Back to Journals » OncoTargets and Therapy » Volume 13
WDR5 Promotes Proliferation and Correlates with Poor Prognosis in Oesophageal Squamous Cell Carcinoma
Authors Huang D, Chen X, Chen X, Qu Y , Wang Y, Yang Y, Cheng Y
Received 15 October 2019
Accepted for publication 11 September 2020
Published 15 October 2020 Volume 2020:13 Pages 10525—10534
DOI https://doi.org/10.2147/OTT.S234773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Di Huang,1 Xue Chen,1 Xuan Chen,1 Yan Qu,1 Yuanyuan Wang,2 Yafei Yang,3 Yufeng Cheng1
1Department of Radiation Oncology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, 250012, People’s Republic of China; 2Department of Oncology, People’s Hospital of Linyi County, Dezhou, Shandong 251500, People’s Republic of China; 3Department of Hepatobiliary Surgery, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, 250012, People’s Republic of China
Correspondence: Yufeng Cheng Department of Radiation Oncology
Qilu Hospital, Cheeloo College of Medicine, Shandong University, 107 West Wenhua Road, Jinan, Shandong 250012, People’s Republic of China
, Email [email protected]
Background: The WD40 protein family member WD repeat domain 5 (WDR5) plays significant roles in the tumorigenesis and development of multiple organ tumours. However, the correlation between WDR5 expression and oesophageal squamous cell carcinoma (ESCC) has not been elucidated.
Methods: WDR5 mRNA expression data were acquired from The Cancer Genome Atlas (TCGA) database, and the expression and prognostic potential of WDR5 were assessed by immunohistochemistry (IHC) and Western blot. The cell counting kit-8 (CCK-8), colony formation assay and cell cycle evaluation were performed to verify the WDR5 function in vitro. The xenograft model was used to verify WDR5 function in vivo.
Results: The mRNA and protein expression levels of WDR5 were significantly upregulated in ESCC tissues compared with expression in adjacent normal tissues. Kaplan–Meier analysis showed that high WDR5 expression in ESCC patients was associated with poor overall survival (P=0.004). Multivariate analysis revealed that WDR5 overexpression emerged as an independent predictor of poor overall survival (P=0.013) in ESCC. The in vitro and in vivo experiments revealed that downregulation of WDR5 expression blocked cell proliferation of ESCC. Mechanistically, we found that WDR5 may influence ESCC proliferation by targeting the PI3K/AKT/mTOR signalling pathway.
Conclusion: Our data demonstrate that overexpression of WDR5 was associated with poor prognosis in patients with ESCC and that WDR5 may act as a potential novel prognostic biomarker for ESCC.
Keywords: WDR5, oesophageal squamous cell carcinoma, prognosis, proliferation
Introduction
Oesophageal cancer is one of the most common malignancies globally. This disease ranks seventh in terms of incidence (572,000 new cases) and sixth in mortality overall (509,000 deaths) worldwide.1 The most common oesophageal cancer histologic subtype in China is squamous cell carcinoma, which usually comprises over 90% of all oesophageal cancer cases.2 Oesophageal squamous cell carcinoma (ESCC) is difficult to treat successfully and has a poor prognosis as a result of the difficulty of early diagnosis; additionally, many patients have metastases at diagnosis. The 5-year overall survival (OS) rate ranges from 15% to 25%.3 Hence, there is an urgent need to find predictive, prognostic and therapeutic markers for oesophageal cancer.
WDR5 is one of the most famous proteins in the WD40 protein family and plays significant roles not only in the development of vertebrates4,5 but also in the tumorigenesis and development of multiple organ tumours.6,7 WDR5, together with the histone methyltransferase MLL1 and SET1 families,8 forms a functional complex, which catalyses the methylation of H3K49 and then promotes gene transcription and influences many biological behaviours of tumours, such as proliferation and invasion.7 In addition, WDR5 can regulate the expression of the p5310 and notch11 signalling pathways or interact with MYC protein,12,13 a principal driver of the development and progression of tumours. However, the correlation between WDR5 expression and ESCC has not been elucidated.
In the present study, we aimed to evaluate the expression of WDR5 in ESCC as well as its prognostic implications.
Materials and Methods
Cell Lines and Materials
The ESCC cell line KYSE-150 was purchased from the China Centre for Type Culture Collection (Beijing, China). KYSE-150 cells were cultured in RPMI-1640 medium (Gibco, Life Technologies Inc., Grand Island, NY, USA) supplemented with 10% foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified 5% CO2 atmosphere.
Colony Formation Assay
Transfected KYSE-150 cells were trypsinized into single-cell suspensions and plated in six-well culture plates at 500 cells/well. After the cells were cultured for two weeks, they were fixed with methanol and then stained with crystal violet. We then calculated the number of colonies. At least 50 cells were defined as clone formation.
Cell Cycle Analysis
Each group of transfected cells was inoculated into 6-well plates at 3 × 10^5 cells/well. Cells were washed twice with pre-cooled PBS. Next, the cells were fixed with pre-cooled 70% ethanol and stored at −20°C for 1 hour. The cells were washed twice with pre-cooled PBS. Then, 200 μL of pre-cooled PBS was used to prepare the cell suspension. The cells were mixed with 20 µL of RNase A solution in a 37°C water bath for 30 min. Then, 400 µL of PI was added to each tube and incubated at 4°C for 30 min in the dark. The cycle distribution of each group of cells was measured by flow cytometry. The results were analysed by the ModFit software.
Western Blot Analysis
Different groups of cells were treated with RIPA lysate, and the extracted protein concentration was determined by the BCA protein concentration assay kit. The protein sample was mixed with the loading buffer and denatured in a boiling water bath for 10 minutes. Samples were electrophoresed on 10% SDS-PAGE gels and then transferred to PVDF membranes on ice. After blocking in 5% dry milk for 90 minutes, membranes were incubated with the primary antibody overnight at 4°C on a shaker. Membranes were incubated with horseradish peroxidase-labelled secondary antibody at room temperature for 1 h. The bands were detected by the chemiluminescence detection system. The primary antibodies included WDR5 (1 µg/mL, AF5810, R&D), β-actin (1:1000 dilution, sc-58673, Santa Cruz Biotechnology), mTOR (1:1000, 2983, Cell Signalling Technology), p-mTOR (1:1000, 5536, Cell Signalling Technology), PI3K (1:1000, 4249, Cell Signalling Technology), p-PI3K (1:1000, ab182651, abcam), AKT (1:2000, 2920, Cell Signalling Technology), p-AKT (1:2000, 4060, Cell Signalling Technology), P70S6K (1:1000, 2708, Cell Signalling Technology), p-P70S6K (1:1000, 9204, Cell Signalling Technology), S6 (1:1000, 2217, Cell Signalling Technology), p-S6 (1:2000, 4858, Cell Signalling Technology), and Cyclin D1 (1:1000, 55506, Cell Signalling Technology).
Cell Counting Kit-8 (CCK-8) Assay
The two groups of cells were inoculated into a 96-well cell culture plate at 1 x 10^4 cells/mL, and 200 μL of cell suspension was added to each well and then cultured at 37°C in a 5% CO2 incubator for 0, 1, 2, 3, and 4 days. Ten micrilitre of CCK-8 reagent were added to each well, and after incubation at 37°C for 2 h, the absorbance was measured on a microplate reader at 450 nm, and the optical density (OD) values of each group were recorded. The cell proliferation rate was calculated as follows: Cell proliferation rate = (transfection group cell OD/control cell OD) × 100%
Immunohistochemistry (IHC)
The oesophageal cancer tissue was soaked with 10% formaldehyde and embedded in paraffin to make a 4μm paraffin section. Paraffin sections were routinely dewaxed and rehydrated, and citrate-EDTA buffer (2 mM EDTA, 10 mM citric acid, 0.05% Tween 20, pH 6.2) was used for antigen retrieval. Sections were incubated at room temperature with hydrogen peroxide to block peroxidase. Then, the anti-WDR5 antibody (5 µg/mL, AF5810, R&D) or anti-Ki-67 (1:800, 9449, Cell Signalling Technology) was applied to the sections at 4°C overnight. The sections were incubated with biotinylated secondary antibodies to react with horseradish peroxidase (HRP)-labelled streptomycin anti-biotin proteins. The sections were then stained with DAB and stained with haematoxylin. Two pathologists assessed the staining intensity of the stained slides using a blind method without knowing the patient’s basic information or clinical characteristics. Five fields were randomly selected under high power microscopy, and the number of positive cells was counted and averaged. The staining intensity and number of positive cells were used to calculate the IHC score. The staining degree was divided into the following groups: 0 (no staining), 1 (weak staining), 2 (medium staining), and 3 (strong staining). The number of positive cells was divided into 0 (<5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), and 4 (>75%). The arithmetic product of the two scores is designated as the final score: 0–1 (-), 2–4 (+), 5–8 (++) and 9–12 (+++). Samples with a 9–12 (+++) score were regarded as overexpressed.
Cell Transfection
We purchased lentiviruses (GenePharma, Inc.) expressing a shRNA form of WDR5. The ESCC cell lines were seeded in 6-well plates (3*10^5/well) and incubate for 24h. The shRNA targeting WDR5 lentiviruses was transfected into cells with 5mg/mL polybrene (GenePharma). Puromycin selection (2 mg/mL) was applied to select stably transduced cells. Western blotting was performed to confirm the knockdown of WDR5. The sequences of shRNA were as follows:
shNC sense sequences: 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ; shNC anti-sense sequences: 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ; shWDR5 sense sequences: 5ʹ-CACCUGUGAGCCAAACUATT-3ʹ; shWDR5 anti-sense sequences: 5ʹ-UAGUUUGGCUUCACAGGUGTT-3ʹ
Patients and Tissue Samples
Primary tumour tissues and corresponding adjacent tissues were obtained from 104 patients diagnosed with ESCC at Qilu Hospital of Shandong University from January 2008 to December 2008. Patients were selected according to the following inclusion criteria: (i) patients who underwent radical resection with a clear surgical margin; (ii) patients with available formalin-fixed tumour tissues, follow-up information and complete medical records; (iii) no patients received neoadjuvant therapy; and (iv) patients with no history of other malignancies. All samples were verified by pathological evaluation. All 104 patients were followed for at least 5 years. The tumour staging was in accordance with the American Joint Committee on Cancer (AJCC, 7th edition).
Human Oesophageal Cancer Xenograft
Ten female athymic nude mice (6–8 weeks old, BALB/c-nu/nu) were randomly assigned to one of two groups of 5 mice each: KYSE-150-shNC and KYSE-150-shWDR5. ESCC cells (4 x 10^6 in 0.1 mL of complete medium) were injected subcutaneously into the right dorsal side of each mouse. The mice were euthanized after three weeks to harvest the tumours. All mouse protocols were approved by the Animal Care and Use Committee of Shandong University, and conducted according to the guidelines of the National Animal Welfare Law of China.
Statistical Analysis
Statistical analysis was performed using SPSS software (Statistical Package for Social Sciences, Chicago, IL, USA). The correlation between WDR5 and clinicopathological factors was determined by the bilateral Χ2 test. Survival curves were obtained using the Kaplan-Meier method and log-rank test, and differences between subgroups were compared. Univariate and multivariate Cox regression analyses were used to assess risk ratios (HRs). P <0.05 was considered statistically significant.
Ethics Statement
The research protocol of the present study was approved by the Ethics Committee of Qilu Hospital, and all patients signed written informed consent before enrolment. Patient information was anonymized and unidentifiable prior to analysis.
Results
WDR5 Was Overexpressed in ESCC Tissues
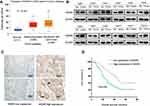
According to the TCGA database (http://ualcan.path.uab.edu/cgi-bin/TCGAExResultNew2.pl?genenam=WDR5&ctype=ESCA), WDR5 is upregulated in ESCC tissues compared with that in normal tissues (Figure 1A, P<0.001). We used Western blot analysis to detect protein levels between 12 ESCC tissues and 12 adjacent normal tissue samples. It was found that the protein levels of WDR5 expression in 8 of the 12 pairs of ESCC tissues were significantly higher than those in adjacent normal tissues (Figure 1B). Then, we measured WDR5 expression in 104 ESCC tissue samples by immunohistochemical staining and found that WDR5 was expressed at low levels in 48 (46.15%) ESCC samples, and the remaining 56 (53.85%) samples maintained high-expression levels (Figure 1C). These results indicate that the expression of WDR5 was significantly upregulated in ESCC tissues.
Relationship Between WDR5 Expression and Corresponding Clinical Pathological Features
The study included 104 patients, and all patients were from Qilu Hospital. Among the 104 ESCC patients, 56 patients were in the WDR5 high-expression group (score 9–12), and 46 were in the low-expression group (score ≤8). To assess the clinical significance of WDR5, we used the chi-square test to analyse the association between WDR5 expression and clinical pathological features in ESCC. The results showed that high expression of WDR5 in ESCC tissues was associated with T stage (P = 0.032) and TNM stage (P = 0.038). However, no significant correlation was found between WDR5 expression and other clinicopathological features, including age, sex, smoking, drinking, lymphatic metastasis, distant metastasis, and degree of differentiation (Table 1).
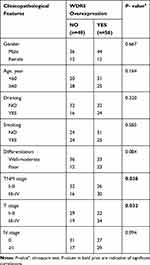 |
Table 1 The Correlations of Clinicopathologic Features of ESCC with WDR5 Expression in FFPE Cancerous Tissues |
High Expression of WDR5 Predicts Poor Prognosis in ESCC
To investigate the prognostic impact of WDR5, Kaplan-Meier survival curves and log rank tests were used to analyse the overall survival (OS) of ESCC patients. The results showed that for all 104 samples, high expression of WDR5 was negatively correlated with OS (P = 0.004) (Figure 1D). In addition, univariate and multivariate analyses were performed to confirm that WDR5 may be an independent risk factor for poor prognosis in 104 ESCC patients. Univariate Cox regression analysis showed that WDR5 expression (P=0.004), differentiation (P=0.033), T stage (P=0.001), N stage (P=0.001), and TNM stage (P=0.001) were significantly associated with overall survival (OS) (Table 2). Multivariate Cox regression analysis confirmed that WDR5 expression (P=0.013), T stage (P=0.008) and TNM stage (P=0.009) were independent predictors of OS in WDR5 patients (Table 2). That is, patients with low expression of WDR5 have a longer survival time than do patients with high WDR5 expression.
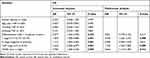 |
Table 2 Univariate and Multivariate Analyses Showing the Overall Survival in Esophageal Cancer |
WDR5 Promotes ESCC Cell Proliferation and Enhances the Cell Cycle in vitro
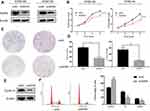
The KYSE-150 and KYSE-140 cell lines with high WDR5 expression were selected to knockdown the expression of WDR5, and we detected the transfection efficiency of shWDR5 using Western blotting (Figure 2A). The WDR5 protein was lower in the shWDR5 transfected group than that in the shNC group. The biological function of WDR5 in ESCC cell proliferation was detected by the CCK-8 and colony formation assays. The CCK-8 assay results showed that the proliferation index of the shWDR5 group was significantly lower than that of the shNC group (Figure 2B). This finding in the CCK-8 assay is consistent with the colony formation assay, and the number of clones was lower when WDR5 was downregulated (Figure 2C and D). We found that cyclin D1 in KYSE-150 shWDR5 group was lower than that in the shNC group (Figure 2E), and we detected the status of the cell cycle in these two groups. As a result of flow cytometry, we found that downregulated expression of WDR5 could lead to G1/S phase arrest (Figure 2F).
WDR5 Promotes ESCC Cell Tumorigenicity in vivo
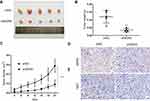
To further verify the role of WDR5 in vivo, 10 mice were divided randomly into 2 groups. Then, shNC and shWDR5 KYSE-150 cells were injected subcutaneously into BALB/c nude mice. As shown in Figure 3A–C, the volume and weight of xenograft shWDR5 tumours were obviously smaller than those in the control group. In addition, we further examined the WDR5 expression levels in tumour tissues from nude mouse tumours by IHC analyses (Figure 3D). Compared to in vitro studies, WDR5 expression was observed to have a similar trend in in vivo experiments, which also indicates that WDR5 regulates the proliferation of ESCC. IHC demonstrated that the proliferation marker Ki-67 (Figure 3E) in the shWDR5 group was reduced in xenograft models.
WDR5 May Enhance ESCC Cell Proliferation via the PI3K/AKT/mTOR Pathway
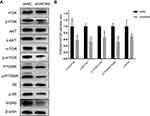
Given that WDR5 is associated with the PI3K/AKT pathway in other tumours14 and that the PI3K/AKT pathway is associated with proliferation of ESCC,15 we next examined the effect of knockdown of WDR5 on the PI3K/AKT pathway in KYSE-150 by Western blot (Figure 4A). As expected, Western blotting showed a decrease in p-AKT/AKT, p-PI3K/PI3K, p-mTOR/mTOR, p-P70S6K/P70S6K and p-S6/S6 after knockdown of WDR5 (Figure 4B). These results indicate that WDR5 affect the expression of PI3K/AKT/mTOR pathway. We speculate that WDR5 enhance ESCC cell proliferation may via the PI3K/AKT/mTOR pathway, but the specific regulatory mechanisms need to be further studied.
Discussion
Oesophageal cancer is one of the most common cancers worldwide. In recent years, with the increase in awareness and treatment of oesophageal cancer, morbidity and mortality have decreased, but oesophageal cancer is still the sixth leading cause of cancer death in the world.1 In China, the mortality of ESCC ranks fourth among all human tumours.16
WDR5 is a core subunit of the human MLL1-4 histone H3K4 methyltransferase complex.9 WDR5 forms complexes with MLL1, RBBP5, ASH2L and DPY30 and plays a key role in histone H3K4 trimethylation, chromatin remodelling, transcriptional activation of target genes, normal biology and diseases such as MLL rearrangement of leukaemia.7,17–19 WDR5 has been reported to play a role in many types of cancer. For instance, WDR5 has been shown to be clearly associated with colorectal cancer metastasis.14 WDR5 expression is increased in leukaemia patients, and high expression of WDR5 is associated with high-risk leukaemia.20 Although WDR5 has been shown to play a role in a variety of cancers, its role in human oesophageal cancer remains unclear.
In this study, we first discovered that WDR5 is overexpressed in human ESCC tissues. Survival analysis showed that patients suffering from ESCC with high levels of WDR5 expression had shorter survival than patients with low WDR5 expression. Univariate and multivariate analysis showed that WDR5 may be an independent prognostic marker for ESCC. This suggests that WDR5 has a highly sensitive and specific prognostic value in ESCC. In addition, T staging and TNM staging are also correlated with the prognosis of ESCC patients. Our study also confirmed the correlation between T staging and TNM staging and WDR5 expression. These results raise the possibility of WDR5 serving as an ESCC oncogene.
To reveal the effect of WDR5 in ESCC in vitro, we performed CCK-8 and colony formation assays. The proliferation index and number of colonies were lower in the shWDR5-transfected groups. These results indicated that knockdown of WDR5 expression can inhibit the proliferation of ESCC cells. Furthermore, this study suggests that the anti-cancer effect after knockdown of WDR5 may occur by inhibiting the cell cycle in KYSE-150. Cyclin D1 protein levels were reduced, and flow cytometry also demonstrated that the cell cycle was arrested in G1/S phase after knockdown of WDR5 expression. Our results are consistent with previous studies in other tumours.21 The PI3K-AKT pathway, which is widely known as an important intracellular signalling pathway, plays a dominant role in apoptosis and proliferation of tumour cells by regulating the activation of downstream targets.22,23 Precisely, the PI3K-AKT pathway has been shown to correlate with the genesis and progression of tumours as well as aggressive biological behaviours. In a previous study of colon cancer, WDR5 was found to be closely related to the PI3K-AKT pathway.14 Our previous studies also confirmed that RACK1 induces chemoresistance in ESCC by upregulating the PI3K/AKT pathway and Bcl-2 expression.15 Here, we report that WDR5 may regulate the development of ESCC through the PI3K/AKT/mTOR signalling pathway.
However, we only demonstrated the correlation between the WDR5 knockdown and the activation of PI3K/Akt/mTOR pathway, no cause-and-effectiveness study was attempted. It is indeed possible that WDR5 may also target other signalling pathways or mechanisms in addition to the PI3K/AKT/mTOR pathway. Previous study showed that WDR5 regulates Cyclin D1 expression by H3K4me3 leading to cancer cell proliferation in gastric cancer cells.21 In this study, we also found knockdown the expression of WDR5 decrease the expression of Cyclin D1. These results are consistent with previous studies in other tumours. Therefore, we speculate that WDR5 may induces histoneH3K4 trimethylation at the Cyclin D1 gene promoter and Cyclin D1 gene transcription in ESCC, which may be one of the reasons for ESCC proliferation. A deeper understanding of tumorigenicity may be required to glean other potential functions of WDR5 in ESCC.
In conclusion, WDR5 is critical for cell development, and its dysregulation is particularly important in ESCC. We demonstrate that WDR5 is upregulated in ESCC and promotes ESCC proliferation may through the PI3K/AKT/mTOR signalling pathway and Cyclin D1. This study shows that WDR5 is an important marker of ESCC and plays a key role in the development of ESCC.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All experiments were performed according to the protocol approved by the Ethics Committee of Qilu Hospital (KYLL-2015-069)
Funding
We appreciate all subjects who participated in this study. The authors declare no conflict of interest. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was funded by the National Nature Science Foundation of China (No. 81972850, No. 81773228) and Special Fund for Taishan Scholar Project(NO.ts20190973).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.21492
2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262
3. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi:10.1016/S0140-6736(12)60643-6
4. Gori F, Friedman LG, Demay MB. Wdr5, a WD-40 protein, regulates osteoblast differentiation during embryonic bone development. Dev Biol. 2006;295:498–506. doi:10.1016/j.ydbio.2006.02.031
5. Gori F, Zhu ED, Demay MB. Perichondrial expression of Wdr5 regulates chondrocyte proliferation and differentiation. Dev Biol. 2009;329:36–43. doi:10.1016/j.ydbio.2009.02.006
6. Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu Rev Biochem. 2012;81:65–95. doi:10.1146/annurev-biochem-051710-134100
7. Lu K, Tao H, Si X, Chen Q. The histone H3 lysine 4 presenter WDR5 as an oncogenic protein and novel epigenetic target in cancer. Front Oncol. 2018;8:502. doi:10.3389/fonc.2018.00502
8. Avdic V, Zhang P, Lanouette S, et al. Structural and biochemical insights into MLL1 core complex assembly. Structure. 2011;19:101–108. doi:10.1016/j.str.2010.09.022
9. Li Y, Han J, Zhang Y, et al. Structural basis for activity regulation of MLL family methyltransferases. Nature. 2016;530:447–452. doi:10.1038/nature16952
10. Xie Q, Li Z, Chen J. WDR5 positively regulates p53 stability by inhibiting p53 ubiquitination. Biochem Biophys Res Commun. 2017;487:333–338. doi:10.1016/j.bbrc.2017.04.060
11. Chung CY, Sun Z, Mullokandov G, et al. Cbx8 acts non-canonically with Wdr5 to promote mammary tumorigenesis. Cell Rep. 2016;16:472–486. doi:10.1016/j.celrep.2016.06.002
12. Thomas LR, Wang Q, Grieb BC, et al. Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC. Mol Cell. 2015;58:440–452. doi:10.1016/j.molcel.2015.02.028
13. Thomas LR, Foshage AM, Weissmiller AM, Tansey WP. The MYC-WDR5 nexus and cancer. Cancer Res. 2015;75:4012–4015. doi:10.1158/0008-5472.CAN-15-1216
14. Tan X, Chen S, Wu J, et al. PI3K/AKT-mediated upregulation of WDR5 promotes colorectal cancer metastasis by directly targeting ZNF407. Cell Death Dis. 2017;8:e2686. doi:10.1038/cddis.2017.111
15. Liu B, Wang C, Chen P, Cheng B, Cheng Y. RACKI induces chemotherapy resistance in esophageal carcinoma by upregulating the PI3K/AKT pathway and Bcl-2 expression. Onco Targets Ther. 2018;11:211–220. doi:10.2147/OTT.S152818
16. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
17. Karatas H, Li Y, Liu L, et al. Discovery of a highly potent, cell-permeable macrocyclic peptidomimetic (MM-589) targeting the WD repeat domain 5 protein (WDR5)-mixed lineage leukemia (MLL) protein-protein interaction. J Med Chem. 2017;60:4818–4839. doi:10.1021/acs.jmedchem.6b01796
18. Dou Y, Milne TA, Ruthenburg AJ, et al. Regulation of MLL1 H3K4 methyltransferase activity by its core components. Nat Struct Mol Biol. 2006;13:713–719. doi:10.1038/nsmb1128
19. Zhang P, Lee H, Brunzelle JS, Couture JF. The plasticity of WDR5 peptide-binding cleft enables the binding of the SET1 family of histone methyltransferases. Nucleic Acids Res. 2012;40:4237–4246. doi:10.1093/nar/gkr1235
20. Ge Z, Song EJ, Kawasawa YI. WDR5 high expression and its effect on tumorigenesis in leukemia. Oncotarget. 2016;7:37740–37754. doi:10.18632/oncotarget.9312
21. Sun W, Guo F, Liu M. Up-regulated WDR5 promotes gastric cancer formation by induced cyclin D1 expression. J Cell Biochem. 2018;119:3304–3316. doi:10.1002/jcb.26491
22. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi:10.1038/nrd1902
23. Wang J, Feng W, Dong Y, Mao X, Guo F, Luo F. MicroRNA‑495 regulates human gastric cancer cell apoptosis and migration through Akt and mTOR signaling. Oncol Rep. 2018;40:3654–3662.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.