Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
What is the impact of GOLD 2017 recommendations in primary care? – a descriptive study of patient classifications, treatment burden and costs
Authors Gayle A , Dickinson S, Morris K, Poole C, Mathioudakis AG , Vestbo J
Received 10 May 2018
Accepted for publication 23 July 2018
Published 23 October 2018 Volume 2018:13 Pages 3485—3492
DOI https://doi.org/10.2147/COPD.S173664
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Alicia Gayle,1 Scott Dickinson,2 Kevin Morris,1 Chris Poole,1 Alexander G Mathioudakis,3 Jørgen Vestbo3,4
1Market Access, Boehringer Ingelheim Ltd, Bracknell, UK; 2Medical and Scientific Affairs, Boehringer Ingelheim Ltd, Bracknell, UK; 3Division of Infection, Immunity and Respiratory Medicine, University of Manchester, Manchester, UK; 4Manchester Academic Health Sciences Centre, Manchester University NHS Foundation Trust, Manchester, UK
Purpose: The changes in grading of disease severity and treatment recommendations for patients with COPD in the 2017 GOLD strategy may present an opportunity for reducing treatment burden for the patients and costs to the health care system. The aim of this study was to assess the implications of the GOLD 2017 grading system in terms of change in distribution across GOLD groups A–D for existing patients in UK primary care and estimate the potential cost savings of implementing GOLD 2017 treatment recommendations in UK primary care.
Patients and methods: Using electronic health record data from the Clinical Practice Research Datalink (CPRD), patients aged ≥35 years with spirometry-confirmed COPD, receiving care during 2016, were included. The cohort was graded according to the GOLD 2017 groups (A–D), and treatment costs were calculated, according to corresponding recommendations, to observe the difference in actual vs predicted costs.
Results: When applying GOLD 2013 criteria, less than half of the cohort (46%) was assigned to GOLD A or B, as compared to 86% when applying the GOLD 2017 grading. The actual mean annual maintenance treatment cost was £542 per patient vs a predicted £389 for treatment according to the 2017 GOLD strategy.
Conclusion: There is a potential to make significant cost savings by implementing the grading and treatment recommendations from the 2017 GOLD strategy.
Keywords: COPD, GOLD, severity, economics
Erratum for this paper has been published.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) a prevalent and burdensome global health problem, is complex, heterogeneous1 and difficult to manage. The Global Initiative for Chronic Obstructive Lung Disease (GOLD)2,3 regularly produces a management strategy document offering recommendations that have increasingly differed from the National Institute for Health and Care Excellence (NICE) 2010 guideline (CG101).4
In 2011, GOLD updated its management strategy, moving away from solely grading disease severity based on airflow limitation (predicted FEV1 [FEV1%]) to a model that also included symptoms and exacerbation history. The most recent 2017 GOLD strategy report went one step further and incorporated an updated severity grading tool, which recognizes the diagnostic and predictive value of spirometry, but utilizes only the degree of symptom and exacerbation frequency to guide treatment.5 This change attracted much debate, as the new classification criteria will reclassify a proportion of COPD C patients to A and D patients to B. It could be hoped that the adoption of a simpler classification, not requiring specialized tests, might promote adherence to guidelines, especially among nonspecialists, who are frequently treating COPD suboptimally.6 In addition, de-escalation of treatment could lead to a limitation of the extensive health care expenditure for these patients.
Of the total estimated economic burden of COPD in the UK, 47.5% is accounted for by expenditure on pharmaceutical treatments.7 In 2010, £497,665,559 (€584,469,239) was spent on long-acting β2 agonist/inhaled corticosteroid (LABA/ICS) combination inhalers in England (National Health Service Business Authority, Freedom of Information Request, 110407 Booth 515237, April 7, 2011). To date, the economic burden of maintenance therapy has not been described, and as costs increase substantially as disease severity moves from moderate to severe,8 it is important to quantify these costs using an exacerbation risk approach as recommended in the GOLD strategy report.
Given that the NICE COPD guideline is now 8 years old, the more recent GOLD recommendations are being increasingly adopted in the UK.9 There is a need to understand the implications of the 2017 GOLD strategy report recommendations on COPD management in the UK considering not only the new classification of disease severity but also treatment costs arising from the updated treatment pathway. The aim of this study was to examine the extent to which a contemporary cohort of COPD patients could be classified using primary care electronic health records into the newly defined GOLD categories. It reported on how the distribution of current inhaled therapy prescriptions differs from those recommended in the GOLD 2017 report and evaluated the treatment cost implications of adopting the 2017 GOLD recommendations.
Patients and methods
This was a descriptive, population-based cross-sectional study using routinely collected health care data provided by the Clinical Practice Research Datalink (CPRD). CPRD provides electronic primary care records from general practice in the UK and is one of the largest and most thoroughly validated databases of longitudinal medical records from primary care globally.10–13 Linkage between CPRD and Hospital Episode Statistics (HES) data is also available, enabling individual anonymized patient records to be followed across care sectors.11–13
For inclusion in the study, patients were required to be registered with practices for the period of observation.14 The study population included prevalent COPD patients, primarily including patients with an existing coded diagnosis for COPD15 prior to the study period (January 1, 2016 to December 31, 2016). COPD patients aged ≥35 years with at least 1 full year of data prior to the study start date were included to ensure that prevalent patients were captured with sufficient medical history to classify historical comorbidities and clinical characteristics. Diagnosis of COPD was based on any record of a diagnostic Read code for COPD in addition to spirometry confirmation of the diagnosis (FEV1/FVC ratio <0.7). The accuracy of COPD diagnosis in CPRD has been previously validated.15,16 Patients with a Read code for participation in a clinical trial in the year prior to the study start were excluded.
Clinical and demographic characteristics were assessed at the study start (January 1, 2016). Each patient was categorized in our cohort according to GOLD classifications (2013 and 2017) using records from the year prior to the study start; the mMRC dyspnea scale was used for the majority of patients for the assessment of symptoms as COPD assessment test (CAT) score was recorded in few patients. Patient records were linked using their postcode to Index of Multiple Deprivation, a combined measure of deprivation based on a total of 37 separate indicators in which each area is given a score to identify how deprived an area is relative to others; each quintile will describe the distribution of patients by the GOLD group according to their area deprivation.
Exacerbations were defined, using an adapted version of a validated algorithm for use in CPRD data,17 as antibiotic and oral corticosteroid prescriptions for 5–14 days were calculated using the date of prescription and drug pack information or coded lower respiratory tract infection or coded acute exacerbation.
As part of the sensitivity analysis, a sub-cohort of patients was identified who were eligible for linkage with HES data to assess whether the inclusion of HES recorded exacerbations (defined as ICD-10 code J44.1 and J44.0 in any position of hospital episode) significantly changed the proportion of patients who would be classified as having more severe COPD. If records occurred within 1 calendar week in both HES and CPRD, only the HES record was included as a hospitalized exacerbation.
Prescribed maintenance therapies for COPD were identified in 3 months after the study start to approximate the prescribed maintenance therapy medications as patients may regularly switch/escalate therapy over a 1-year period. The investigated treatments were those recommended by GOLD as monotherapy or combination therapy: short-acting β2 agonists (SABA), short-acting muscarinic antagonist (SAMA), LABAs, long-acting muscarinic antagonist (LAMA) and ICSs. Thirty-day medication cost was calculated for each inhaler type using Drug Tariff prices and COPD maintenance treatment dose for each drug as per the summary of product characteristics recommendations. Mean monthly costs for each drug class were then used to estimate annual costs, assuming 100% compliance. To estimate treatment costs for the same cohort according to GOLD 2017 recommendations, we developed a list of acceptable treatments for each GOLD stage and guidance on how to adjust non-recommended treatments (S1). Given the paucity of available clinical information, we adopted a pragmatic approach excluding short-acting/rescue therapy, short courses of oral corticosteroids and off-licensed therapies. The difference in actual vs predicted costs was calculated. The cost of medication used was defined using drug, dose, device (where applicable) and brand recorded.
Statistical analyses
Comorbidities and demographic information were described for the groups of patients by GOLD 2017 and GOLD 2013 classification. Summary statistics included mean ± SD or median and IQR for continuous data and number (percentage) for categorical data comparing across 2013 and 2017 GOLD strategies. Between-group differences were tested for statistical significance using either chi-squared test for categorical data or one-way ANOVA for continuous data (with α=0.05 used to denote significance throughout).
Total drug costs were summarized for the overall study cohort and by the GOLD groups at baseline. Estimates of drug costs according to recommended treatments by the GOLD group are presented as total costs and per patient. The UK drug costs for individual products within each drug class were used to estimate drug class costs. Summary statistics included mean ± SD annual costs.
As part of the planned sensitivity analysis, additional HES-linked data were used to understand the proportional change in GOLD C/D patients when including additional hospital data to confirm the number of hospitalized exacerbations. In addition, we assessed GOLD 2017 after excluding comorbid asthma patients. Finally, we reestimated treatment costs per patient per year using mean drug class costs to understand whether the assumptions that the underlying distribution of costs was non-normal impacted our estimates.
These analyses were performed in accordance with relevant regulations/guidelines. This study was reviewed and approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research (Ref: 17_075R) and an internal scientific committee of the study sponsor. As this was a non-interventional study using anonymized data, no patient consent was necessary.
Results
Upon application of the inclusion criteria to the CPRD data, 19,268 patients were eligible for inclusion (S2). The average age was 70 years, and 53% were male (Table 1). Most were either former (45%) or current (38%) smokers, and one-third (36%) were either overweight or obese (body mass index >25 kg/m2). Patient characteristics by GOLD 2013 are given in S3.
There was a significant shift toward less severe grading when applying GOLD 2017 compared to GOLD 2013. When applying GOLD 2013 criteria, less than half of the cohort (46%) was assigned to GOLD A or B, as compared to 86% when applying GOLD 2017 (Figure 1). The majority of patients moved from group D to B (65%) and C to A (74%) when applying GOLD 2017 (S4). Fifteen percentage (n=2,939) of the total cohort could not be classified into the GOLD group due to missing symptom data (mMRC or CAT). The GOLD 2017 groups by airflow limitation are given in S5. The results of sensitivity analyses restricted to patients eligible for linkage with HES were consistent with those of the main analysis. We detected no major difference (χ2 P-value = 0.3722) in the proportion of patients classified as GOLD 2017 C/D among these patients (22% n=1,321).
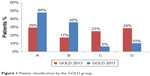  | Figure 1 Patient classification by the GOLD group. |
Figure 2 shows the treatment classes prescribed in 3 months following the study start by the GOLD group; 17% (n=3,327) of the total cohort did not receive any prescriptions for maintenance treatment and 9% (n=1,788) received short-acting therapy (SABA or SAMA) only. In total, 32% of all patients were prescribed triple (ICS/LABA/LAMA) combination therapy: 22% of GOLD A, 43% of GOLD B, 45% of GOLD C and 62% of GOLD D patients. Over half of the patients (53%, n=10,191) received a therapy containing an ICS. After conducting a sensitivity analysis defining 3 months of prescription information as 1.5 months before study start and 1.5 months after, no difference was observed in the proportion of patients who would be allocated to each drug class group overall and by the GOLD group. Detailed figures of therapies prescribed by the GOLD group are given in S6.
Total costs per year for these study patients under their current therapy were estimated to be £8,614,020 across the UK. Applying the GOLD allocations for recommended treatments (S1) this figure would be reduced to £6,151,361: a difference of ~£2.5 million (a 29% decrease). The actual mean annual maintenance treatment cost was £542 per patient vs a predicted £389 for treatment according to 2017 GOLD guidelines. Actual and predicted annual treatment costs for the whole cohort by the GOLD group are shown in Figure 3.
  | Figure 3 Comparison of estimated treatment costs compared to GOLD 2017 recommendations. |
Discussion
In this nationwide study of COPD in the UK, the majority of patients classified as being at high risk (GOLD C and D) according to GOLD 2013 were classified as low risk (GOLD A and B) when applying GOLD 2017 criteria. Consequently, just over a tenth of patients with COPD in the UK are classified as high symptoms and high risk (GOLD group D).
The GOLD 2017 classification has already been evaluated in several other cohorts with study populations ranging between 200 and 33,765 patients with COPD.18–22 While a significant shift toward groups A and B was observed in all cohorts, there was significant heterogeneity in the distribution in the previous studies. Importantly, all previous studies recruited patients from secondary care, and, therefore, they might have been less sensitive toward milder patients whose disease is well managed within primary care settings.
This study shows that reviewing patients using a GOLD strategy is possible using medical records in clinical practice. Revision of maintenance therapy as a result of this may improve clinical outcomes through a reduction in inappropriate prescribing of ICS therapies. A sizable proportion of patients were reclassified from group D to B following the new GOLD strategy; for them, ICS would no longer be the recommended therapy. According to several studies, ICS may safely be withdrawn in this group of patients.23 While the GOLD strategy does not make specific recommendations on ICS withdrawal, recent evidence derived from clinical trials and observational studies may help to guide clinicians. Furthermore, the resulting recommended maintenance treatment implementation is an affordable option when compared to current maintenance treatment regimens being used in the UK.
Combination therapy with ICS/LABA or LAMA/LABA (if ICS is not tolerated) and LAMA monotherapy is recommended by both 2010 NICE guidelines and in the GOLD 2017 report3 for the management of severe stable COPD, while a combination of ICS/LABA/LAMA is recommended to be considered in cases of persistent exacerbations or breathlessness. Among patients with COPD classified in the GOLD 2017 D category, 50% received recommended medications at study start. However, 10% of patients received monotherapies (other than LAMA) or other combination medications that are not recommended for severe stable COPD, indicating that they were in early stages of disease management. Interestingly, for 6% of severe cases, no prescriptions for COPD medication were found. These results suggest possible undertreatment of the high-risk patient population in real-world practice in the UK.
In this study, 37% of patients had a concomitant diagnosis of asthma. ICS is a key component of asthma treatment, and although a large proportion of our overall cohort may have been considered appropriate in this study to step down from the GOLD groups C and D, they may be more appropriately treated with ICS due to their asthma diagnosis. After excluding these patients, the proportion of patients who were classified as C or D decreased from 53% using GOLD 13% to 16% using GOLD 2017, consistent with our main findings suggesting that spirometry alone is not sufficient in distinguishing patients with overlap syndrome.
A major strength of the current study is the use of CPRD data that offer a large sample size, validated in prior studies, and well known to be representative of the demographic breakdown and primary care for the whole UK population. The likelihood of misdiagnosis was low because national incentive programs had driven better recording of COPD diagnosis in primary care and because the presence of chronic airflow limitation was mandatory for enrolment in this cohort. However, there are some limitations to the analysis. Variables of interest are not always widely available in the database; we used mMRC dyspnea score to calculate GOLD stage for the majority of patients (with mMRC ≥1 defining the lower symptom patients as GOLD A or C), as COPD CAT score was only available for 10% of patients (Table 1); however, a previous pan-European observational study reported that the cut point of mMRC grade ≥1 and CAT score ≥10 was approximately equivalent in determining low-symptom patients.24 The algorithm used to detect exacerbations has been shown to accurately predict 85% of events17 but did not distinguish hospitalized exacerbations; however, results from a subgroup of patients with linked HES data show a nonsignificant <10% difference in the ratio of patients in the high-risk groups (GOLD C and D) vs low-risk (GOLD A and B). In addition, data are not available to the same extent and level of detail in all UK regions, particularly notable was the absence of data from the East Midlands region and the relatively small proportion of patients from the Yorkshire and North East England areas (S7). Regional results will need to be interpreted with caution; however, all four countries of the UK are well represented, and this is less likely to lead to bias or affect the direction of the overall results.
To estimate the drug costs per GOLD 2017, we developed a list of acceptable and unacceptable treatments. However, we only had limited access to patients’ clinical information and for this reason we had to adopt a pragmatic approach and were only able to exclude treatments that were clearly incompatible with each GOLD stage in our estimation of maintenance therapy costs; this infers the assumption that patients were stable on their current therapies and that any cost differences observed would be a result of GOLD classification changes independent of treatment adherence and efficacy which should be considered when reviewing patients’ therapy. However, we expect better adherence to treatment guidelines, which are now much simpler to follow. We also used exact national drug costs applicable in England and Wales. While this improves the generalizability of our findings, the actual costs in local settings may differ from national estimates, thereby limiting their applicability to local health economies.
Conclusion
This study shows the impact of the changes in the GOLD 2017 strategy when it comes to classifying COPD patients into treatment groups. A significant proportion of patients classified as being high risk (GOLD C and D) using the GOLD 2013 criteria were reclassified as low risk (GOLD A and B). This study also shows that prescribing the maintenance treatments recommended by GOLD 2017 for each group is likely to save money when compared to the current UK maintenance treatment prescribing. Physicians should consider whether the GOLD 2017 reclassification represents an opportunity to review patients and optimizes their maintenance treatment accordingly.
Data sharing statement
Data are available on request from the CPRD. Their provision requires the purchase of a license, and our license does not permit us to make them publicly available to all. We used data from the version collected in June 2017 and have clearly specified the data selected in the “Patients and methods” section. To allow identical data to be obtained by others, via the purchase of a license, we will provide the code lists on request. Licenses are available from the CPRD (http://www.cprd.com): The Clinical Practice Research Data link Group, The Medicines and Healthcare products Regulatory Agency, 5th Floor, 151 Buckingham Palace Road, Victoria, London SW1W 9SZ, UK.
Acknowledgment
This study was funded by Boehringer Ingelheim Ltd.
Author contributions
All the authors contributed to the conception or design of the work or the acquisition, analysis or interpretation of data for the work. KM and SD contributed to the costing methods. AG and CP contributed to the analysis. All the authors contributed to the interpretation of data and reviewed the manuscript. All the authors have been involved in drafting the work or revising it critically for important intellectual content and have approved the final version to be published. Finally, all the authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
AG, SD, KM and CP are employees of Boehringer Ingelheim Ltd. JV has received honoraria from AstraZeneca, Boehringer-Ingelheim, Chiesi and Novartis within the last 3 years for advising and presenting. AGM has received honoraria from Boehringer-Ingelheim and GlaxoSmithKline within the last 3 years. The authors report no other conflicts of interest in this work.
References
Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. | ||
Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease, Inc.; 2016. | ||
NICE [homepage on the Internet]. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [cited 2016 6/12/2016]. Available from: https://www.nice.org.uk/guidance/cg101. Accessed September 20, 2018. | ||
NICE. Surveillance report 2016 – Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101. [Willett S, Anderson P, Sparrow K, et al; 2016]. | ||
Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report. 2017. | ||
Sandelowsky H, Natalishvili N, Krakau I, Modin S, Ställberg B, Nager A. COPD management by Swedish general practitioners – baseline results of the PRIMAIR study. Scand J Prim Health Care. 2018;36(1):5–13. | ||
Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2 Suppl):5S–9S. | ||
Starkie HJ, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future. Int J Chron Obstruct Pulmon Dis. 2008;3(1):71–88. | ||
Sehl J, O’Doherty J, O’Connor R, O’Sullivan B, O’Regan A. Adherence to COPD management guidelines in general practice? A review of the literature. Ir J Med Sci. 2018;187(2):403–407. | ||
Kousoulis AA, Rafi I, de Lusignan S. The CPRD and the RCGP: building on research success by enhancing benefits for patients and practices. Br J Gen Pract. 2015;65(631):54–55. | ||
Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. | ||
Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60(572):e128–e136. | ||
Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23(5):686–689. | ||
Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. | ||
Quint JK, Müllerova H, Disantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. | ||
Soriano JB, Maier WC, Visick G, Pride NB. Validation of general practitioner-diagnosed COPD in the UK General Practice Research Database. Eur J Epidemiol. 2001;17(12):1075–1080. | ||
Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the Recording of Acute Exacerbations of COPD in UK Primary Care Electronic Healthcare Records. PLoS One. 2016;11(3):e0151357. | ||
Högman M, Sulku J, Ställberg B, et al. 2017 Global Initiative for Chronic Obstructive Lung Disease reclassifies half of COPD subjects to lower risk group. Int J Chron Obstruct Pulmon Dis. 2018;13(13):165–173. | ||
Kahnert K, Alter P, Young D, et al. The revised GOLD 2017 COPD categorization in relation to comorbidities. Respir Med. 2018;134:79–85. | ||
Sun L, Chen Y, Wu R, Lu M, Yao W. Changes in definition lead to changes in the clinical characteristics across COPD categories according to GOLD 2017: a national cross-sectional survey in China. Int J Chron Obstruct Pulmon Dis. 2017;12:3095–3102. | ||
Cabrera López C, Casanova Macario C, Marín Trigo JM, et al. Comparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on Grouping and Outcomes. Am J Respir Crit Care Med. 2018;197(4):463–469. | ||
Marçôa R, Rodrigues DM, Dias M, et al. Classification of Chronic Obstructive Pulmonary Disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: Comparison with GOLD 2011. COPD. 2018;15(1):21–26. | ||
Harlander M, Barrecheguren M, Turel M, Miravitlles M. Should Patients Switched from D to B in the GOLD 2017 Classification be Discontinued from Inhaled Corticosteroids? COPD. 2017;14(5):465–468. | ||
Jones PW, Adamek L, Nadeau G, Banik N. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J. 2013;42(3):647–654. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


