medication; and, 3) Motivating factors for adoption and adherence to heart healthy behaviors. Table 2.
 |
Table 2 Summary of Qualitative Themes |
Theme 1: CVD Prevention Preferences and Practices
Depending on the type of CVD risk factor, some participants agreed and followed their healthcare providers’ recommendations while others disagreed with the recommendations and described their preferred approach to CVD prevention. There were four subthemes identified including: Discordance between providers’ recommendations and PLWH preferred strategy for CVD prevention; CVD prevention knowledge inconsistent with PLWH CVD risk behavior; Experienced or perceived medication side effects; and perceived symptoms as cues to taking medication.
Discordance Between Providers’ Recommendations and PLWH Preferred Strategy for CVD Prevention
Participants reported that their providers were more likely to prescribe medication than provide counseling for lifestyle modification even though they would have preferred the latter. For lifestyle modification counseling, a majority of participants reported that their provider recommendations did not come with clear instructions, benchmarks or directions. Others also emphasized a dissatisfaction with more prescription medication and questioned providers’ motivations in prescribing these medications. For example, a FGD participant at UH said,
My concern is I don’t wanna take any more medications than I absolutely, positively have to. And so often you go to a doctor, and you walk outta there with two or three possible prescriptions; and I’m like, ‘No, I don’t want it, I don’t want any more prescriptions!.’
Another FGD participant from UH said,
Well, the idea of taking more, the fact that it makes me question the motivation for some of these prescriptions, and it’s like there’s evidence that the more medications that you take, the more risks that you have for heart disease and other stuff!.
CVD Prevention Knowledge Inconsistent with PLWH CVD Risk Behavior
An overwhelming majority of participants were generally knowledgeable about CVD-risk reduction strategies from personal experience, research, or provider recommendations, though they exhibited a divergence towards acting on and applying this knowledge to engage in CVD prevention. For example, participants were aware of the CVD risk associated with the use of tobacco products but were unable to achieve smoking cessation due to other perceived benefits. One FGD participant at UH said,
That’s one of my bad habits, smoking. My doctor’s already said, ‘You need to quit smoking.’ Smoking, to me, is not just a habit but it’s something I enjoy … When I get stressed out, that’s when I smoke.
Some participants intentionally modified the frequency of taking their CVD-related medications. Participants often noted optimal adherence to ART citing its importance in their overall life and health, though expressed having a more relaxed attitude towards taking CVD-related medication as prescribed. For example, a FGD participant at MH said,
Because you know [ART] are keeping us alive. The other pills, we can kind of play with. We know we need to take them but I’m pretty sure, with me, the high blood pressure, take it – the cholesterol, I play with it. I might take it once a week, twice a week.
Experienced or Perceived Medication Side Effects
A majority of participants cited medication side effects as a reason for not adhering to CVD-prevention medication. Side effects experienced by patients in the past due to medication included allergic reactions, muscle and joint pains, and headaches. For example, a FGD participant at MH said,
The diuretics, a lot of times, they pull a lot of your fluids out and they cause you to cramp.
Other participants also discussed medication interactions as the source of perceived side effects. This was especially heard from patients who were taking cholesterol medications. For example, FGD participant at MH said,
The only thing I really worry about is the statin is part of your liver and the HIV meds are hard on your liver. I just worry about the liver. I’d rather have a heart attack than a liver problem.
Perceived Symptoms as Cues to Taking Medication
Some participants reported that they had experienced a physical symptom or discomfort that they perceived was related to their cardiovascular system before they took their CVD-preventative medication. These symptoms included swelling, increased heart rate, lightheadedness and distorted vision. These symptoms were triggers for them to take medication, but they did not continue taking medication when the symptoms subsided. For example, FGD participant at UH said,
I haven’t needed any blood pressure [medication] but … – last night it looked like my legs and feet were swollen, so I said, ‘I better try to take this high blood pressure medication,’ so I started back taking that.
Another FGD participant at MH said,
But I skip my high blood pressure as well. Unless I can feel my heart beating real hard, I don’t take it.
Theme 2: Impact of Long-Term HIV Care on Adherence to CVD Medication
An overwhelming majority of participants used their experience with ART medication taking as reference points of guidance for CVD prevention. For some, there were similarities between the reasons and process behind ART adherence and CVD medications. For example, a participant at DUMC said,
Like I said, it was like with the HIV I was like kind of in denial and then I started taking my medications and then I seen the results of taking it and the results of not taking it. So, I mean, when I found out about the heart it was kind of hard for me to accept that. But then I got to thinking, well if my HIV was – HIV medication was working, my heart medication will work.
Many participants described the differences they perceived between the outcomes of ART and CVD medication adherence. While ART adherence offered short-term outcomes in the form of reduction of viral load, the outcomes for CVD prevention medications adherence were not the same and this led to nonadherence. For example, FGD participant at MH said,
It’s easier to remember to take your HIV meds because you know what’s going to happen if you don’t. But the blood pressure, the cholesterol … I’ll take it today. I ain’t taking it tomorrow. … I don’t think we really see the now from yesterday’s report when I got my blood pressure checked.
When reflecting on their HIV care especially with respect to the healthcare providers who had supported them to the point of achieving viral suppression, some participants observed that as a result of achieving viral suppression, their providers were not as committed to them compared to when they were first diagnosed with HIV. Participants reported that most of the focus was on newly diagnosed patients. As one FGD participant at UH said,
“[Doctors] have a tendency to push you aside as you get older, and you get more progressive in being undetectable, and you’re an older patient. And they kinda shine on the new ones coming in because there’s this one over here who’s just getting started”.
Theme 3: Motivating Factors for Adoption and Adherence to Heart Healthy Behaviors
A majority of participants discussed reasons for engaging in CVD risk reduction including medication adherence. For some participants, engaging in heart healthy behaviors was for their own internal sense of satisfaction. For example, some expressed their desire to stay alive as long as possible, or the need to avoid further significant health problems and acknowledgment that taking medication could prevent further health complications. For example, one FGD participant at DUMC said;
I haven’t been told I have heart disease, per se, but blood pressure and cholesterol can lead to heart disease. That’s why I take my medication on blood pressure and cholesterol.
Another participant from DUMC said;
Mainly I just want to live and have a healthy – live a good life and knowing that that’s a part of your living is to have a happy, healthy heart. So whatever I have to do, I’ll do to make sure that I can be here a while.
For other participants, motivation for engaging in heart healthy behaviors came from social connections through family, friends, and healthcare providers. Familial experiences with adverse CVD-related health events were a leading motivator for CVD medication adherence. Similarly, participants were motivated to live longer to care for their children and grandchildren. Some participants also discussed the support they received from their healthcare providers, especially those that had established long-term relationships through the course of their HIV management. For example, one participant from DUMC said,
I want to be here to see my grandkids off to school, off to college. I wanna be able to get out and play with my grandkids. So, I got to do something to make my life better in order for me to be able to do this.
Another FGD participant from UH said,
Every time I see my doctor she emphasizes – I think she emphasizes [my CVD-risk] more than the HIV.
Apart from relationship-based motivators, some participants described memory aids and medication organizers as reminder tools that helped with the timing of taking their medication and helped to streamline the process of taking multiple medications.
Discussions
Through interviews with treatment-experienced PLWH who were virally suppressed, we explored factors that were related to adherence to medications used to reduce the risk of CVD. In order to achieve CVD prevention goals, participants described issues related to their healthcare providers' recommendation, self-management, and the impact of their long-term experience with HIV care.
In this study, participants preferred approaches to CVD prevention that were not aligned with their healthcare providers' recommendations. Misalignment of strategies for CVD prevention maybe reflect inadequate discussions of patient preferences and healthcare providers' rationale for recommended therapies.36 Optimal patient–provider communication has been shown to increase patients' understanding of the logic of treatment plans and ability to obtain needed information in order to increase participation in care.37 Future studies should focus on developing strategies to enhance PLWH-provider communication on CVD prevention and particularly CVD medication adherence.
Participants in this study expressed adequate knowledge of CVD prevention including optimal CVD medication adherence. However, their CVD prevention knowledge and their behaviors toward CVD prevention were not consistent. Discordance between CVD risk perception and CVD prevention knowledge among PLWH has been documented in previous studies.22,38 Future interventions should focus on individualizing CVD risk for PLWH and development of strategies to overcome barriers in engaging in CVD prevention behaviors.23
Some participants in this study intentionally adjusted the frequency of taking their CVD medications. This occurred when they experienced physical symptoms which they attributed to CVD, when they perceived or experienced side effects, and when they compared the importance of ART relative to CVD medications. Previous studies have found that patients may not take their CVD medications as prescribed because of their beliefs about the efficacy of the medication, dosing frequency, side effects and because they do not experience any adverse symptoms.39–42 Given that study participants had developed a habit of ART medication taking with different side effects, dosage frequency, and adverse HIV infection symptoms, it is critical for future research to evaluate and quantify the relative influence of CVD medication attributes on intentional CVD medication nonadherence for PLWH who have achieved viral suppression.43
Participants described the differences in timing and certainty of benefits between ART and CVD medications. While they linked short-term outcomes of ART adherence to reduction in viral load, participants did not associate CVD medications adherence to reduction of CVD risk or severe CVD events. Compared to other conditions, participants in this study reported that their HIV self-management was a major priority. This is consistent with the idea that patients are more adherent to treatments for chronic conditions that they perceive to be more severe.44 However, since they had achieved viral suppression, there may be an opportunity to leverage their interactions with their healthcare providers to support the management of other comorbidities. For example, since HIV care guidelines recommend that HIV providers monitor and support ART adherence,20,21 future research should focus on adapting and tailoring these evidence-based practices to support CVD medication adherence for PLWH who have achieved viral suppression.
These findings should be interpreted in light of the study’s limitations. Even though participants were reassured that their responses would have no impact on their clinical care to limit social desirability bias, they may still have been inclined to respond in a positive manner. The use of viral load suppression may be an imperfect indicator for ART adherence since PLWH who experience HIV drug resistance may be highly adherent, yet have detectable viral load.45 Another limitation of this study was the lack of including healthcare providers’ perspective on CVD medication adherence. Finally, the perspectives presented in this study were from PLWH at 3 large academic medical centers and may not be generalizable to other health systems.
Notwithstanding limitations, this study contributes to a growing body of literature on CVD prevention among PLWH. To our knowledge, this is the first study to characterize contextual factors associated with adherence to CVD medications in treatment experienced PLWH who were virally suppressed. Specifically, our finding suggests that future research should focus on developing interventions to enhance patient–provider communication in order to elicit beliefs, concerns and preferences for CVD prevention strategies. Future research should also seek to leverage and adapt established evidence-based practices in HIV care to support CVD medication adherence in order to meet CVD treatment guidelines.
Data Sharing Statement
Corresponding author has access to the data and is able to provide them upon request.
Acknowledgments
We would like to thank the study participants for their time and willingness to provide this information.
Disclosure
Dr. Bosworth reports research grants from Sanofi, Novo Nordisk, and Improved Patient Outcomes, Otsuka as well as consulting from Novartis, Abbott, and Sanofi; also personal fees from Preventric Diagnostics outside the submitted work. Dr. Longenecker reports research grants from Gilead Sciences and served on an advisory board for Esperion Therapeutics and grants from Medtronic Philanthropy, outside the submitted work. Dr Kelley Jones reports grants from National Institutes of Health, during the conduct of the study. The remaining authors declare that they have no conflicts or competing interests.
References
1. Hall HI, Brooks JT, Mermin J. Can the United States achieve 90–90–90? Curr Opin HIV AIDS. 2019;14(6:464–470. doi:10.1097/coh.0000000000000578.
2. UNAIDS. 90-90-90 treatment for all; 2020. Available from: https://www.unaids.org/en/resources/909090.
3. Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2012;26:335–343. doi:10.1097/QAD.0b013e32834dcec9
4. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi:10.1001/jamainternmed.2013.3728
5. Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9:54–62. doi:10.1097/coh.0000000000000015
6. De Socio GV, Ricci E, Maggi P, et al. Prevalence, awareness, treatment, and control rate of hypertension in HIV-infected patients: the HIV-HY study. Am J Hypertens. 2013;27:222–228. doi:10.1093/ajh/hpt182
7. Burkholder GA, Tamhane AR, Safford MM, et al. Racial disparities in the prevalence and control of hypertension among a cohort of HIV-infected patients in the southeastern United States. PLoS One. 2018;13:e0194940–e0194940. doi:10.1371/journal.pone.0194940
8. Clement ME, Park LP, Navar AM, et al. Statin utilization and recommendations among HIV- and HCV-infected Veterans: a cohort study. Clin Infect Dis. 2016;63:407–413. doi:10.1093/cid/ciw289
9. Burkholder GA, Tamhane AR, Salinas JL, et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55:1550–1557. doi:10.1093/cid/cis752
10. Monroe AK, Rowe TL, Moore RD, et al. Medication adherence in HIV-positive patients with diabetes or hypertension: a focus group study. BMC Health Serv Res. 2013;13(1):488. doi:10.1186/1472-6963-13-488
11. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi:10.1016/j.ahj.2007.12.011
12. Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi:10.1093/cid/ciq243
13. Bangsberg DR. Monitoring adherence to HIV antiretroviral therapy in routine clinical practice: the past, the present, and the future. AIDS Behav. 2006;10(3:249–251. doi:10.1007/s10461-006-9121-7.
14. Croome N, Ahluwalia M, Hughes LD, et al. Patient-reported barriers and facilitators to antiretroviral adherence in sub-Saharan Africa. AIDS. 2017;31(7):995–1007. doi:10.1097/QAD.0000000000001416
15. Langness J, Cook PF, Gill J, et al. Comparison of adherence rates for antiretroviral, blood pressure, or mental health medications for HIV-positive patients at an academic medical center outpatient pharmacy. J Managed Care Pharm. 2014;20(8):809–814. doi:10.18553/jmcp.2014.20.8.809
16. Batchelder AW, Gonzalez JS, Berg KM. Differential medication nonadherence and illness beliefs in co-morbid HIV and type 2 diabetes. J Behav Med. 2014;37:266–275. doi:10.1007/s10865-012-9486-1
17. Monroe AK, Pena JS, Moore RD, et al. Randomized controlled trial of a pictorial aid intervention for medication adherence among HIV-positive patients with comorbid diabetes or hypertension. AIDS Care. 2018;30:199–206. doi:10.1080/09540121.2017.1360993
18. Weiss JJ, Konstantinidis I, Boueilh A, et al. Illness perceptions, medication beliefs, and adherence to antiretrovirals and medications for comorbidities in adults with HIV infection and hypertension or chronic kidney disease. J Acquired Immune Deficiency Syndromes. 2016;73:403–410. doi:10.1097/qai.0000000000001075
19. Okeke NL, Webel AR, Bosworth HB, et al. Rationale and design of a nurse-led intervention to extend the HIV treatment cascade for cardiovascular disease prevention trial (EXTRA-CVD). Am Heart J. 2019;216:91–101. doi:10.1016/j.ahj.2019.07.005
20. Weiser J, Beer L, Brooks JT, et al. Delivery of HIV antiretroviral therapy adherence support services by HIV care providers in the United States, 2013 to 2014. J Int Assoc Provid AIDS Care. 2017;16:624–631. doi:10.1177/2325957417729754
21. Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International association of physicians in AIDS care panel. Ann Intern Med. 2012;156:
22. Cioe PA, Crawford SL, Stein MD. Cardiovascular risk-factor knowledge and risk perception among HIV-infected adults. J Assoc Nurses AIDS Care. 2014;25:60–69. doi:10.1016/j.jana.2013.07.006
23. Webel A, Davey CH, Schexnayder J, et al. The impact of perceived cardiovascular risk on cardiovascular disease prevention behaviors in people with and without HIV infection. J Acquired Immune Deficiency Syndromes. 2020. doi:10.1097/qai.0000000000002290
24. Elliott RA, Ross-Degnan D, Adams AS, et al. Strategies for coping in a complex world: adherence behavior among older adults with chronic illness. J Gen Intern Med. 2007;22:805–810. doi:10.1007/s11606-007-0193-5
25. Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the necessity-concerns framework. J Psychosom Res. 2008;64:41–46. doi:10.1016/j.jpsychores.2007.05.004
26. Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22:313–356. doi:10.1007/bf02850081
27. NHLBI. Implementation research to develop interventions for people living with HIV (preclude) (U01); 2017. Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-HL-18-007.html.
28. Gamble-George JC, Longenecker CT, Webel AR, et al. Implementation research to develop interventions for people living with HIV (the preclude consortium): combatting chronic disease comorbidities in HIV populations through implementation research. Prog Cardiovasc Dis. 2020. doi:10.1016/j.pcad.2020.03.006
29. Aifah A, Okeke NL, Rentrope CR, et al. Use of a human-centered design approach to adapt a nurse-led cardiovascular disease prevention intervention in HIV clinics. Prog Cardiovasc Dis. 2020. doi:10.1016/j.pcad.2020.02.013
30. Palinkas LA, Horwitz SM, Green CA, et al. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42:533–544. doi:10.1007/s10488-013-0528-y
31. Schreier M. Qualitative Content Analysis in Practice. London: Sage; 2012.
32. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. doi:10.1177/1049732305276687
33. Glaser B, Strauss A. The Discovery of Grounded Theory. London: Weidenfield & Nicolson.; 1967.
34. software D. Web application for managing, analyzing, and presenting qualitative and mixed method research data; 2019. Available from: https://www.dedoose.com/.
35. Final NIH policy on the use of a single institutional review board for multi-site research. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-094.html.
36. Roter DL. Patient participation in the patient-provider interaction: the effects of patient question asking on the quality of interaction, satisfaction and compliance. Health Educ Monogr. 1977;5:281–315. doi:10.1177/109019817700500402
37. Alegría M, Sribney W, Perez D, et al. The role of patient activation on patient-provider communication and quality of care for US and foreign born Latino patients. J Gen Intern Med. 2009;24(Suppl 3):534–541. doi:10.1007/s11606-009-1074-x
38. Temu TM, Kirui N, Wanjalla C, et al. Cardiovascular health knowledge and preventive practices in people living with HIV in Kenya. BMC Infect Dis. 2015;15:421. doi:10.1186/s12879-015-1157-8
39. Ogedegbe G, Harrison M, Robbins L, et al. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis. 2004;14:3–12.
40. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
41. Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39:1151–1162. doi:10.1016/s0005-7967(00)00091-7
42. Benson J, Britten N. Patients’ decisions about whether or not to take antihypertensive drugs: qualitative study. BMJ. 2002;325:873. doi:10.1136/bmj.325.7369.873
43. Lamiraud K, Geoffard PY. Therapeutic non-adherence: a rational behavior revealing patient preferences? Health Econ. 2007;16:1185–1204. doi:10.1002/hec.1214
44. AJ C. Patient Adherence to Medical Treatment Regimens: Bridging the Gap Between Behavioral Science and Biomedicine. New Haven, CT: Yale University Press; 2004.
45. WHO. Global action plan on HIV drug resistance 2017–2021: 2018 progress report; 2018. Available from: https://www.who.int/hiv/pub/drugresistance/gap-hivdr-progress2018/en/.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
