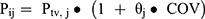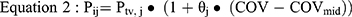Back to Journals » Drug Design, Development and Therapy » Volume 15
What to Do About Missed Doses? A Retrospective Study of Olanzapine in the Elderly
Authors Xiao T, Wang Z, Li G, Huang S, Zhu X, Liu S, Li X, Hu J, Shang D, Wen Y
Received 20 April 2021
Accepted for publication 2 July 2021
Published 4 August 2021 Volume 2021:15 Pages 3411—3423
DOI https://doi.org/10.2147/DDDT.S316110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Tao Xiao,1,2,* Zhanzhang Wang,1,2,* Guanlie Li,3 Shanqing Huang,1,2 Xiuqing Zhu,1,2 Shujing Liu,1,2 Xiaolin Li,1,2 Jinqing Hu,1,2 Dewei Shang,1,2 Yuguan Wen1,2
1Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, 510370, People’s Republic of China; 2Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, 510370, People’s Republic of China; 3Key Laboratory of Molecular Target & Clinical Pharmacology, School of Pharmaceutical Sciences, Guangzhou Medical University, Guangzhou, 511436, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuguan Wen; Dewei Shang
Department of Pharmacy, The Affiliated Brain Hospital of Guangzhou Medical University, 36 Mingxin Road, Guangzhou, 510370, People’s Republic of China
Tel +86-020-81268389
Fax +86-020-81891391
Email [email protected]; [email protected]
Background: Schizophrenia is characterized by a high disease burden. Olanzapine is a common drug used in antipsychotic medication. Little is known about the population pharmacokinetics of olanzapine in elderly patients. Missed doses are a common and unavoidable issue during the treatment of psychiatric diseases, especially in elderly patients. This study aimed to identify what an elderly person should do if doses are inadvertently missed.
Methods: Data were collected from 140 elderly psychiatric patients (aged ≥ 65 years) who received olanzapine therapy. Olanzapine concentrations were determined by high pressure liquid chromatographic tandem mass spectrometry (HPLC-MS/MS) and a population-based approach was used to quantify the characteristics of elderly patients. A non-linear mixed-effects model was used for data analysis. Simulations based on the final model were applied to predict situations involving a single missed dose or three consecutive missed doses under several remedial regimens.
Results: A total of 474 samples from 140 elderly patients were included in the therapeutic drug monitoring (TDM) data analysis. A one-compartment model, with no significant covariates, was developed to describe the population pharmacokinetics of olanzapine in elderly patients. The population predicted systematic clearance (CL/F) and volumes of distribution (V/F) were 18 L/h and 785 L, respectively. The simulation demonstrated that in a missed dose situation, elderly patients should take the regular dose immediately; the refill dose used at the second remedial time point depends on the length of the time delay.
Conclusion: Here, we used a simulation to provide a remedial regimen for missed doses of olanzapine in the elderly population. Our simulation can provide valuable suggestions for individualized therapy in elderly patients.
Keywords: olanzapine, elderly patients, missed doses, simulation, remedial regimen
Introduction
Schizophrenia is a severely disabling chronic mental illness characterized by hallucinations, delusions, confusion, negative symptoms, behavioral disorders, or impaired executive function.1 It has been estimated that the lifetime prevalence of schizophrenia in the elderly is approximately 0.5% to 1%.2 Many patients will gradually lose their social functions, thus, causing a heavy burden on their family and society.3 Schizophrenia requires long-term and even life-long antipsychotic drug treatment.
Olanzapine is an atypical antipsychotic drug that is widely used in China.4 The recommended daily dose of olanzapine is 5 to 20 mg and the main adverse reactions associated with this drug are drowsiness, weight gain, elevated serum prolactin, extrapyramidal reactions, tardive dyskinesia, and metabolic disorders. The most serious reaction is diabetes or hyperlipidemia caused by abnormal metabolism.5 The therapeutic range is described as 20 to 80 ng/mL in the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatri (AGNP) guidelines.6 Olanzapine is well absorbed orally and is mainly metabolized into 10-N, 4ʹ-N-glucuronides and 4ʹ-N-desmethylolanzapine via CYP1A2 in the liver, and into olanzapine-N-oxide via flavin-containing monooxygenase 3. The drug concentration in the plasma reaches its peak, and a steady-state, after 6 hours and 7 days, respectively. Approximately 93% of the drug is bound to plasma proteins; this is metabolized through CYP2D6 to 2-hydroxymethyl olanzapine in a secondary pathway.7 Reports have shown that serum olanzapine concentrations can be affected by several factors, including age, smoking habits, co-medications, and gender. A previous meta-analysis showed that olanzapine dose-related concentrations in smokers were significantly lower than in non-smokers.8 Another study demonstrated that age had a significant impact on the concentrations of olanzapine; when prescribing olanzapine, the dose-related concentration of olanzapine increased with increasing age, even the lower doses could be used in elderly patients.9 Furthermore, the concentration of olanzapine showed a significant difference between genders, with a 26.1% higher concentration in women than men.9 Research has also shown that the coadministration of valproate can reduce the concentration of olanzapine.10
Missed doses are a common and unavoidable issue when treating patients with antipsychotic drugs. In the process of long-term medication for mental illness, patients may not take medication strictly according to their regular plans; this can occur for a variety of reasons. A previous randomized trial found that the non-persistent use of antipsychotic drugs was significantly associated with higher age.11 Elderly patients are a special population and commonly show weak compliance, high drug concentrations, with significant and unexplainable variations.12 A previous report demonstrated that the prevalence of missed medications ranged from 41% to 43%; forgetting to take medications was a key self-reported reason for non-persistence (55%). Other factors related to patients forgetting to take their medications, included age, educational level, employment status, and a lack of attention to medication treatment.13
Skipping a dose of a drug may make the plasma level of the medication drop off.14 The non-persistent use of antipsychotic drugs may cause relapse and persistent symptoms, both of which may lead to increased costs.15 A previous study demonstrated that the risk of hospitalization may increase significantly in the first 10 days after missing a remedial dose.16 A patient’s missed dose behavior may affect the choice of medicine and dosing regimen, thus, leading to differences in drug choices and the prescribing process. The request for advice on ‘what to do when I miss a dose’ is general in clinical practice.
Due to changes in pharmacodynamics and pharmacokinetics, the increased combination of different medications, and more concomitant diseases, the factors that influence pharmacokinetics in the elderly population are complex and individual differences are obvious.17 A previous study compared adult and elderly populations and found that the corrected dose of olanzapine in the elderly (> 60 years old) was 27% higher than younger patients.10 Compared with younger patients, elderly patients may be more sensitive to the side effects of antipsychotic drugs; this may lead to poor efficacy, weight gain, and disorders related to glucose and lipid metabolism.18,19
The classic pharmacokinetic model method is simple but needs a complete set of data at each time point; however, parameters are poorly estimated. A population pharmacokinetic model approach could incorporate different numbers of data points into a single model and distinguish inter-individual and intra-individual variability.12 Although many studies have used sparse sample concentration strategies to describe the population pharmacokinetics of olanzapine, in children, adolescents, and adults, none of these previous studies have specifically focused on the population pharmacokinetics of olanzapine in elderly patients.20 Given the lower clearance and highly variable characteristics of elderly patients, there is a clear need to carry out population pharmacokinetic studies in this critical population.
In addition, we must develop a strategy to correct for missed doses; patients are very concerned about this issue. However, there is no remedial strategy for missed doses; patients generally take their medication on a subjective basis. This arbitrary use of medication is more likely to lead to poor efficacy or serious adverse reactions. The application of population pharmacokinetic methods can quantitatively evaluate the impact of missed doses and calculate the best remedial treatment plan. Our goal was to use a population-based approach to develop an appropriate remedial strategy.
Materials and Methods
Subjects, Study Design, and Drug Dosing
We performed a retrospective study on therapeutic drug monitoring (TDM) data acquired from psychiatric inpatients treated with olanzapine in the Affiliated Brain Hospital of Guangzhou Medical University (China) between 1st January 2019 and 31st May 2020. The exclusion criteria were as follows: (1) non-Chinese, (2) tended to have poor drug compliance or had serum samples taken at inappropriate times, for example, some patients are temporarily sampled because of an emergency, and (3) serum olanzapine concentration was zero, below the lower limit of quantification, or higher than the upper limit of quantification. Patients were eligible for recruitment if they (1) took oral olanzapine as a treatment, (2) had undergone serum drug concentration monitoring and were psychiatric patients aged>65 years old (including boundary value), (3) if multiple serum concentration data could be selected under different dosage schemes; and (4) if complete medical records were available.
For each patient, we recorded a range of demographic data (age, gender, body weight) and clinical data, including dosage regimen, serum olanzapine concentration, liver function (alanine transaminase, aspartate aminotransferase, γ-glutamyl transferase, creatine kinase, alkaline phosphatase), renal function (uric acid, creatinine, retinol-binding protein, β2-microglobulin), blood lipids (triglyceride, total cholesterol, low-density lipoprotein), inflammatory indicators (neutrophil, white blood cell, eosinophils, hypersensitive c-reactive protein), combined medication history (valproic acid, omeprazole, risperidone), glucose, the plasticity of prolactin, and angiotensin-converting enzyme. Inpatients were dosed at the scheduled time. Samples were collected 10–22.5 h after the last dose and before the next dose, on an empty stomach in the early morning. Most patients were monitored once a month. The trough concentration occurred during the elimination phase, especially at the end of the elimination phase. Even if there may be an error in the serum collection time, the difference in trough concentration was not obvious. After confirmation of the data, 434 of the 474 concentrations (91.56%) referred to the steady-state trough concentration. Most patients took olanzapine once or twice per day.
Statistical Analysis
Statistical analyses were performed using SPSS (version 25.0, IBM Corporation, Armonk, NY). Demographic and physiological characteristics are presented as mean ± standard deviation (SD) and range.
Determination of Olanzapine Concentration
Serum olanzapine concentrations were measured on an Agilent 6410 Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clara, CA, USA) by HPLC-MS/MS at the Affiliated Brain Hospital of Guangzhou Medical University, China. Separation was carried out on an Agilent Eclipse XDB C18 column (4.6 × 50 mm, 1.8 µm) at 35°C. The mobile phase consisted of A: B (1: 1, v: v); A was methanol: water (75:25, containing 5 mmol·L−1 ammonium format), B was pure acetonitrile, and the flow rate was 0.5 mL·min−1. The calibration curve involved concentrations of 2, 4, 40, 80, 120, 160, and 200 ng·mL−1. The lower limit of quantification, and quality control samples of the low, medium, and high concentrations were 2, 6, 60, and 150 ng·mL−1, respectively. The relative standard deviation of inter-day and intra-day accuracy were both within 15% of the quality control sample.10,21
The Population Pharmacokinetic Model
Model Development
For this part of our research, we used NONMEM software (version 7, level 3.0, Icon Development Solutions, Ellicott City, MD, USA). A non-linear mixed-effects model was used for all analyses involving olanzapine data. The model was established by first-order elimination with the interaction option (FOCE-I), and Pirana software (version 2.9.0) was used to run the model. The absorption rate constant (Ka) was fixed to values that were published in the previous literature because of the lack of serum samples around the absorption phase.22 Similarly, the absorption lag time could not be evaluated. We did not use the algebraic equation, and differential equation. There were few absorption data in our study, and the concentration data was sparse; data was not enriched. Concentrations were between 10–22.5 h, and most patients scored 2–3 points; therefore, we did not apply the two-compartment model, or different types of absorption models. The one-compartment model of subroutine ADVAN2 and TRANS2 is well described.
Covariate Testing
The following continuous covariates were evaluated: age, body weight, dosage regimen, serum olanzapine concentration, alanine transaminase, aspartate aminotransferase, γ-glutamyl transferase, creatine kinase, alkaline phosphatase, the plasticity of prolactin, angiotensin-converting enzyme, uric acid, creatinine, retinol-binding protein, β2-microglobulin, triglyceride, total cholesterol, and low-density lipoprotein. In addition, the following categorical variables were evaluated: gender, infection, glucose, and combination medication.
The categorical covariates (such as gender and co-medications) were evaluated using a linear equation (equation 1):
where COV represents the covariate value for a patient, for the gender covariate COV = 0 (representing a female) and COV = 1 (representing a male). For the co-medication covariate: if the patient had certain concomitant drugs such as valproic acids, sertraline, perphenazine, and fluoxetine at the corresponding olanzapine sampling time point, then COV = 1; if the patient did not receive concomitant drugs, then COV = 0. We investigated the impact of combined medication, including valproic acids, sertraline, perphenazine, and fluoxetine; this practice can affect the serum concentration of olanzapine, as described previously.
Continuous covariates were then linearly introduced into the model using equation 2.
where COV represents the continuous covariate, such as alanine transaminase, and COVmid represents the middle of the corresponding covariate. θj was used as a factor to adjust the jth PK parameter.
To assess the effect of continuous and categorical covariates on olanzapine pharmacokinetics, we first established a pharmacokinetic model by forward selection and then simplified the model using backward elimination. When a certain covariate was included in the basic model, and the model reduced the objective function value (OFV) by more than or equal to 3.84 (P < 0.05, degrees of freedom = 1), then the covariate was identified as being significant and should be retained in the base model. The next significant covariate was eliminated from the model one by one and we evaluated the model by comparing OFV values from models with and without each covariate. If a covariate was eliminated, and the OFV value increased by more than 6.63 (P < 0.01, degrees of freedom = 1), then the covariate had a significant effect on the model parameters and was included in the final model.
Model Evaluation
The final model was guided by OFV, parameter estimation, diagnostic plots, successful minimization and rationality, and the accuracy of the shrinkage value.
The parameter estimation accuracy of the final model was evaluated by the application of a non-parametric bootstrap resampling method (n=1000); this established 95% confidence intervals. We used GraphPad Prism for diagnostic plots (v. 8.0.1). The normalized prediction distribution error (NPDE) was simulated 1000 times with Pearl-speaks-NONMEM (PsN) (v. 3.4.2). We also used the R add-on packages Xpose4 (v. 4.3.2) and NPDE (v.2.0) to visualize NPDE plots.
Model Simulation
By analyzing the olanzapine data, we found that most elderly patients used 10 mg as the dose to be taken at nine o’clock PM before sleep. In the AGNP guidelines, the recommended therapeutic reference range for olanzapine as an antipsychotic drug is 20 to 80 ng/mL.6 However, this range is only for adults; a therapeutic range for elderly patients has yet to be established. By collating TDM data from elderly patients, we were able to calculate a reference range for the observed plasma concentrations at the 68% confidence interval (8.1 to 44.68 ng/mL). The treatment reference range was defined as the 68% confidence interval of the concentration range, by the AGNP guidelines.
Monte Carlo simulation was carried out in NONMEM software. To provide a suitable remedial regimen selection for elderly patients when they miss doses, we carried out simulations for situations when patients miss a single dose or triple doses. All situations assumed certain factors, as given below.
We assumed that elderly patients had been prescribed a 60-day course of olanzapine and needed to take a 10 mg dose of olanzapine at nine o’clock each night. Simulations were performed using the final model parameters and based on one bootstrap sample to achieve a steady-state concentration at 25 ng/mL; this allowed us to define the mean concentration of olanzapine in elderly patients taking a dose of 10 mg on a nightly basis. We simulated a single missed dose situation by assuming that a typical elderly patient forgot a dose of olanzapine on the 30th day, within hours of the time that it was usually taken. A triple missed dose situation was defined as when a typical elderly patient forgot three doses of olanzapine (on the 30th, 31st, and 32nd day) within hours of the time that was usually taken. Then, we simulated three scenarios, as follows.
Scenario 1: The missed doses were skipped and the regular dose was taken at the next scheduled time.
Scenario 2: The patient took the regular dose immediately. We then simulated whether another remedial dose was needed and how many doses were needed to restore the previous steady-state concentration of olanzapine based on the shortest required time.
Scenario 3: The patient omitted the subsequent dose. Simulations were used to identify how long it would take the concentration of olanzapine to fall below the detection limit (assumed to be less than the concentration after six half-lives) after a single or triple missed dose.
Considering the different tablet sizes of olanzapine in clinical use, the remedial dose was accurate to 1.25 mg and 2.5 mg (a quarter of 5 or 10 mg tablet) for selecting a remedial dosing regimen.
Results
Demographic Information
Five samples from two patients were excluded as a result of the patients smoking. Finally, we included a total of 140 elderly patients with psychosis; these underwent treatment between 1st January 2019 and 31st May 2020. The mean ±SD age of the patients was 73.4 ± 7.26 years (range: 65–96 years) and the mean ± SD body weight was 60.60 ± 11.28 kg (range: 35–90 kg). The detailed demographic characteristics of these elderly patients are shown in Table 1. A total of 474 olanzapine serum concentrations (91.56% were steady-state trough concentrations) were available for population pharmacokinetic analyses. Fifty of the 140 patients (35.71%) experienced changes in their dosage regimen.
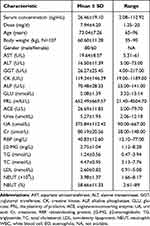 |
Table 1 Subject Characteristics |
Population Pharmacokinetic Modeling
Finally, a one-compartment pharmacokinetic model, with the first-order absorption, was identified as the best fitting model to describe the olanzapine data. Based on a previously published meta-analysis,22 Ka of olanzapine was fixed at 0.868 h−1. Typical CL/F and V/F were 18 L/h and 785 L, respectively. No covariates had a significant effect on the population pharmacokinetic parameters of olanzapine. The population pharmacokinetic parameters and bootstrap are shown in Table 2.
 |
Table 2 Population PK Parameters Estimates and Bootstrap Results for Olanzapine |
Model Evaluation
The goodness of fit plots for the final model are shown in Figure 1. The NPDE method was used to validate the final population pharmacokinetic model for olanzapine; 1000 simulations were performed to evaluate the cumulative distribution of olanzapine (Figure 2). Figure 2A and B show a quantile-quantile (Q-Q) plot and histogram, respectively; the NPDE method produced a mean of 0.02 and a variance of 0.99 (P = 0.064). There was no trend for NPDE over time (Figure 2C) or NPDE trends with regards to the predicted concentrations of olanzapine (Figure 2D). These results indicate that the population pharmacokinetic model for olanzapine was relatively precise and reliable.
 |
Figure 2 Results of the NPDE for the final model of olanzapine in elderly patients. Notes: (A) Normal Q-Q plots for NPDE; (B) Histogram of NPDE; (C) NPDE vs time; (D) NPDE vs population predicted concentration of olanzapine (predicted DV). The Q-Q plots of NPDE based on observations vs the theoretical standard normal distribution N (0, 1). The line y=x with 95% confidence interval represents a theoretical distribution. A normal distribution is represented by dashed lines in the histogram of NPDE. The NPDE for data above the limit of quantification is presented by blue closed circles. The blue and pink lines with dots in the scatterplot represent the 10th, 50th and 90th percentiles of the NPDE corresponding to the observed data. The shaded light blue and pink areas are 95% prediction intervals for the selected percentiles corresponding to simulated data.28,29 |
Model Simulation
In the first scenario, we evaluated single or triple missed doses in the final model using dose regimens of 10 mg quaque nocte for 60 days; we assumed the dose was skipped, but the patient continued to take the scheduled dose regimen thereafter. Simulation indicated that a skipped missed dose would result in a steady-state concentration (24.58 ng/mL) within 14 days (Figure 3). Table 3 shows the situation with a single or triple missed dose but without a remedial dose; serum concentrations were detected on the 31st, 32nd, and 33rd day.
 |
Table 3 Estimated concentrations if the elderly patient forgot to take a single or triple doses |
 |
Figure 4 The patient omitted the subsequent dose. Notes: The simulated time of olanzapine population predicted concentrations after a single or triple missed dose. |
In the second scenario, we simulated different time points for the remedial dose. We investigated a remedial strategy in which the first remedial dose (10 mg) was taken immediately and that this was followed by a second remedial dose at the next scheduled time (nine o’clock at night) with the regular dose taken at the subsequent scheduled time. The concentration shown in Table 4 assumes two remedial doses were taken followed by a subsequent scheduled dose. We defined the regimen with the shortest time to achieve a steady-state concentration as the most suitable remedial regimen; dosing recommendations for the first remedial dose and the second remedial dose are shown in Tables 4 and 5. Based on the simulation, we found that if a single dose was missed, and if the dose was delayed by 1 to 3 hours, then patients could receive 10 mg immediately and 10 mg at the next scheduled time. In cases when triple doses were missed, we found that if the patient took their drugs between 1 to 2 or 3 hours after their missed doses, they could refill with 10 mg immediately, and 18.75 or 17.5 mg at the next scheduled time. If a single or triple dose had been missed, we found that if the patients remembered to take the drug, then they could refill the missed doses immediately; however, the next remedial dose recommendation was based on the length of the delay. With an increasing delay, there was a reduction in the remedial dose.
 |
Table 4 Dosing Recommendations After Missed Doses of Olanzapine |
 |
Table 5 Summary of Dosing Recommendations After Missed Doses of Olanzapine |
In addition, according to the size of the tablets prescribed in the hospital, the remedial dose was chosen based on the minimum limit of a quarter of a tablet, representing 5 mg and 10 mg. For example, if a patient was prescribed 10 mg olanzapine and missed three doses within 9 h of the time that it was usually taken, then the patient should take the first remedial 10 mg immediately, and the 12.5 or 15 mg at the second remedial time.
In the third scenario, after 7 days, the patient’s serum olanzapine concentration was below the detection limit and treatment efficacy was relatively weak. Thus, the next course of treatment needed to be restarted (Figure 4).
Discussion
To our knowledge, this is the first population pharmacokinetics study to evaluate the impact of a missed dose on the pharmacokinetics of olanzapine based on TDM data from elderly psychiatric patients. First, we developed a one-compartment model to describe the pharmacokinetic parameters and demonstrated that the model was precise and could accurately predict the concentrations of olanzapine. Secondly, we used population pharmacokinetics, based on a small number of sample concentrations, was used to investigate the influence of factors on the pharmacokinetics of olanzapine. Finally, the model, based on TDM data, was applied to investigate the effects of a single or triple missed dose and determine strategies for how this situation could be remedied.
We found that the population pharmacokinetic model parameters agreed with a previous meta-analysis, which covered multiple age groups.22 All estimates of parameters were relatively accurate (relative standard errors < 40%). Given the lack of data for the absorption phase, and because the estimated Ka was not reliable, we decided to fix the Ka from a previously reported value. Our research showed that we could predict the concentration within 10–22.5 h. Furthermore, our methodological comparisons showed that a fixed value of Ka did not affect the predictive value. We tested several factors, using forward inclusion and backward elimination methodology, and showed that several covariates did not significantly affect the pharmacokinetics of olanzapine, including age, gender, co-medication, and body weight. There are several possible reasons for these results. One factor is that the smoking covariate was not included. At the beginning of our study, a total of 142 elderly patients were recruited; however, two of these patients smoked. This had a significant effect on the concentration of the drug in the serum. However, due to the small number of patients, and strict tobacco control in our hospital, these two patients were excluded. Therefore, we did not use smoking status as a covariate in our study. Another factor is that the gender covariate was not included. After simulating a missed dose, we found that a women’s consumption of 2.5 mg was in line with the consumption by men (3 mg) when reaching the same serum concentration. Considering the size of the tablets and drug efficacy, the difference between males and females was negligible; therefore, elderly patients may not require dose adjustment according to gender. The effect of these doses on V/F was not clinically significant; therefore, gender was not considered in the final model. The conventional dose used for elderly patients is relatively small; consequently, the actual serum concentration of olanzapine may not fluctuate much. In addition, males generally smoked more excessively than females; consequently, the gender effect seen in previous studies may be due, in part, to smoking.23 In the present study, we excluded patients who smoked; this may have weakened the effect of gender.
Some studies have described the significant impacts of smoking, gender, race, body weight, formulation, and co-medications on the clearance of olanzapine and the volume of distribution.24 The pharmacokinetics of olanzapine may differ between the elderly and adults. In adults, previous TDM data analysis revealed a significant impact for smoking on the elimination of olanzapine; the CL/F of male smokers was 1.53-fold higher than that of non-smokers.22 Furthermore, gender has also been found to be a significant factor that is able to influence the clearance of olanzapine. Compared to females, male patients generally smoke more excessively; consequently, smoking and gender may have an interactive effect on the concentration of olanzapine in the serum. It is important to note that in our pharmacokinetics analysis of elderly patients, we restricted our patient cohort to non-smokers, thus, making it less likely that we would detect a significant impact of these co-covariates on the pharmacokinetics of olanzapine. When compared to a previous study, the elderly patients in our study had similar values for each of the olanzapine pharmacokinetic parameters studied.22 We identified a small difference (similar parameters) between elderly patients and adults; this was most likely due to patient demographics, including the fact that the elderly cohort exclusively involved non-smokers.
One of the main aims of clinical work is to guide patients to take medications accurately in accordance with doctor’s instructions. Psychiatric patients often need to take long-term medications; however, in actual clinical practice, patients often miss medications, thus, causing indirect effects on health. For such cases, we propose a treatment strategy that involves the patient taking the missed dose immediately; a second remedial dose should be taken after a period of 24 hours with the next dose being taken at the subsequent scheduled time. By applying this strategy, the drug concentration can return quickly to the therapeutic range. Otherwise, if there is a break in the drug course, the drug concentration will fall below the computational concentration range; thus, the therapeutic effect will be compromised following missed doses.14,25 We also propose a double remedial dosage regimen, rather than a single remedial regimen to be taken 24 hours after the missed dose. This strategy may generate an excessively larger dosage at one time, thus, leading to adverse reactions. At the scheduled time, or the next scheduled time, double doses can often be used to make up for missed doses. For example, if patients missed one dose, they would take 20 mg at the regular time after 24 hours; however, this method can easily lead to adverse reactions and is not recommended. Similarly, if patients missed the third dose, then they should not take 30 mg at the regular time as they would have already exceeded the daily dose range of olanzapine according to the manufacturer’s instructions (5 to 20 mg). If this happens to inpatients, then a precise treatment can be provided; TDM can be used to calculate accurate dosage.
Due to the ethical regulations, patients should not intentionally miss a dose of a given drug; consequently, it is difficult to perform prospective clinical research in this area.26 Thus, it is difficult to accurately collate information relating to missed doses from patients and conduct prospective analyses. Population pharmacokinetics can simulate various scenarios of missed doses, based on the known drug dose and the drug concentration in the body. It is then possible to investigate the impact of missed doses on drug efficacy, formulate remedial dosing plans, and maximize the compensatory effect to over-ride the harm caused by missed doses.27 Therefore, population pharmacokinetics can play a unique role and is of significant value for research and clinical application in the area of missed doses.
The 2017 AGNP consensus guidelines report that the therapeutic reference of olanzapine ranges from 20 to 80 ng/mL for patients who are 18 to 65 years of age.6 In contrast, the mean (±SD) serum concentration of olanzapine in our elderly population was 26.46 ± 19.10 ng/mL; this does not concur with the AGNP guidelines. This indicates that the currently recommended range may not be suitable for elderly patients. We calculated that the mean (±SD) serum concentration of olanzapine was 25.94 ± 19.32 ng/mL in our elderly patients who had been administered with olanzapine alone and not combined with other psychiatric drugs. It is vital that we establish an appropriate reference range for the elderly population; we recommend that future research should urgently target this unmet niche.
The study has some limitations that need to be considered. First, the study was retrospective in nature and collated data from the TDM. Consequently, some data might be missing from our analysis. However, this did not have any impact on our ability to predict trough concentrations. Although olanzapine may not be the best test case, olanzapine is used in many elderly patients in our hospital, and the dose used is generally lower than that given to younger adults. However, there is a clear problem with missed doses. In the future, we will use appropriate drugs and apply this methodology further. Second, this study was not able to assess the influence of smoking. Due to strict tobacco control in our inpatients, smoking was not considered in our current model. The CL/F of olanzapine for male smokers is known to be 1.53-fold higher than for male non-smokers. The effects experienced by elderly smokers could be simulated using fixed parameters from the literature, but it is not possible to verify these results clinically.22 Third, the number of patients involved in our current analysis was relatively small. We believe that a larger sample size would establish a more accurate model.
Conclusion
In conclusion, we used a simulated model to provide a remedial regimen for elderly patients who missed a single or triple dose of olanzapine. The relatively precise and reliable one-compartment model, with no significant covariates, can provide valuable suggestions for individualized therapy in elderly patients. Clinical parameters for children, adults, and adolescents do not differ significantly, thus suggesting that demographics have little effect on the pharmacokinetics of olanzapine in these particular age cohorts. It is vital that we establish an appropriate reference range for plasma olanzapine for the elderly population.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University (approval number: 2020-005). Standard informed consent was provided by each patient after admission. We confirm that this study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We are grateful to all participants for their willingness to participate in this study. We thank International Science Editing for editing this manuscript. Tao Xiao and Zhanzhang Wang are considered co-first authors.
Funding
This study was supported by grants from the Natural Science Foundation of Guangdong Province (2018A0303130074 and 2021A1515011325), Guangzhou Municipal Science and Technology Project for Medicine and Healthcare (20201A011047 and 20202A011016), Science and Technology Plan Project of Guangdong Province (2019B030316001), and Guangzhou Municipal Key Discipline in Medicine (2021–2023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5):e141. doi:10.1371/journal.pmed.0020141
2. Andreas S, Schulz H, Volkert J, et al. Prevalence of mental disorders in elderly people: the European MentDis_ICF65+ study. Br J Psychiatry. 2017;210(2):125–131. doi:10.1192/bjp.bp.115.180463
3. Sanders JL. A distinct language and a historic Pendulum: the evolution of the diagnostic and statistical manual of mental disorders. Arch Psychiatr Nurs. 2011;25(6):394–403. doi:10.1016/j.apnu.2010.10.002
4. Li A, Ji SM, Yue WH, et al. Development of a population pharmacokinetic model of olanzapine for Chinese health volunteers and patients with schizophrenia. BMJ Open. 2018;8(8):e020070. doi:10.1136/bmjopen-2017-020070
5. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. doi:10.1002/wps.20252
6. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–2):e1. doi:10.1055/s-0037-1600991
7. Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine. pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37(3):177–193. doi:10.2165/00003088-199937030-00001
8. Tsuda Y, Saruwatari J, Yasui-Furukori N. Meta-analysis: the effects of smoking on the disposition of two commonly used antipsychotic agents, olanzapine and clozapine. BMJ Open. 2014;4(3):e004216. doi:10.1136/bmjopen-2013-004216
9. Castberg I, Westin AA, Skogvoll E, et al. Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatr Scand. 2017;136(5):455–464. doi:10.1111/acps.12794
10. Deng SH, Wang ZZ, Lu HY, et al. A retrospective analysis of steady-state olanzapine concentrations in chinese patients using therapeutic drug monitoring: effects of valproate and other factors. Ther Drug Monit. 2020;42(4):636–642. doi:10.1097/FTD.0000000000000738
11. Stentzel U, Berg NVD, Schulze LN, et al. Predictors of medication adherence among patients with severe psychiatric disorders: findings from the baseline assessment of a randomized controlled trial (Tecla). BMC Psychiatry. 2018;18(1):155. doi:10.1186/s12888-018-1737-4
12. Bigos KL, Bies RR, Pollock BG. Population pharmacokinetics in geriatric psychiatry. Am J Geriatr Psychiatry. 2006;14(12):993–1003. doi:10.1097/01.JGP.0000224330.73063.6c
13. Sajatovic M, Levin J, Fuentes-Casiano E, Cassidy KA, Tatsuoka C, Jenkins JH. Illness experience and reasons for nonadherence among individuals with bipolar disorder who are poorly adherent with medication. Compr Psychiatry. 2011;52(3):280–287. doi:10.1016/j.comppsych.2010.07.002
14. Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88(4):457–459. doi:10.1038/clpt.2010.171
15. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. doi:10.2147/PROM.S42735
16. Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69(1):47–53. doi:10.4088/JCP.v69n0107
17. Davies EA, O’Mahony MS. Adverse drug reactions in special populations - the elderly. Br J Clin Pharmacol. 2015;80(4):796–807. doi:10.1111/bcp.12596
18. Krause M, Huhn M, Schneider-Thoma J, Rothe P, Smith RC, Leucht S. Antipsychotic drugs for elderly patients with schizophrenia: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(12):1360–1370. doi:10.1016/j.euroneuro.2018.09.007
19. Iwata Y, Nakajima S, Caravaggio F, et al. Threshold of dopamine d2/3 receptor occupancy for hyperprolactinemia in older patients with schizophrenia. J Clin Psychiatry. 2016;77(12):e1557–e1563. doi:10.4088/JCP.15m10538
20. Maharaj AR, Wu H, Zimmerman KO, et al. Population pharmacokinetics of olanzapine in children. Br J Clin Pharmacol. 2021;87(2):542–554. doi:10.1111/bcp.14414
21. Ni XJ, Wang ZZ, Shang DW, Lu HY, Zhang M, Wen YG. Simultaneous analysis of olanzapine, fluoxetine, and norfluoxetine in human plasma using liquid chromatography-mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1092:506–514. doi:10.1016/j.jchromb.2018.05.026
22. Yin AY, Shang DW, Wen YG, Li L, Zhou TY, Lu W. Population pharmacokinetics analysis of olanzapine for Chinese psychotic patients based on clinical therapeutic drug monitoring data with assistance of meta-analysis. Eur J Clin Pharmacol. 2016;72(8):933–944. doi:10.1007/s00228-016-2040-2
23. Bigos KL, Pollock BG, Coley KC, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48(2):157–165. doi:10.1177/0091270007310385
24. Jovanović M, Vučićević K, Miljković B. Understanding variability in the pharmacokinetics of atypical antipsychotics - focus on clozapine, olanzapine and aripiprazole population models. Drug Metab Rev. 2020;52(1):1–18. doi:10.1080/03602532.2020.1717517
25. Wang CY, Jiao Z, Ding JJ, Yu EQ, Zhu GX. Remedial dosing recommendations for delayed or missed doses of valproic acid in patients with epilepsy based on Monte Carlo simulations. Epilepsy Behav. 2020;111:107265. doi:10.1016/j.yebeh.2020.107265
26. Ding JJ, Zhang YJ, Jiao Z, Wang Y. The effect of poor compliance on the pharmacokinetics of carbamazepine and its epoxide metabolite using Monte Carlo simulation. Acta Pharmacol Sin. 2012;33(11):1431–1440. doi:10.1038/aps.2012.135
27. Bonate PL. A brief Introduction to monte carlo simulation. Clin Pharmacokinet. 2001;40(1):15–22. doi:10.2165/00003088-200140010-00002
28. Nguyen TH, Comets E, Mentré F. Extension of NPDE for evaluation of nonlinear mixed effect models in presence of data below the quantification limit with applications to HIV dynamic model. J Pharmacokinet Pharmacodyn. 2012;39(5):499–518. doi:10.1007/s10928-012-9264-2
29. Wang ZZ, Zhang YF, Huang WC, et al. Effects of comedication and genetic factors on the population pharmacokinetics of Lamotrigine: a prospective analysis in Chinese patients with epilepsy. Front Pharmacol. 2019;10:832. doi:10.3389/fphar.2019.00832
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.