Back to Journals » International Medical Case Reports Journal » Volume 13
When Chondroma Happens in an Unexpected Location: A Case Report of Intra-Axial Intracranial Chondroma
Authors Gharib M, Nikfarjam Z
Received 8 May 2020
Accepted for publication 3 July 2020
Published 24 July 2020 Volume 2020:13 Pages 275—278
DOI https://doi.org/10.2147/IMCRJ.S260817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Masoumeh Gharib,1 Zahra Nikfarjam2
1Department of Pathology, Mashhad University of Medical Sciences, Mashhad, Iran; 2Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran
Correspondence: Zahra Nikfarjam
Department of Pathology, Ghaem Educational, Research, and Treatment Center, Ahmadabad Avenue, Mashhad, Khorasan Razavi 99199-91766, Iran
Tel +98 51 38400000
Fax +98 51 38453239
Email [email protected]
Abstract: Chondroma is a benign tumor of mature hyaline cartilage that is often found in the long bones and may be rarely diagnosed in other parts of the body. Here, we present a young patient with the definitive diagnosis of intra-axial intracranial chondroma and without dural connection. The presenting symptoms of the patient were headache and impaired vision. The brain magnetic resonance imaging (MRI) revealed a huge enhancing parasagittal brain mass. The diagnosis was confirmed by immunohistochemistry, which was positive for S100.
Keywords: case report, intracranial chondroma, rare malignant tumors
Introduction
Chondroma is a benign tumor of mature hyaline cartilage, which is often found in the long bones of the upper and lower limbs or pelvis.1,2 Intracranial chondromas are rare and account for only 0.2% of intracranial primary tumors.3 Most of the reported cases of intracranial chondromas are sporadic, although they have also been reported in the settings of Maffucci’s and Ollier’s syndromes.4 The first case of intracranial chondroma was reported by Hirschfeld in 1851, who at the time believed that the tumor originates from the remnants of embryonic chondrogenic cells along the basement membrane of synchondrosis. So far, several hypotheses describe the pathogenesis of this lesion. Intracranial chondromas reported so far have often been extradural and were located in the spheno-occipital area at the skull base and with dural connection, although less frequent cases of intracranial chondroma in the paranasal sinuses, choroid plexus, or dura have also been reported.5–8 Interestingly, a very limited number of reported intracranial chondromas have been intraparenchymal without dural connection.9,10 Here, we present a young patient with the definite diagnosis of intra-axial intracranial chondroma without dural connection.
Case Presentation
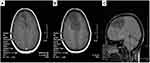
A 36-year-old woman presented with the complaint of occasional headaches since one year ago that has exacerbated since one week before the presentation and accompanied by blurred vision. She had no significant past medical or drug history. As the physical examination was unremarkable, brain MRI without gadolinium-based contrast agents was requested, which revealed a large, low-signal, heterogeneous mass in T2 and T1 MRI sequences in the right frontal lobe, extending to the right parietal lobe, and with significant midline shift to the left. To determine the nature of the mass, MRI with contrast injection was performed, showing a peripheral, punctuated enhancing lesion. Given the imaging findings, the differential diagnoses of low-grade glioma and brain lymphoma were rendered (Figure 1). The patient underwent craniotomy and the tissue sample was sent for histologic examination. Macroscopic examination showed multiple fragmented, pale-blue tissue fragments with elastic consistency, resembling cartilage, and totally measuring 8 * 8 * 3 cm. Primary microscopic examination described a neoplastic lesion with cartilaginous lobules and the appearance of whorls and gave the final diagnosis of metaplastic meningioma, WHO grade I. As radiology did not correlate with pathology, the specimen was sent to a neuroradiologist and a neuropathologist for second opinion. The neuroradiologist described a huge intra-axial mass in the frontoparietal area with compression and midline shifts that was non-contrast enhancing. The diagnosis of meningioma and hemangiopericytoma was excluded radiologically as they are contrast-enhancing and the definite radiologic diagnosis was not given. Examination of the specimen by the neuropathologist described a mesenchymal neoplasm, consisting of chondroid lobules without atypia, or necrosis. There was no evidence of brain parenchyma or dura (Figure 2A). As there was no brain tissue or dura in the specimen, the possibility of specimen displacement in the operating room was suggested. However, there was no related surgery in that day. To determine the exact nature of the mass, immunohistochemical staining for EMA, CK, GFAP, and S100 markers was performed, which was positive for S100 and negative for the other three markers (Figure 2B). The final diagnosis was intracranial chondroma.
 |
Figure 2 (A) Hematoxylin and eosin staining, 400×, showing cartilaginous lobules without atypia, mitosis, or necrosis and (B) positive IHC staining of S100 marker. |
Discussion
Primary intracranial chondroma is quite rare and consists of only 0.2% of primary intracranial tumors.3 To the best of our knowledge, 163 cases of intracranial chondroma have been reported, which were mostly originated from the skull base, falx, and dura. The skull and later the fossa and dura area originated (Table 1). Only a few of these cases were similar to this case and there was no association between the tumor and the dura. There was no significant gender predilection in intracranial chondromas.11–14 The reported cases mostly presented in the third decade of life,15 although the age range was 15 months to 60 years.16 Due to the slow-growing nature of the tumor, patients with intracranial chondromas often present with a large mass and a long history of symptoms at the time of presentation, which may vary depending on the location of the tumor.17 The most commonly reported symptoms are headache and seizures (Table 2). However, focal neurological deficits have also been reported as a primary symptom, as well as Parkinson-like symptoms.18
 |
Table 1 Origin of Intracranial Chondromas in the Literature |
 |
Table 2 Common Symptoms of Intracranial Chondromas in the Literature16 |
Imaging characteristics of intracranial chondromas can be hypo or hyperdense, based on its degree of calcification. On MRI, chondromas appear iso to hypointense in T1 and as a mixture of iso to hyperintense in T2 images.19 Sullivan et al showed that most of the lesions were hypointense in T1 weighted brain MRI with a heterogeneous appearance in T2 weighted brain MRI.16
As most cases of intracranial chondromas are dura-attached (unlike this patient), one of the important differential diagnoses is meningioma. Intracranial chondroma, like meningioma, presents as a well-circumscribed, extra-axial, and dura-based mass, although in very rare cases, chondromas have no dural connection and have heterogeneous enhancement, which differentiates these lesions radiologically from meningiomas.5 The differentiation of chondromas from other soft-tissue tumors that can involve the CNS and have similar imaging findings (such as chordoma, chondrosarcoma, and hemangiopericytoma) is very important. Chordoma originates from notochord remnants and is often located in the midline. Chondrosarcoma also tends to be situated in the midline, although it is sometimes found in the lateral hemisphere, and has malignant histological features (hypercellularity, cellular atypia, and increased mitotic activity) with progressive and destructive borders in imaging and histology.20
In all reported cases, intracranial chondromas were white- gray, multilobulated masses with calcification, similar to this case. Microscopic examination reveals a well-circumscribed mass with a fibrotic capsule, consisting of single or binary chondrocytes within the lacunae in a chondroid matrix. In the reported cases, which used immunohistochemical staining as well, tumoral cells were positive for S100 and vimentin, and negative for EMA, and CK.16 The important point in the pathologic study of these lesions is to consider its important differential diagnoses, including meningiomas, chordoma, osteochondroma, and well-differentiated chondrosarcoma.20 There have also been reports of simultaneous occurrence of two different types of tumors in one patient and in one anatomic location (collision tumor), which included the simultaneous occurrence of choroid plexus papilloma and chondroma.21
Intracranial chondromas may occur singly or in association with systemic chondromatosis such as Maffucci’s syndrome or Ollier’s disease.22–25 Both Maffucci’s syndrome and Ollier’s disease are caused by a somatic mutation in the IDH-1 and IDH-2 genes, encoding the isocitrate dehydrogenase 2 enzymes. However, according to the previous studies, in sporadic intracranial chondromas, no mutation in the IDH genes was reported.26,27 Multiple theories have been presented about the origin of intracranial chondromas, most of which consider its origin from the embryonic chondrogenic cells remnants along the basement membrane of synchondrosis.28 Although the theory of metaplasia of meningeal fibroblasts or perivascular mesenchymal tissue fits for dura-attached cases, other theories such as ectopic embryologic rest of cartilaginous cells or the inappropriate replacement of cartilaginous elements during trauma are also given.11,28
Conclusion
Intracranial chondroma should be considered as a differential diagnosis in the brain parenchyma and tissue sampling should be performed for histopathologic confirmation of these rare entities.
Ethical Consideration
Approval of institutional review board from the Ethics Committee of Mashhad University of Medical Sciences was obtained proactively and a written informed consent document was obtained from the patient for the details and images to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Unni KK, Inwards CY. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases. Lippincott Williams & Wilkins; 2010.
2. Liwei Z, Jianping D, Xuzhu C, Linsen M, Junmei W. An intracranial chondroma with intratumoral and subarachnoidal hemorrhage. Neurol India. 2011;59(2):310–313. doi:10.4103/0028-3886.79170
3. Berkmen YM, Blatt ES. Cranial and intracranial cartilaginous tumours. Clin Radiol. 1968;19(3):
4. Ghogawala Z, Moore M, Strand R, Kupsky WJ, Scott RM. Clival chondroma in a child with ollier’s disease. Case report. Pediatr Neurosurg. 1991;17(1):
5. Abeloos L, Maris C, Salmon I, et al. Chondroma of the dural convexity: a case report and literature review. Neuropathology. 2012;32(3):306–310. doi:10.1111/j.1440-1789.2011.01264.x
6. Alpers BJ. Cerebral osteochondroma of dural origin. Ann Surg. 1935;101(1):27–37. doi:10.1097/00000658-193501000-00005
7. Palacios E. Intracranial solitary chondroma of dural origin. Am J Roentgenol Radium Ther Nucl Med. 1970;110(1):67–70. doi:10.2214/ajr.110.1.67
8. Verbrugghen A, Learmonth J. Chondroma of the falx cerebri. J Nervous Mental Dis. 1932;76(5):463–466. doi:10.1097/00005053-193211000-00003
9. Chorobski JJ, Jarzymski J, Ferens E. Intracranial solitary chondroma. Surg Gynecol Obstet. 1939;68:677–686.
10. Zhan RY, Pan XF, Wan S, et al. Solitary intracerebral chondroma without meningeal attachment: a case report with review of the literature. J Int Med Res. 2011;39:675–681.
11. Dutton J. Intracranial solitary chondroma. Case report. J Neurosurg. 1978;49(3):460–463. doi:10.3171/jns.1978.49.3.0460
12. Fountas KN, Stamatiou S, Barbanis S, Kourtopoulos H. Intracranial falx chondroma: literature review and a case report. Clin Neurol Neurosurg. 2008;110:8–13. doi:10.1016/j.clineuro.2007.08.020
13. Xin Y, Hao S, Zhang J, et al. Microsurgical treatment of intracranial chondroma. J Clin Neurosci. 2011;18:1064–1071. doi:10.1016/j.jocn.2010.12.028
14. Weng JC, Li D, Li H, et al. Surgical management and outcomes of intracranial chondromas: a single-center case series of 66 patients. World Neurosurg. 2017;108:264–277. doi:10.1016/j.wneu.2017.08.151
15. Mapstone TB, Wongmongkolrit T, Roessman U, et al. Intradural chondroma: a case report and review of the literature. Neurosurgery. 1983;12(1):111–114. doi:10.1227/00006123-198301000-00019
16. Sullivan JC, Goldsmith J, Rojas R, Varma H, Kasper EM. Intracranial dural parafalcine chondroma: case report and systematic review of the literature. World Neurosurg. 2019;122:
17. Sebbag M, Schmidt V, Leboucq N, et al. Dura mater chondroma. A case report and review of the literature. J Radiol. 1990;71(8–9):495–498.
18. Connolly ID, Johnson E, Lummus S, Hayden Gephart M. Massive intradural chondroma masquerading as lower body parkinsonism. Cureus. 2018;10(1):e2099. doi:10.7759/cureus.2099
19. Korczyn AD. Vascular parkinsonism—characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11(6):319–326. doi:10.1038/nrneurol.2015.61
20. Weber AL, Brown EW, Hug EB, Liebsch NJ. Cartilaginous tumors and chordomas of the cranial base. Otolaryngol Clin North Am. 1995;28:453–471.
21. Salazar J, Vaquero J, Aranda IF, Menèndez J, Jimenez MD, Bravo G. Choroid plexus papilloma with chondroma: case report. Neurosurgery. 1986;18:781–783. doi:10.1227/00006123-198606000-00018
22. Rogers DJ, Boseley ME, Stephan MJ, et al. Enchondroma of the skull base secondary to generalized enchondromatosis: a case report and review of the literature. Ear Nose Throat J. 2011;90(11):535–537.
23. van Nielen KM, de Jong BM. A case of ollier’s disease associated with two intracerebral lowgrade gliomas. Clin Neurol Neurosurg. 1999;101:106–110. doi:10.1016/S0303-8467(98)00072-9
24. Chakrabortty S, Tamaki N, Kondoh T, Kojima N, Kamikawa H, Matsumoto S. Maffucci’s syndrome associated with intracranial enchondroma and aneurysm: case report. Surg Neurol. 1991;36:216–220. doi:10.1016/0090-3019(91)90116-Q
25. Nakase H, Nagata K, Yonezawa T, Morimoto T, Sakaki T. Extensive parasellar chondroma with ollier’s disease. Acta Neurochir (Wien). 1998;140:100–101. doi:10.1007/s007010050066
26. Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in ollier disease and maffucci syndrome. Nat Genet. 2011;43:1256–1261. doi:10.1038/ng.1004
27. Amary MF, Damato S, Halai D, et al. Ollier disease nd maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011;43:1262–1265. doi:10.1038/ng.994
28. Acampora S, Troisi F, Fusco G, et al. Voluminous intracranial chondroma. Surg Neurol. 1982;18(4):254–257. doi:10.1016/0090-3019(82)90335-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.