Back to Journals » Psychology Research and Behavior Management » Volume 8
What does best evidence tell us about the efficacy of group cognitive–behavioral therapy for obsessive–compulsive disorder? Protocol for a systematic review and meta-analysis
Authors Pozza A, Andersson G, Dèttore D
Received 3 March 2015
Accepted for publication 4 May 2015
Published 6 August 2015 Volume 2015:8 Pages 225—230
DOI https://doi.org/10.2147/PRBM.S83872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Igor Elman
Andrea Pozza,1,2 Gerhad Andersson,3 Davide Dèttore2,4
1Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; 2Miller Institute of Behavioural and Cognitive Psychotherapy, Genoa, Italy; 3Department of Behavioural Sciences and Learning, Linköping University, Linköping, Sweden; 4Department of Health Sciences, University of Florence, Florence, Italy
Abstract: Group cognitive–behavioral therapy (GCBT) may be a cost-effective alternative modality for the treatment of obsessive–compulsive disorder (OCD). In the last decade, a great deal of research has been conducted to evaluate the efficacy of GCBT for OCD. Despite promising results, studies have produced inconclusive evidence. The current paper will present a protocol for a systematic review and meta-analysis of randomized controlled trials assessing the efficacy of GCBT compared with control conditions or individual CBT at post-treatment and follow-up on OCD symptoms, anxiety, depression, obsessive beliefs, quality of life, and functioning. Another aim will be to compare the levels of early drop out from GCBT relative to control conditions or individual CBT. Finally, the study will investigate potential outcome moderators (age, sex, OCD severity, severity of concurrent depression, comorbid personality disorders, duration of OCD symptom onset, duration of treatment, intensity of treatment, generation cohort, methodological quality, and publication date). A systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines will be conducted using random-effects meta-analyses. Online databases and trial registries will be searched, the corresponding authors will be contacted, and conference proceedings and relevant journals will be hand-searched to locate published and unpublished studies. Risk of bias will be assessed using the Cochrane Collaboration’s tool.
Keywords: obsessive–compulsive disorder, cognitive–behavioral therapy, meta-analysis, group therapy
Introduction
Obsessive–compulsive disorder (OCD) is a chronic psychological condition with a lifetime prevalence of 2% in the general population.1 OCD consists of intrusive thoughts, impulses or mental images, and repetitive behaviors or mental compulsions, which can strongly affect the quality of life of the individual.2 The World Health Organization has ranked OCD as the tenth leading cause of disability of all health conditions in the industrialized world.3
Several well-controlled studies demonstrated that cognitive–behavioral therapy (CBT) with exposure and response prevention (ERP) is the most effective psychological treatment for OCD.4 ERP entails confrontation with obsessional stimuli and refraining from compulsions to demonstrate that feared consequences will not occur.4 Improvement in OCD symptoms is mediated by habituation of anxiety response and a reduction in exaggerated probabilities associated with feared consequences as a result of repeated disconfirmation of the expected harm.5
Research suggests that individual CBT for OCD produces statistically significant improvements in approximately 75% of patients.5 However, when the reliable change criterion is used, only 25% of cases do achieve a full recovery status.5 Moreover, ERP is associated with a 25% refusal rate, even in clinical trials in which treatment is offered at no cost,6 presumably due to the apprehension about the time, effort, or perceived distress associated with the treatment. The importance of tailored treatment approaches with the aim of targeting frequent relapses in OCD patients was also put forward.6 However, only few patients receive ERP,7 and access to psychotherapy is limited by the high costs of individual sessions and long waiting lists.8,9
Group (G)CBT may be a cost-effective modality of treatment. In the last decade, a great deal of research has been conducted to evaluate the efficacy of GCBT for OCD.10 Despite promising results, studies have produced inconclusive evidence.11 To our knowledge, only one systematic review was conducted. Jónsson and Hougaard12 performed a meta-analysis of 13 studies, including randomized controlled trials (RCTs) and open trials, which compared GCBT versus waitlist control conditions. They reported a large mean pre–post effect size (Cohen’s d=1.18) and a large between-groups effect size (Cohen’s d=1.13). However, the authors assessed the effects of GCBT versus control conditions exclusively on OCD symptoms as outcomes, and they did not examine therapeutic gains at follow-up. In addition, due to the limited number of studies (one trial), the authors did not conduct a meta-analysis directly comparing GCBT versus individual CBT.
To date, a systematic review including only RCTs and assessing GCBT relative to control conditions or individual CBT does not exist. Therefore, the current paper will present a protocol for a systematic review and meta-analysis of RCTs. The research will aim to assess the following:
- the efficacy of GCBT compared with control conditions (waitlist or active control conditions) at post-treatment and follow-up on OCD symptoms, anxiety, depression, obsessive beliefs, quality of life, and functioning; follow-up assessments ranging from 1 to 6 months will be pooled to evaluate the maintenance of treatment gains at midterm follow-up; and follow-up assessments longer than 6 months will be pooled to evaluate the maintenance of treatment gains at long-term follow-up;
- the efficacy of GCBT compared with individual CBT on the aforementioned outcomes;
- to examine levels of early dropout from GCBT relative to control conditions or individual CBT; and
- to investigate potential outcome moderators (age, sex, OCD severity, severity of concurrent depression, comorbid personality disorders, duration of OCD symptoms, duration of treatment, intensity of treatment, concurrent pharmacological treatments, generational cohort coded as adult samples and children/adolescent samples, methodological quality, and publication date).
Methods/design
Eligibility criteria
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,13 the criteria considered for inclusion of the studies will involve the following characteristics: 1) participants; 2) interventions; 3) comparators; 4) outcomes; and 5) study/design.
Characteristics of participants
Studies will be included if they were conducted on patients with a primary diagnosis of OCD, and if the diagnosis was made through a semistructured interview based on standardized diagnostic criteria, such as the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (SCID-I).14 Studies will be included if they involved only patients with a current primary diagnosis of OCD. Studies will be included if they used either adult or adolescent/children samples. Diagnoses of comorbid disorders considered as exclusion criteria will have to be made through structured interviews as well. Studies on primary compulsive hoarding will be excluded. The main reason for this is that the treatment for hoarding differs from CBT for OCD, and hoarding is a separate diagnosis in the DSM-5.2 Studies where all the patients had OCD and a specific comorbid psychological or medical disorder (eg, comorbid major depressive disorder) will be excluded. Studies where all the participants had a comorbid mood/anxiety disorder will be excluded since these studies could use a special population of patients with OCD (ie, all the patients have OCD and comorbid generalized anxiety disorder) who are believed to have clinical characteristics different from those of patients with OCD alone. Thus, studies where only some patients had a comorbid disorder will be included since co-occurrence of other disorders is relatively common in OCD. In addition, since comorbid depressive symptoms (but not the diagnosis of mood/anxiety disorders) may be relatively common in OCD; thus, studies on patients with these types of symptoms will be allowed. Concurrent personality disorders will not be a reason for exclusion, and diagnoses will have to be made through structured interviews.
A concurrent pharmacological treatment will not be considered as a reason for exclusion. However, to control for the effects of a concurrent pharmacological treatment, the proportion of patients on psychotropic medications will be used as a moderator (see the “Coding of moderators” section). Studies on so-called treatment-resistant OCD will be included.
Characteristics of interventions
Studies will be included if they assessed the efficacy of GCBT. GCBT is defined as a group psychotherapeutic treatment mainly based on at least one of the following cognitive–behavioral techniques: psychoeducation, ERP, and cognitive restructuring. Eligible studies will have to focus on GCBT as the main intervention. Thus, studies will not be included if GCBT is used exclusively as an augmentation strategy or an adjuvant component in the context of individual CBT.
Characteristics of comparators
Studies will be included if they compared GCBT for OCD with a control condition (no treatment, waitlist), an active control condition (eg, treatment as usual or attention/relaxation controls), or individual CBT without group sessions.
Characteristics of outcomes
Studies will be included if they used validated outcome measures of OCD symptoms, obsessive beliefs, anxiety, depression, quality of life and functioning, and satisfaction with treatment. Measures may be self-report instruments or interviews. Eligible outcomes will have to be measured at post-treatment at 1-month follow-up or longer. Studies will be screened for inclusion if they reported sufficient information about the results to allow for effect size calculation. In cases where insufficient information is available from the paper, the authors will be contacted to provide additional information. Where no further data will be provided, studies will not be included.
Characteristics of study/design
Studies will only be included if they used a RCT design, with random allocation to at least two conditions. Studies conducted on the same data of previously published trials will result in exclusion. No language restrictions will be applied.
Information sources and search procedure
The following search strategies will be used to identify studies for inclusion.
Electronic search
Studies will be retrieved through online systematic literature searches, in which keywords related to OCD (“obsessive compulsive disorder”, “obsessions”, “compulsions”, “obsessive beliefs”, “anxiety disorder”) will be combined through the Boolean operator “AND” with keywords and text words indicative of GCBT construct (“group therapy”, “group treatment”, “group cognitive behavioural therapy”).
The following online databases will be searched: PsycINFO; PubMed; Science Direct; CINAHL; Biological Abstracts; PsycLIT; Embase; and the Cochrane Central Register of Controlled Trials. No date restriction will be applied to the databases. The online search will be conducted on published records from December 1966 to January 2014.15–18
Corresponding authors
To request any further papers, either published or unpublished, all the corresponding authors of the included studies will be contacted.
Hand-searching
Conference proceedings, doctoral theses, and dissertations will be hand-searched for the abstract books of the following international associations relevant to the issue of OCD, occurring up to January 2014: European Association of Behavioural and Cognitive Therapies (EABCT), British Association of Behavioural and Cognitive Psychotherapy (BABCP), American Psychological Association (APA), Australian Association for Cognitive Behaviour Therapy (AACBT), and Association for Behavioral and Cognitive Therapies (ABCT).
An issue-by-issue examination of some relevant journals for this field from January 1990 to January 2014 will be conducted. The following journals will be hand-searched: American Journal of Psychiatry; Behaviour Research and Therapy; Behavior Therapy; Psychotherapy and Psychosomatics; Journal of Obsessive Compulsive and Related Disorders; Cognitive Behaviour Therapy; Journal of Behavior Therapy and Experimental Psychiatry; Psychological Medicine; Journal of Affective Disorders; Journal of Clinical Psychiatry; Journal of Clinical and Consulting Psychology.
Study selection
Studies will be assessed according to the eligibility criteria by two independent reviewers (AP and DD) during three different stages. During the first and the second stage, studies will be examined with regard to the inclusion criteria after reading the title and abstract, respectively. During these stages, studies will be retained when there is no agreement on inclusion between the reviewers. Finally, the remaining studies will be assessed in terms of the eligibility criteria after the reading the full-text article. After each stage, the reviewers will discuss reasons for inclusion, and potential discrepancies in judgment will be addressed during meetings with an independent reviewer (GA) with the aim of obtaining a shared pool of included studies for the meta-analysis. Between-reviewer agreement of study inclusion will be calculated by computing Cohen’s kappa index.19
Assessment of risk of bias
The methodological quality of the included RCTs will be assessed using the Cochrane Collaboration’s tool for risk of bias assessment.20 Two of the reviewers (AP and DD) will conduct the risk of bias assessments independently. Each discrepancy will be discussed and resolved in meetings. Each study will be rated for risks of bias owing to selection bias (random sequence generation and allocation concealment), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other biases. Risk of bias due to blinding and incomplete outcome data will be separately assessed within each included study for different outcomes. Since the blinding of participants can be critical in trials on the efficacy of psychotherapy, we did not use this item to assess the quality of RCTs on GCBT.
Risk of bias assessment will be conducted within each included trial and across the included trials. According to guidelines provided by Higgins and Green,20 each domain is rated as high, low, or unclear. For within-trial assessments, risk of bias will be classified as low if it is regarded as low by the two independent reviewers for all domains, it will be classified as unclear if it is regarded as low or unclear for all the domains, and it will be classified as high if it is regarded as high for one or more domains. For between-trial assessment, risk of bias will be classified as: 1) low, if most information is from trials at low risk of bias; 2) unclear, if most information is from trials at low or unclear risk of bias; and 3) high, if the proportion of information from trials at high risk of bias is sufficient to affect interpretation of the results.20
Coding of moderators
If the inconsistency analyses indicate large and significant heterogeneity between effect sizes, the role of the moderators will be investigated. Two independent reviewers (DD and AP) will code the moderators, extract the data from the primary studies, and insert them in an Excel worksheet. Subsequently, during meetings between the two reviewers, insertion of the data in the worksheets will be checked for accuracy, and each potential discrepancy will be discussed and resolved. The following variables will be coded as moderators:
- Participants’ characteristics: 1) mean age of the sample; 2) sex of the sample (coded as the percentage of female participants); 3) co-occurence of comorbid personality disorders (percentage of participants with comorbid personality disorders included in the sample); 4) OCD symptom severity (coded as a continuous variable based on the scores on the Yale–Brown Obsessive Compulsive Scale [Y-BOCS]); 5) severity of concurrent depressive symptoms (coded as a continuous variable based on the scores on the Beck Depression Inventory [BDI]-II); 6) duration of OCD symptoms coded as the number of years from the first diagnosis of OCD made by a mental health professional, or as the age of onset self-reported by the patient; and 7) generational cohort (categorical variable: children/adolescent versus adult samples).
- Treatment characteristics: 1) duration of treatment (coded as the number of weeks); 2) intensity of treatment (coded as the number of sessions per week and the number of treatment hours per week); and 3) proportion of patients on concurrent medication.
- Study characteristics: 1) date of publication; 2) methodological quality (as a continuous variable based on the scores on the Cochrane Collaboration’s tool).
If these data are not reported in the paper, the authors of the study will be contacted to request the data.
Statistical analysis
Power calculations
An a priori power analysis was performed with the aim of investigating the number of studies requested to achieve statistically powerful analyses in order to identify a small effect size, as defined in Lipsey and Wilson.21 Power calculations were conducted according to the guidelines provided by Borenstein et al.22 Calculations suggested that we would need to include at least 20 studies with a mean sample size of 30 (15 participants per condition) to be able to detect an effect size of 0.30, assuming a medium-level study variance, a statistical power of 0.80, and a criterion for significance set at 0.05 (two-tailed test). Alternatively, we would need 15 studies including 40 participants each, to detect an effect size of 0.30. The power analysis was conducted using the Power and Precision software version 4.00.
Data extraction and summary measures
Before calculating the effect sizes, potential outlier studies will be identified through the sample-adjusted meta-analytic deviance method.23
As we expect noticeable heterogeneity across the included studies, effect sizes will be calculated using a random-effects model. Random-effects models assume that the included studies are drawn from populations of studies that systematically differ from each other. According to these models, the effect sizes derived from included studies differ not only because of the random error within studies (as in the fixed-effects model), but also because of the true variation in effect sizes from one study to the other.23 Analyses will be conducted separately for studies comparing GCBT versus control conditions (no treatment or waitlist) for studies comparing GCBT versus active control conditions, and for studies comparing GCBT versus individual CBT.
The data requested for the calculation of the effect sizes (Hedges’ g)24 will be extracted independently by two meta-analysts (DD and AP) through the following formula provided in Equation 1:
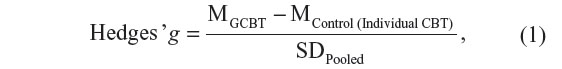
where MGCBT is the mean of the groups of patients treated with GCBT, MControl (Individual CBT) is the mean number of patients assigned to control conditions or individual CBT, and SDPooled is the pooled standard deviation.
The effect size for each study will be weighted through the application of the following correction formula provided in Equation 2:
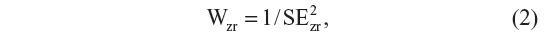
where SE2zr is the standard error of the effect size computed for each study.
Effect sizes will be estimated using a 95% confidence interval and interpreted according to the criteria suggested by Cohen.19 Thus, effect sizes of 0.80 or more will be assumed to be large, 0.50 moderate, and 0.20 small.20 According to Hedges and Olkin,24 Hedges’ correction for small sample bias will be applied to all effect sizes.
A global effect size will be calculated as a mean effect size obtained by combining effect sizes related to all the considered outcomes. Effect sizes will be pooled for self-report instruments and interviews in a first phase. Subsequently, analyses will be performed separately for self-report instruments and then for interviews. In addition, effect sizes will be calculated separately as well for each of the aforementioned outcomes.
Inconsistency analysis
In order to assess between-studies heterogeneity, two complementary indices will be used: the I2 index21 and the Q statistic,24 respectively. The I2 index determines, in percentage, the degree of heterogeneity in the effect sizes of the included studies.21 A value approximating zero suggests homogeneity, whereas values of 25%–50%, 50%–75%, and 75%–100% represent low, medium, and large heterogeneity, respectively.21
Analysis of moderators
If the inconsistency analysis suggests large heterogeneity, an analysis of the aforementioned moderators will be conducted using a mixed-model analysis of variance and weighted least squares meta-regressions.
Publication bias
In order to investigate the likelihood that the effect sizes are subjected to publication bias, Orwin’s fail-safe N method25 and a visual inspection of the funnel plot will be used.
Statistical analysis will be performed using the Power and Precision software version 4.00 and the Comprehensive Meta-Analysis software version 2.00 to conduct power calculations and meta-analyses, respectively.
Disclosure
The authors report no conflicts of interest in this work.
References
Crino R, Slade T, Andrews G. The changing prevalence and severity of obsessive-compulsive disorder criteria from DSM-III to DSM-IV. Am J Psychiatry. 2005;162(5):876–882. | |
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed. Washington, DC: American Psychiatric Association; 2013. | |
Murray CJ, Lopez AD. The utility of DALYs for public health policy and research: a reply. Bull World Health Organ. 1997;75(4):377–381. | |
Gava I, Barbui C, Aguglia E, et al. Psychological treatments versus treatment as usual for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2007;(2):CD005333. | |
Fisher PL, Wells A. How effective are cognitive and behavioral treatments for obsessive-compulsive disorder? A clinical significance analysis. Behav Res Ther. 2005;43(12):1543–1558. | |
Taylor S, Abramowitz JS, McKay D. Non-adherence and non-response in the treatment of anxiety disorders. J Anxiety Disord. 2012;26(5):583–589. | |
Mancebo MC, Eisen JL, Pinto A, Greenberg BD, Dyck IR, Rasmussen SA. The brown longitudinal obsessive compulsive study: treatments received and patient impressions of improvement. J Clin Psychiatry. 2006;67(11):1713–1720. | |
Baer L, Minichiello WE. Reasons for inadequate utilization of cognitive-behavioral therapy for obsessive-compulsive disorder. J Clin Psychiatry. 2008;69(4):676. | |
Lind C, Boschen MJ, Morrissey S. Technological advances in psychotherapy: implications for the assessment and treatment of obsessive compulsive disorder. J Anxiety Disord. 2013;27(1):47–55. | |
Sousa MB, Isolan LR, Oliveira RR, Manfro GG, Cordioli AV. A randomized clinical trial of cognitive-behavioral group therapy and sertraline in the treatment of obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(7):1133–1139. | |
Himle JA, Rassi S, Haghighatgou H, Krone KP, Nesse RM, Abelson J. Group behavioral therapy of obsessive-compulsive disorder: seven vs twelve-week outcomes. Depress Anxiety. 2001;13(4):161–165. | |
Jónsson H, Hougaard E. Group cognitive behavioural therapy for obsessive-compulsive disorder: a systematic review and meta-analysis. Acta Psychiatr Scand. 2009;119(2):98–106. | |
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. | |
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P).New York: Biometrics Research, New York State Psychiatric Institute, 2002. | |
clinicaltrials.gov [homepage on the Internet]. US National Library of Medicine. Available from: https://clinicaltrials.gov/. Accessed July 16, 2015. | |
who.int/ictrp [homepage on the Internet]. World Health Organization. Available from: http://who.int/ictrp/. Accessed July 16, 2015. | |
isrctn.com [homepage on the Internet]. ISRCTN Registry, BioMed Central Ltd. Available from: http://isrctn.com/. Accessed July 16, 2015. | |
anzctr.org.au [homepage on the Internet]. The Australian New Zealand Clinical Trials Registry NHMRC Clinical Trials Centre. Available from: http://anzctr.org.au/. Accessed July 16, 2015. | |
Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1977. | |
Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons Ltd; 2008. | |
Lipsey MW, Wilson DB. Practical Meta-Analysis (Applied Social Research, Methods Series, Vol 49). Thousand Oaks, CA: Sage Publications; 2001. | |
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2009. | |
Huffcutt AI, Winfred A. Development of a new outlier statistic for meta-analytic data. J Appl Psychol. 1995;80(2):327–334. | |
Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press, Inc.; 1985. | |
Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Behav Stat. 1983;8(2):157–159. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
