Back to Journals » Vascular Health and Risk Management » Volume 15
When to withhold oral anticoagulation in atrial fibrillation – an overview of frequent clinical discussion topics
Authors Seelig J , Pisters R, Hemels ME, Huisman MV , ten Cate H, Alings M
Received 28 June 2019
Accepted for publication 28 August 2019
Published 17 September 2019 Volume 2019:15 Pages 399—408
DOI https://doi.org/10.2147/VHRM.S187656
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Magnus Bäck
Jaap Seelig,1 Ron Pisters,1 Martin E Hemels,1,2 Menno V Huisman,3 Hugo ten Cate,4,5 Marco Alings6
1Department of Cardiology, Rijnstate Hospital, Arnhem, The Netherlands; 2Department of Cardiology, Radboud University Medical Center, Nijmegen, The Netherlands; 3Department of Thrombosis and Hemostasis, Leiden University Medical Center, Leiden, The Netherlands; 4Department of Internal Medicine, Cardiovascular Research Institute Maastricht, Maastricht, The Netherlands; 5Anticoagulation Clinic Maastricht, Maastricht, The Netherlands; 6Department of Cardiology, Amphia Hospital, Breda, The Netherlands
Correspondence: Martin E Hemels
Department of Cardiology, Rijnstate Hospital, Wagnerlaan 55, Arnhem, AD 6815, The Netherlands
Tel +31 64 126 8279
Email [email protected]
Abstract: Stroke prevention with oral anticoagulants in patients with atrial fibrillation predisposes for bleeding. As a result, in select patient groups anticoagulation is withheld because of a perceived unfavorable risk-benefit ratio. Reasons for withholding anticoagulation can vary greatly between clinicians, often leading to discussion in daily clinical practice on the best approach. To guide clinical decision-making, we have reviewed available evidence on the most frequently reported reasons for withholding anticoagulation: previous bleeding, frailty and age, and an overall high bleeding risk.
Keywords: hemorrhage, frail elderly, age, anticoagulants, atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with heart failure, mortality, and ischemic stroke.1 Stroke prevention with anticoagulants predisposes AF patients for bleeding. As a result, in select patient groups anticoagulation is withheld because of a perceived unfavorable risk-benefit ratio.2–4 However, these choices cannot always be justified based on available evidence.
With an aging population, AF is becoming even more prevalent. Decision-making concerning withholding or (re-)initiating anticoagulation is a growing challenge for physicians.5 In parallel, AF patients are likely to have more comorbidities, and consequently are at higher risk of both stroke and bleeding.6,7 Increasingly common factors such as previous bleeding, frailty, and an overall high bleeding risk are amongst the most frequently reported reasons for withholding anticoagulation.2,8
In this review, evidence and gaps in the current knowledge of the benefits and risks of anticoagulation in AF are discussed, with a focus on high bleeding risk, previous bleeding, and frailty.
Anticoagulation and high bleeding risk
Due to an increase in comorbidities, patients with AF will more often be at an increased bleeding risk. Decision-making regarding anticoagulation can be particularly challenging in these patients, especially when both stroke and bleeding risk are high.2,9 Oral anticoagulants (OAC) used for stroke prevention in AF are vitamin K antagonists (VKA), such as warfarin, or the non-vitamin K oral anticoagulants (NOAC) dabigatran, rivaroxaban, apixaban, and edoxaban.1 As described below, available evidence suggests the clinical benefit of anticoagulation is higher than is often perceived.
In patients with a CHA2DS2-VASc stroke risk score of ≥2 (male) or ≥3 (female), anticoagulation is indicated by current AF-guidelines, and it should be considered in patients with a CHA2DS2-VASc of one (male) or two (female).1,10 In the GARFIELD-AF registry, 30% of the patients with CHA2DS2-VASc ≥2 were not treated with oral anticoagulation (OAC).2 The strongest predictors for withholding OAC were concomitant antiplatelet therapy (odds ratio (OR) 15.0 [95% confidence interval (CI) 14.1–15.8]) and a history of bleeding (OR 2.5 [95% CI 2.2–3.0]).2 Compared to patients on OAC, patients withheld from OAC had an increased risk of all-cause mortality (5.3% vs 3.9%, p<0.001), ischemic stroke or systemic embolism (1.6% vs 1.1%, p<0.001), but a decreased risk of major bleeding (0.5% vs 0.8%, p<0.001). Data from the NCDR PINNACLE, a prospective United States-based registry focusing on quality-improvement, showed an even higher proportion of 42% of the patients with CHA2DS2-VASc ≥2 not treated with OAC.11 In a multivariable model, lower CHA2DS2-VASc scores and higher HAS-BLED scores were both associated with OAC non-prescription.11,12 Similar observations were derived from German insurance databases, where 40.5–48.7% of AF patients were classified as “definite OAC under-use”.13
A Spanish, prospective, observational study in 1361 AF patients with stable anticoagulation control with VKA observed an annual cessation rate of 1.54%/year.14 In 80% of them, OAC was stopped because of a major bleeding or at the health care providers’ discretion. Cox regression analysis showed that the occurrence of major bleeding, heart failure, cancer, or renal impairment during follow-up was all independently associated with early OAC cessation. The authors conclude that many factors associated with bleeding also predispose to OAC cessation. OAC cessation, however, was associated with an increase in ischemic stroke (Hazard Ratio (HR) 1.85 [95% CI 1.17–2.94]) and all-cause mortality (HR 1.30 [95% CI 1.02–1.67]).
In a Dutch retrospective study, 45 out of 89 patients (51%) with a history of AF and admitted with a first ischemic stroke were insufficiently anticoagulated prior to their stroke.15 Taken into consideration the increased occurrence of intracranial hemorrhage (ICH) as a result of increased OAC use, strict adherence to AF-guidelines could have prevented an estimated 20 out of 89 (22%) ischemic strokes. In the Registry of the Canadian Stroke Network, 90% of the 597 patients admitted with ischemic stroke and known AF with increased stroke risk were not therapeutically anticoagulated, or not anticoagulated at all.16 These data demonstrate the perceived difficulties of real-world anticoagulation management, and the importance of good anticoagulation control. Thus, it is of utmost importance to know in which high-risk patient OAC can still safely be prescribed.
To reduce AF-related events, more frequent monitoring of high bleeding risk patients for presence of lower hemoglobin levels and/or active (minor) bleeding, changes in renal function, therapy adherence, and modifiable stroke and/or bleeding risk factors, such as hypertension or alcohol abuse, are likely to result in safer OAC use.1 The use of accurate bleeding prediction models could diminish under- or overtreatment with OAC in AF. Unfortunately, bleeding prediction has been shown difficult. Over the years, multiple bleeding risk scores, such as the HAS-BLED, ATRIA, GARFIELD-AF risk tool, or HEMORR2HAGES, have been developed to help clinical decision-making.12,17–19 However, these risk scores have only moderate predictive accuracy, especially in the elderly.20 Further complicating matters is the fact that an increased bleeding risk is correlated with an increased stroke risk, since strong bleeding risk factors such as increasing age, vascular disease, or prior stroke are the most important risk factors for ischemic stroke.21–23
In an effort to improve the prediction of bleeding, the ABC-bleeding risk score (Age, Biomarkers (high-sensitive troponin T, GDF-15, and hemoglobin), Clinical history) has been developed, which had a only slightly higher c-statistic (0.68 [95% CI 0.66–0.70]) than the HAS-BLED (0.61 [95% CI 0.59–0.63]) or the ORBIT score (0.65 [95% CI 0.62–0.67]).24,25 Since the ABC-bleeding risk scores require the assessment of GDF-15, a cytokine which is upregulated in conditions of systemic inflammation or oxidative stress, the score is currently not implemented in daily clinical practice.26 An interesting aspect of GDF-15 is that increased levels are not associated with an increased risk of stroke, while it is strongly predictive of bleeding.27 It will be interesting to see if GDF-15, and perhaps other biomarkers, can guide clinicians with decision-making on anticoagulation (re-)initiation.
Management of patients with a high bleeding risk
Several studies have focused on the question whether AF patients with a high bleeding risk are better off when OAC is withheld. However, based on current literature, anticoagulation is especially important in patients at a very high stroke risk, regardless of HAS-BLED scores.
To assess the benefit of OAC in AF, a net clinical benefit (NCB) using the method of Singer et al, is often calculated: NCB = (ischemic strokeoff OAC – ischemic strokeon OAC) – 1.5 * (intracranial hemorrhage rateon OAC – intracranial hemorrhageoff OAC), in which the factor −1.5 is to compensate for the often greater clinical impact of intracranial bleeding.28 A NCB >0 indicates that the benefit of less ischemic stroke with OAC outweighs the risk of ICH. A NCB for warfarin was calculated for each CHA2DS2-VASc score in a large Swedish study of 182,678 patients with AF.29 For CHA2DS2-VASc 0 (ie, male without risk factor), there was no NCB of warfarin treatment (NCB 0.0 [95% CI −0.1–0.1]). In patients with CHA2DS2-VASc ≥1, a positive NCB was observed. The NCB was highest in the patients at the highest risk of stroke, regardless of HAS-BLED scores. Similar results were seen in a large Danish study, where VKA (with or without aspirin) vs no antithrombotic treatment had a positive NCB in patients with a CHA2DS2-VASc ≥2.30 The NCB with VKA was greater in patients with HAS-BLED ≥3 vs HAS-BLED <3 on VKA (NCB 2.21 [95% CI 1.93–2.50] vs NCB 1.19 [95% CI 1.07–1.32]), and VKA + aspirin (NCB 1.97 [95% CI 1.62–2.32] vs 0.81 [95% CI 0.56–1.07]), respectively.30 High bleeding risk and high ischemic stroke risk are positively correlated. In individuals with a high bleeding risk, the risk reduction of ischemic stroke with OAC supersedes the small increase in the risk of ICH.30 In a different Danish study, the NCB was calculated for warfarin, dabigatran, rivaroxaban, and apixaban vs no anticoagulation.31 A positive NCB was observed in both VKA or NOAC treated patients with CHA2DS2-VASc ≥2. The NCB was even greater in the subgroup of patients with HAS-BLED ≥3, irrespective of treatment with VKA or NOAC.
However, there are some limitations to these studies. Confounding by indication could have played an important role in these analyses, as patients on different anticoagulation strategies may differ in terms of stroke and bleeding risk, possibly overestimating NCB counts.29,30,32 Furthermore, non-intracranial major or non-major clinically relevant bleeding is not a part of the used NCB formula, although they often play an important role in clinical decision-making. However, despite these limitations, the evidence for prescribing OAC despite high bleeding risk remains strong.
The treatment of high-risk patients should not only focus on the antithrombotic strategy, but also on reducing the risk of bleeding. A flowchart to help reduce bleeding risk is shown in Table 1. Although many important bleeding risk factors are non-modifiable, treatment should focus on currently known modifiable risk factors for bleeding, including hypertension, labile international normalized ratio (INR), concomitant drug-use, including over the counter drugs like non-steroidal anti-inflammatory drugs (NSAID), and alcohol abuse.1 A systolic blood pressure of >140 mmHg is associated with an increased bleeding risk, and adequate blood pressure control is therefore recommended to reduce bleeding risk.1,33 In patients with labile INR, switching to a NOAC should be considered.1 The concomitant use of antiplatelet drugs, NSAIDs, and drugs inhibiting OAC metabolism can strongly increase bleeding risk, and therefore their use should be avoided if possible.34–39 Drugs affecting metabolism and increasing bleeding risk in NOACs are primarily P-gp and CYP3A4 inhibitors, and in VKA primarily CYP2C9 and CYP3A4 inhibitors.40 Alcohol abuse (ie, ≥8 units/week) shows conflicting results regarding bleeding risk.12,21,41 However, suspected heavy drinking is an important reason for clinicians to withhold OAC.2 Since alcohol abuse is also associated with an increased risk of stroke in AF patients and medication non-adherence, addressing a patients’ alcohol usage is nonetheless an important element of the management of AF patients.21,33,42 However, there is no substantial evidence to withhold OAC in alcohol abusers without significant hepatic impairment.
 |
Table 1 Flowchart to help reduce bleeding risk in high-risk AF patients |
In patients at risk for gastrointestinal (GI) bleeding, proton pump inhibitors (PPI) can be prescribed to reduce bleeding risk. In a retrospective cohort study in Medicare beneficiaries treated with either apixaban, rivaroxaban, dabigatran, or warfarin, PPI co-therapy was associated with a lower risk of hospitalization for upper GI-bleeding.43,44 Only in patients categorized in the lowest GI-bleeding risk decile, no protective effect of PPI therapy was observed.44 Current guidelines recommend that in patients with an elevated GI-bleeding risk PPI should be considered, specifically in patients with a history of GI-bleeding or ulcer, malignancy, or concomitant antiplatelet therapy.9
Combined use of antiplatelet drugs and anticoagulants strongly increases bleeding risk, and is a frequently observed reason for withholding OAC.2,11,38,39 In comparison to VKA monotherapy, single antiplatelet therapy in addition to VKA or NOAC had a HR for major bleeding of 1.82 (95% CI 1.76–1.89) and 1.28 (95% CI 1.13–1.44), respectively.39 Concomitant dual antiplatelet therapy with a NOAC or VKA was associated with a 1.2–3.9-fold and 2.4–5.4-fold higher risk of major bleeding, respectively.39 In a meta-analysis only including patients on low-dose aspirin from the pivotal NOAC trials, rates of stroke or systemic embolism were lower with NOACs (HR 0.78 [95% CI 0.67–0.91]), in comparison to VKAs.45 The rates of major bleeding were similar (HR 0.83 [95% CI 0.69–1.01]). The rates of ICH were lower (HR 0.38 [95% CI 0.26–0.56]). The results from these studies suggest NOACs may be both safer and more effective than VKAs in patients on concomitant antiplatelet therapy. There have only been head-to-head studies between NOAC or VKA and concomitant antiplatelet use in patients after a recent percutaneous coronary intervention (PCI). The WOEST, PIONEER-AF PCI, RE-DUAL PCI, and AUGUSTUS trials all showed less bleeding with dual therapy (NOAC or VKA with a P2Y12 inhibitor) compared to triple therapy (dual therapy plus aspirin), with no significant difference in efficacy.46–49 However, these individual trials were not powered for the efficacy endpoints. A meta-analysis of the WOEST, PIONEER-AF PCI, and RE-DUAL PCI trials suggests the incidence of ischemic events with dual therapy vs triple therapy is equally low.50 The current guidelines provide a good overview and recommend an individualized approach of triple therapy duration based on bleeding and atherothrombotic risk with the aim to keep triple therapy duration as short as possible.9 The optimal antithrombotic regimen beyond 1 year remains undefined in these patients, but will also importantly depend on risk factors for bleeding.
Although the far majority of AF patients with increased stroke risk will benefit from OAC, the risks can outweigh the benefits in some patients (e.g. patients with a non-treatable cause of (recurrent) major bleeding).9 In these patients, a left atrial appendage (LAA) occluding device or surgical LAA occlusion may be considered according to the current guidelines (class of recommendation IIb, level of evidence C).1 The ASAP study included AF patients with CHADS2≥1 and a contraindication for OAC (in 93%: history or tendency of bleeding), in which a LAA occluding device (Watchman) was implanted.51 After implantation, patients received 6 months of clopidogrel or ticlopidine, and lifelong aspirin. Ischemic stroke rate (1.7%/year) was significantly lower than expected based on the predicted stroke risk of the cohort (7.3%/year). The EWOLUTION trial was a nonrandomized, prospective cohort study in which 1020 patients with a Watchman device were enrolled.52 In this study, 72.2% of the patients had a reported contraindication for OAC. The observed ischemic stroke rate was 1.3 (95% CI 0.8–1.9) per 100 patient-years, which was 83% lower than predicted based on historical data using the CHA2DS2-VASc score. In patients with a previous major bleeding specifically, the risk reduction was similar at 85% (observed risk: 1.2 [95% CI 0.4–2.5]). Unfortunately, there are no randomized data available on LAA occlusion in patients with a contraindication for OAC. However, based on available evidence, LAA occlusion seems to be a safe and effective strategy in patients with a contraindication for OAC.53
(Re-)initiation of anticoagulation after bleeding
One of the most frequently reported reasons to withhold anticoagulation is a history of bleeding, especially a history of ICH.2,3,14,54 Nevertheless, available data indicate a benefit of OAC resumption in patients with AF and a prior major bleeding.
Recently, a meta-analysis was published comprising 5685 AF patients that experienced a major bleeding.55 In comparison with the withholding of OAC after the index bleeding, OAC restarters had a 46% relative risk (RR) reduction of any thromboembolic event, and a 10.8% absolute risk reduction for all-cause mortality.55 Restarting OAC was associated with an increased risk of a recurrent major bleeding (OR 1.85), although no increased risk of recurrence of the index bleeding event (ie, ICH or GI-bleeding) was observed. NCB analysis, including thromboembolic events, major bleeding, and all-cause mortality, demonstrated that restarting OAC was associated with a clinical advantage (NCB 0.11 [95% CI 0.09–0.14]).55 An important limitation, however, is that all included studies were observational, and selection bias in these studies is possible.56 Furthermore, only one study included patients with a history of “any major bleeding”, whereas the other six studies solely focused on either ICH or GI-bleeding. Therefore, these results should be interpreted with caution.
A retrospective analysis of insurance data showed a lower combined risk of ischemic stroke and all-cause mortality with the resumption of warfarin (HR 0.76 [95% CI 0.59–0.97]) or dabigatran (HR 0.66 [95% CI 0.44–0.99]).57 In comparison to no re-initiation, warfarin resumption had an increased risk of major bleeding (HR 1.56 [95% CI 1.10–2.22]), whereas dabigatran resumption was not significantly associated with major bleeding (HR 0.65 [95% CI 0.32–1.33]). The risk-benefit ratio was, therefore, higher for dabigatran than for warfarin. Careful interpretation of these results is warranted, as differences in time to resumption, dosing (75 mg dose was initiated in 9.6% of the dabigatran users), switching, and discontinuation between warfarin or dabigatran treated patients could have strongly influenced outcomes.56
In patients with a history of ICH and AF, an increasing body of evidence shows the benefits of OAC resumption. However, there is substantial controversy regarding the optimal time period for re-initiation.58–60 A pooled analysis of the retrospective AF studies of Kuramatsu et al, and Nielsen et al, showed that OAC restarters had a lower rate of any thromboembolic event (HR 0.45 [95% CI 0.26–0.78]), and that OAC resumption was not significantly associated with recurrent major bleeding (HR 1.65 [95% CI 0.97–2.79]).55,61,62 In a model with any thromboembolic event, major bleeding, and all-cause mortality, OAC resumption after ICH resulted in a positive NCB.55 A meta-analysis from eight studies with a retrospective design comprised of 5306 patients hospitalized for anticoagulation-associated ICH for any indication.63 The re-initiation of OAC resulted in a lower risk of thromboembolic events (RR 0.34 [95% CI 0.25–0.45]), without an increase in recurrent ICH (RR 1.01 [95% CI 0.58–1.77]).63 Not only a lower risk of thromboembolism has been observed, but also an improvement in functional recovery of OAC resumption in ICH survivors. A pooled analysis of three prospective studies in 941 AF patients showed that anticoagulation resumption was associated with improved functional recovery at 1-year post-ICH (OR 1.89 [95% CI 1.32–2.70]).64 Although there is good evidence in favor of VKA resumption from observational studies, data on NOAC resumption after recent ICH are very limited.65,66 Data from randomized controlled trials are not available. APACHE-AF is an ongoing trial focusing on the safety and efficacy of full-dose apixaban vs antiplatelet drugs or no antithrombotic therapy after recent ICH in AF.67 SoSTART is an ongoing trial with a similar design, but the choice of OAC is left to the physician: dabigatran, rivaroxaban, apixaban, edoxaban, warfarin, phenindione, or acenocoumarol.68
Overall, (re-)initiation of OAC in AF patients after a major bleeding seems to be beneficial. However, it is unclear what the optimal moment for (re-)starting OAC therapy is. In a retrospective assessment of insurance data, 1329 patients with AF, a major GI-bleeding, and an interruption of warfarin for 48 hrs were included.69 Warfarin restarters had a reduced risk of thromboembolism (HR 0.71 [95% CI 0.54–0.93]) and all-cause mortality (HR 0.67 [95% CI 0.56–0.81]), compared to non-restarters. Both groups had a comparable risk of recurrent GI-bleeding. Compared to restarting warfarin after 30 days after GI-bleeding, an early restart within 7, 7–15, 15–21, or 21–30 days was not associated with a decreased thromboembolic risk. In contrast, restarting warfarin within 7, 7–15, or 15–21 days was associated with a decreased all-cause mortality risk. Careful interpretation of these results is warranted, as it is likely that the different groups analyzed had different risks of rebleeding and thromboembolism, given the high probability of selection bias. Moreover, in this study, restarting warfarin within 7 days was associated with an increased risk of recurrent GI-bleeding, compared to restarting after 30 days.69 A retrospective study using administrative and clinical databases showed that a restart of warfarin, which was after a median of 4 days (95% CI 2–9), was not related with a recurrence of GI-bleeding.70 However, when a restart within 1–7 days was compared with >7 days, the rate of recurrent GI-bleeding was increased significantly (12.4% and 6.23%, respectively).70 In a prospective study of 197 patients hospitalized for GI-bleeding, it was observed that warfarin resumption after a median of 5 days resulted in lower thromboembolic events (HR 0.12 [95% CI 0.006–0.81]), without increasing the risk of GI-bleeding recurrence (HR 2.17 [95% CI 0.86–6.67]).71,72 All-cause mortality within 90 days after hospital discharge was similar between restarters and non-restarters (HR 0.63 [95% CI 0.22–1.89]). Therefore, it has previously been suggested that warfarin resumption can be considered as early as 7–14 days after GI-bleeding.73 Since data are lacking on the timing of NOAC resumption after GI-bleeding, the authors advised to apply data for warfarin resumption with caution, because of the faster therapeutic onset of NOACs.73
In patients with ICH, “early resumption” (within 2 weeks) of OAC therapy in patients with a high risk of thromboembolism, and “late resumption” (after 4 weeks) in patients with a high risk of ICH, has been suggested.60 The most recent European Heart Rhythm Association guidelines recommend that OAC may be restarted after 4–8 weeks after ICH, if the risk of thromboembolism is high and the risk of recurrent ICH is low.9 In general, the optimal timing of resumption after ICH is still largely unknown, and is dependent on many factors. OAC should not be restarted in patients with cerebral amyloid angiopathy, because of the high recurrent ICH risk.9 In other situations, decision-making is more difficult and should, therefore, be decided in a multidisciplinary team.1,60 For example, lobar bleeding, cerebral microbleeds, a non-traumatic origin, cerebral aneurysm, or lacunar infarcts are associated with an increased risk of recurrent ICH, while a deep cortical bleed has a relatively low recurrence risk.60 As data are limited, further research from preferably randomized controlled trials is essential.
Anticoagulation and frailty
Frailty has been defined as a syndrome of increased aging-associated vulnerability, resulting in a compromised ability to cope with stressors.74 With aging of the population, the incidence of both frailty and AF increases drastically, and is likely to result in an increased incidence of ischemic stroke.9 It is however problematic that multiple reports have shown a 50% lower prescription rate in frail AF patients, compared to non-frail patients.75,76 In a questionnaire distributed amongst treating physicians of AF patients from nursing homes in France, recurrent falls (47%) and cognitive impairment (22%) were the most common reasons for withholding OAC.4 Other studies also found an (excessive) fall risk as an important reason for OAC non-prescription.8,77 However, an increasing body of evidence suggests that OAC should not be withheld based on frailty solely.
A recent prospective study in hospitalized, elderly AF patients in Spain showed that amongst patients with anticoagulation, the incidence of ischemic stroke (2.7% vs 3.2%, p=0.79) and major bleeding (7.5% vs 8.1%, p=0.84) was similar between frail and non-frail patients at 1-year follow-up, respectively.78
Fall risk is an important parameter of frailty. A history of falls or an increased fall risk is associated with all-cause mortality, ischemic stroke, and bleeding.79–81 However, conflicting results have been published on the risk of the most feared complication of anticoagulation in patients with frailty: (traumatic) ICH.79–82 In a retrospective study in AF patients anticoagulated with warfarin, the incidence rate per 100 patient-years of traumatic ICH was 2.0 (95% CI 1.3–3.1)) in high fall risk AF patients, and 0.34 (95% CI 0.27–0.45) in other patients.82 In a post hoc analysis of the ARISTOTLE trial, a history of fall(s) was associated with an increased ICH risk (HR 1.96 [95% CI 1.06–3.61]).80 However, in the ENGAGE-AF TIMI-48 trial and in the Loire Valley AF Project, the presence or absence of fall risk or a history of fall(s), did not increase the incidence of ICH.79,81 The reason for these contradictory results is uncertain. Nevertheless, using a Markov model, it was estimated that patients with AF taking warfarin have to fall more than 295 times in 1 year for the risks of warfarin to outweigh its benefits.83 Also, for both edoxaban and apixaban the relative safety and efficacy profile compared with warfarin were consistent in high fall risk patients.80,81 Fall risk alone should therefore not be a reason to withhold anticoagulation.9
Dementia is another often cited reason for OAC non-prescription in AF.4 However, like fall risk, dementia should not be a general contraindication for OAC.9 Anticoagulation initiation and monitoring in dementia can be challenging, as therapy adherence and a patients’ ability to make decisions are often suboptimal.9 Nonetheless, OAC treatment is correlated with lower ischemic stroke and all-cause mortality rates in these patients.84 Moreover, AF is linked to dementia and cognitive decline, and OAC in AF has been associated with a lower risk of dementia.85,86 Anticoagulation treatment is therefore encouraged, but attention to therapy adherence is especially important.
Conclusion
Anticoagulation management remains an important discussion topic, especially in an aging AF population with progressively more comorbidities. Often, the perceived unfavorable risk-benefit ratio of anticoagulation is overestimated in these patients. Although a careful assessment of risks and benefits is warranted, the benefits of stroke prevention generally outweigh bleeding risk. This holds true specifically in patients with commonly reported reasons for anticoagulation withholding previous bleeding, frailty and age, and high bleeding risk (Table 2). After major bleeding, the optimal timing of anticoagulation resumption is largely unknown, and often requires multidisciplinary assessment.
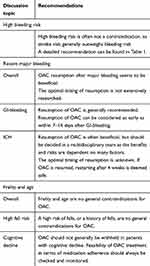 |
Table 2 Summary of recommendations |
Disclosure
J Seelig receives funding from the Netherlands Federation of Anticoagulation clinics. ME Hemels discloses speaker fees from Bayer, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Roche and received a research grant from Netherlands Federation of Anticoagulation clinics. M.V. Huisman reports research grants from Dutch Healthcare Fund, Bayer, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Daiichi Sankyo. H ten Cate received research grants from Bayer and Pfizer; advisory boards for Bayer, Pfizer, Leo; consultancy for Alveron. H ten Cate is an unpaid chairman of the board of the Netherlands Federation of Anticoagulation clinics. M Alings discloses speaker fees from Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Daiichi Sankyo, and Milestone Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
1. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi:10.1093/eurheartj/ehw210
2. Martin A, Siegal D, Verbrugge F, et al. Why do clinicians withhold anticoagulation in patients with atrial fibrillation and CHA2DS2-VASc score ≥2? Arch Cardiovasc Dis Suppl. 2019;11(1):83–84. doi:10.1016/j.acvdsp.2018.10.184
3. Pisters R, van Vugt SPG, Brouwer MA, et al. Real-life use of Rivaroxaban in the Netherlands: data from the Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation (XANTUS) registry. Neth Heart J. 2017;25(10):551–558. doi:10.1007/s12471-017-1009-9
4. Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc. 2015;63(1):71–76. doi:10.1111/jgs.13200
5. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi:10.1093/eurheartj/eht280
6. Son MK, Lim NK, Park HY. Trend of prevalence of atrial fibrillation and use of oral anticoagulation therapy in patients with atrial fibrillation in South Korea (2002-2013). J Epidemiol. 2018;28(2):81–87. doi:10.2188/jea.JE20160149
7. Proietti M, Laroche C, Nieuwlaat R, et al. Increased burden of comorbidities and risk of cardiovascular death in atrial fibrillation patients in Europe over ten years: a comparison between EORP-AF pilot and EHS-AF registries. Eur J Intern Med. 2018;55:28–34. doi:10.1016/j.ejim.2018.05.016
8. O’Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167(4):601–609. e601. doi:10.1016/j.ahj.2013.12.014
9. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393. doi:10.1093/eurheartj/ehy136
10. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi:10.1378/chest.09-1584
11. Lubitz SA, Khurshid S, Weng LC, et al. Predictors of oral anticoagulant non-prescription in patients with atrial fibrillation and elevated stroke risk. Am Heart J. 2018;200:24–31. doi:10.1016/j.ahj.2018.03.003
12. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi:10.1378/chest.10-0134
13. Wilke T, Groth A, Mueller S, et al. Oral anticoagulation use by patients with atrial fibrillation in Germany. Adherence to guidelines, causes of anticoagulation under-use and its clinical outcomes, based on claims-data of 183,448 patients. Thromb Haemost. 2012;107(6):1053–1065. doi:10.1160/TH11-11-0768
14. Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, et al. Cessation of oral anticoagulation is an important risk factor for stroke and mortality in atrial fibrillation patients. Thromb Haemost. 2017;117(7):1448–1454. doi:10.1160/TH16-12-0961
15. Pisters R, van Oostenbrugge RJ, Knottnerus IL, et al. The likelihood of decreasing strokes in atrial fibrillation patients by strict application of guidelines. Europace. 2010;12(6):779–784. doi:10.1093/europace/euq080
16. Gladstone DJ, Bui E, Fang J, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. 2009;40(1):235–240. doi:10.1161/STROKEAHA.108.516344
17. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. doi:10.1016/j.jacc.2011.03.031
18. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713–719. doi:10.1016/j.ahj.2005.04.017
19. Fox KAA, Lucas JE, Pieper KS, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open. 2017;7(12):e017157. doi:10.1136/bmjopen-2017-017157
20. Jaspers Focks J, van Vugt SP, Albers-Akkers MT, et al. Low performance of bleeding risk models in the very elderly with atrial fibrillation using vitamin K antagonists. J Thromb Haemost. 2016;14(9):1715–1724. doi:10.1111/jth.13361
21. Bassand JP, Accetta G, Al Mahmeed W, et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: rationale for comprehensive management of atrial fibrillation. PLoS One. 2018;13(1):e0191592. doi:10.1371/journal.pone.0191592
22. Marcucci M, Lip GY, Nieuwlaat R, Pisters R, Crijns HJ, Iorio A. Stroke and bleeding risk co-distribution in real-world patients with atrial fibrillation: the Euro Heart Survey. Am J Med. 2014;127(10):979–986. (e972). doi:10.1016/j.amjmed.2014.05.003
23. Peacock WF, Tamayo S, Patel M, Sicignano N, Hopf KP, Yuan Z. CHA2DS2-VASc scores and major bleeding in patients with nonvalvular atrial fibrillation who are receiving rivaroxaban. Ann Emerg Med. 2017;69(5):
24. Hijazi Z, Oldgren J, Lindback J, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387(10035):2302–2311. doi:10.1016/S0140-6736(16)00741-8
25. O’Brien EC, Simon DN, Thomas LE, et al. The ORBIT bleeding score: a simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J. 2015;36(46):3258–3264. doi:10.1093/eurheartj/ehv476
26. Shoemaker MB, Stevenson WG. The ABC death risk score: is it time to start measuring GDF-15? Eur Heart J. 2018;39(6):486–487. doi:10.1093/eurheartj/ehx642
27. Hijazi Z, Lindback J, Alexander JH, et al. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker-based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37(20):1582–1590. doi:10.1093/eurheartj/ehw054
28. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. doi:10.7326/0003-4819-151-5-200909010-00003
29. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125(19):2298–2307. doi:10.1161/CIRCULATIONAHA.111.055079
30. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106(4):739–749. doi:10.1160/TH11-05-0364
31. Banerjee A, Lane DA, Torp-Pedersen C, Lip GY. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a ‘real world’ atrial fibrillation population: a modelling analysis based on a nationwide cohort study. Thromb Haemost. 2012;107(3):584–589. doi:10.1160/TH11-11-0784
32. Chan PH, Huang D, Lau CP, et al. Net clinical benefit of dabigatran over warfarin in patients with atrial fibrillation stratified by CHA2DS2-VASc and time in therapeutic range. Can J Cardiol. 2016;32(10):1247e1215–1247 e1221. doi:10.1016/j.cjca.2016.01.016
33. Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients: a position document from the European Heart Rhythm Association, endorsed by the European Society of Cardiology Working Group on Thrombosis. Europace. 2011;13(5):723–746. doi:10.1093/europace/eur126
34. Bayer plc. Xarelto summary of product characteristics; 2018. Available from: https://www.medicines.org.uk/emc/product/2793/smpc.
35. Boehringer Ingelheim Limited. Pradaxa summary of product characteristics; 2018. Available from: https://www.medicines.org.uk/emc/product/4703/smpc.
36. Bristol-Myers Squibb-Pfizer. Eliquis Summary of Product Characteristics; 2018. Available from: https://www.medicines.org.uk/emc/product/2878/smpc.
37. Daiichi Sankyo UK Limited. Lixiana summary of product characteristics; 2019. Available from: https://www.medicines.org.uk/emc/product/6905/smpc.
38. Douros A, Renoux C, Yin H, Filion KB, Suissa S, Azoulay L. Concomitant use of direct oral anticoagulants with antiplatelet agents and the risk of major bleeding in patients with nonvalvular atrial fibrillation. Am J Med. 2019;132(2):191–199 e112. doi:10.1016/j.amjmed.2018.10.008
39. van Rein N, Heide-Jorgensen U, Lijfering WM, Dekkers OM, Sorensen HT, Cannegieter SC. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139(6):775–786. doi:10.1161/CIRCULATIONAHA.118.036248
40. Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug-drug and drug-food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55–61. doi:10.15420/aer.2017.50.1
41. Goodman SG, Wojdyla DM, Piccini JP, et al. Factors associated with major bleeding events: insights from the ROCKET AF trial (rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation). J Am Coll Cardiol. 2014;63(9):891–900. doi:10.1016/j.jacc.2013.11.013
42. Grodensky CA, Golin CE, Ochtera RD, Turner BJ. Systematic review: effect of alcohol intake on adherence to outpatient medication regimens for chronic diseases. J Stud Alcohol Drugs. 2012;73(6):899–910. doi:10.15288/jsad.2012.73.899
43. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151(6):1105–1112. e1110. doi:10.1053/j.gastro.2016.08.054
44. Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320(21):2221–2230. doi:10.1001/jama.2018.17242
45. Bennaghmouch N, de Veer A, Bode K, et al. Efficacy and safety of the use of non-vitamin K antagonist oral anticoagulants in patients with nonvalvular atrial fibrillation and concomitant aspirin therapy: a meta-analysis of randomized trials. Circulation. 2018;137(11):1117–1129. doi:10.1161/CIRCULATIONAHA.117.028513
46. Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107–1115. doi:10.1016/S0140-6736(12)62177-1
47. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–2434. doi:10.1056/NEJMoa1611594
48. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513–1524. doi:10.1056/NEJMoa1708454
49. Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509–1524. doi:10.1056/NEJMoa1817083
50. Piccini JP, Jones WS. Triple therapy for atrial fibrillation after PCI. N Engl J Med. 2017;377(16):1580–1582. doi:10.1056/NEJMe1710753
51. Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61(25):2551–2556. doi:10.1016/j.jacc.2013.03.035
52. Boersma LV, Ince H, Kische S, et al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology. Circ Arrhythm Electrophysiol. 2019;12(4):e006841. doi:10.1161/CIRCEP.118.006841
53. Nishimura M, Sab S, Reeves RR, Hsu JC. Percutaneous left atrial appendage occlusion in atrial fibrillation patients with a contraindication to oral anticoagulation: a focused review. Europace. 2018;20(9):1412–1419. doi:10.1093/europace/eux313
54. Gattellari M, Worthington JM, Zwar NA, Middleton S. The management of non-valvular atrial fibrillation (NVAF) in Australian general practice: bridging the evidence-practice gap. A national, representative postal survey. BMC Fam Pract. 2008;9:62. doi:10.1186/1471-2296-9-62
55. Proietti M, Romiti GF, Romanazzi I, et al. Restarting oral anticoagulant therapy after major bleeding in atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2018;261:84–91. doi:10.1016/j.ijcard.2018.03.053
56. Smit MD, Van Gelder IC. Resumption of anticoagulation after major bleeding decreases the risk of stroke in patients with atrial fibrillation. Evid Based Med. 2017;22(3):107–108. doi:10.1136/ebmed-2017-110694
57. Hernandez I, Zhang Y, Brooks MM, Chin PK, Saba S. Anticoagulation use and clinical outcomes after major bleeding on dabigatran or warfarin in atrial fibrillation. Stroke. 2017;48(1):159–166. doi:10.1161/STROKEAHA.116.015150
58. Xu Y, Shoamanesh A, Schulman S, et al. Oral anticoagulant re-initiation following intracerebral hemorrhage in non-valvular atrial fibrillation: global survey of the practices of neurologists, neurosurgeons and thrombosis experts. PLoS One. 2018;13(1):e0191137. doi:10.1371/journal.pone.0191137
59. Kappelle LJ, Hofmeijer J, Chamuleau SA, van Nieuwenhuizen KM, Hemels ME, Klijn CJ. [Resumption of antithrombotic treatment after an intracerebral haemorrhage]. Ned Tijdschr Geneeskd. 2015;159:A8507.
60. Li YG, Lip GYH. Anticoagulation resumption after intracerebral hemorrhage. Curr Atheroscler Rep. 2018;20(7):32. doi:10.1007/s11883-018-0733-y
61. Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313(8):824–836. doi:10.1001/jama.2015.0846
62. Nielsen PB, Larsen TB, Skjoth F, Lip GY. Outcomes associated with resuming warfarin treatment after hemorrhagic stroke or traumatic intracranial hemorrhage in patients with atrial fibrillation. JAMA Intern Med. 2017;177(4):563–570. doi:10.1001/jamainternmed.2016.9369
63. Murthy SB, Gupta A, Merkler AE, et al. Restarting anticoagulant therapy after intracranial hemorrhage: a systematic review and meta-analysis. Stroke. 2017;48(6):1594–1600. doi:10.1161/STROKEAHA.116.016327
64. Murphy MP, Kuramatsu JB, Leasure A, et al. Cardioembolic stroke risk and recovery after anticoagulation-related intracerebral hemorrhage. Stroke. 2018;49(11):2652–2658. doi:10.1161/STROKEAHA.118.021799
65. Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43(6):1511–1517. doi:10.1161/STROKEAHA.112.650614
66. Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. 2014;45(5):1304–1312. doi:10.1161/STROKEAHA.113.004506
67. van Nieuwenhuizen KM, van der Worp HB, Algra A, et al. Apixaban versus antiplatelet drugs or no antithrombotic drugs after anticoagulation-associated intraCerebral HaEmorrhage in patients with Atrial Fibrillation (APACHE-AF): study protocol for a randomised controlled trial. Trials. 2015;16:393. doi:10.1186/s13063-015-0731-0
68. Start or STop Anticoagulants Randomised Trial (SoSTART). Available from: https://clinicaltrials.gov/ct2/show/NCT03153150. Accessed August 28, 2019.
69. Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113(4):662–668. doi:10.1016/j.amjcard.2013.10.044
70. Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484–1491. doi:10.1001/archinternmed.2012.4261
71. Sengupta N, Feuerstein JD, Patwardhan VR, et al. The risks of thromboembolism vs. recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: a prospective study. Am J Gastroenterol. 2015;110(2):328–335. doi:10.1038/ajg.2014.398
72. Scott MJ, Veitch A, Thachil J. Reintroduction of anti-thrombotic therapy after a gastrointestinal haemorrhage: if and when? Br J Haematol. 2017;177(2):185–197. doi:10.1111/bjh.14599
73. Kido K, Scalese MJ. Management of oral anticoagulation therapy after gastrointestinal bleeding: whether to, when to, and how to restart an anticoagulation therapy. Ann Pharmacother. 2017;51(11):1000–1007. doi:10.1177/1060028017717019
74. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi:10.1016/j.cger.2010.08.009
75. Oqab Z, Pournazari P, Sheldon RS. What is the impact of frailty on prescription of anticoagulation in elderly patients with atrial fibrillation? A Systematic Review and Meta-Analysis. J Atr Fibrillation. 2018;10(6):1870. doi:10.4022/jafib.1870
76. Wilkinson C, Todd O, Clegg A, Gale CP, Hall M. Management of atrial fibrillation for older people with frailty: a systematic review and meta-analysis. Age Ageing. 2018;48(2):196–203. doi:10.1093/ageing/afy180
77. Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37(4):1075–1080. doi:10.1161/01.STR.0000209239.71702.ce
78. Gullon A, Formiga F, Diez-Manglano J, et al. Influence of frailty on anticoagulant prescription and clinical outcomes after 1-year follow-up in hospitalised older patients with atrial fibrillation. Intern Emerg Med. 2019;14(1):59–69. doi:10.1007/s11739-018-1938-3
79. Banerjee A, Clementy N, Haguenoer K, Fauchier L, Lip GY. Prior history of falls and risk of outcomes in atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Am J Med. 2014;127(10):972–978. doi:10.1016/j.amjmed.2014.05.035
80. Rao MP, Vinereanu D, Wojdyla DM, et al. Clinical outcomes and history of fall in patients with atrial fibrillation treated with oral anticoagulation: insights from the ARISTOTLE trial. Am J Med. 2018;131(3):269–275. e262. doi:10.1016/j.amjmed.2017.10.036
81. Steffel J, Giugliano RP, Braunwald E, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol. 2016;68(11):1169–1178. doi:10.1016/j.jacc.2016.06.034
82. Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med. 2005;118(6):612–617. doi:10.1016/j.amjmed.2005.02.022
83. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159(7):677–685. doi:10.1001/archinte.159.7.677
84. Subic A, Cermakova P, Religa D, et al. Treatment of atrial fibrillation in patients with dementia: a Cohort Study from the Swedish Dementia Registry. J Alzheimers Dis. 2018;61(3):1119–1128. doi:10.3233/JAD-170575
85. Alonso A, Arenas de Larriva AP. Atrial fibrillation, cognitive decline and dementia. Eur Cardiol. 2016;11(1):49–53. doi:10.15420/ecr.2016:13:2
86. Friberg L, Rosenqvist M. Less dementia with oral anticoagulation in atrial fibrillation. Eur Heart J. 2018;39(6):453–460. doi:10.1093/eurheartj/ehx579
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
