Back to Journals » Journal of Inflammation Research » Volume 15
Water Extract of Senecio scandens Buch.-Ham Ameliorates Pruritus by Inhibiting MrgprB2 Receptor
Authors Ye F, Jiang Y, Zhang J, Zong Y, Yu M, Chen C, Zhu C, Yang Y, Jia K, Chen G, Tang Z
Received 11 August 2022
Accepted for publication 18 October 2022
Published 27 October 2022 Volume 2022:15 Pages 5989—5998
DOI https://doi.org/10.2147/JIR.S384661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Fan Ye,1,* Yucui Jiang,1,2,* Jian Zhang,1 Yingxin Zong,1 Mei Yu,1,3 Cuihua Chen,2 Chan Zhu,1 Yan Yang,1 Keke Jia,1 Gongxi Chen,4 Zongxiang Tang1
1School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 2School of Chinese Medicine & School of Integrated Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 3Department of Pharmacy, Taizhou Hospital of Traditional Chinese Medicine, Nanjing University of Chinese Medicine, Taizhou, People’s Republic of China; 4School of Pharmaceutical Sciences, Jishou University, Jishou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gongxi Chen; Zongxiang Tang, Email [email protected]; [email protected]
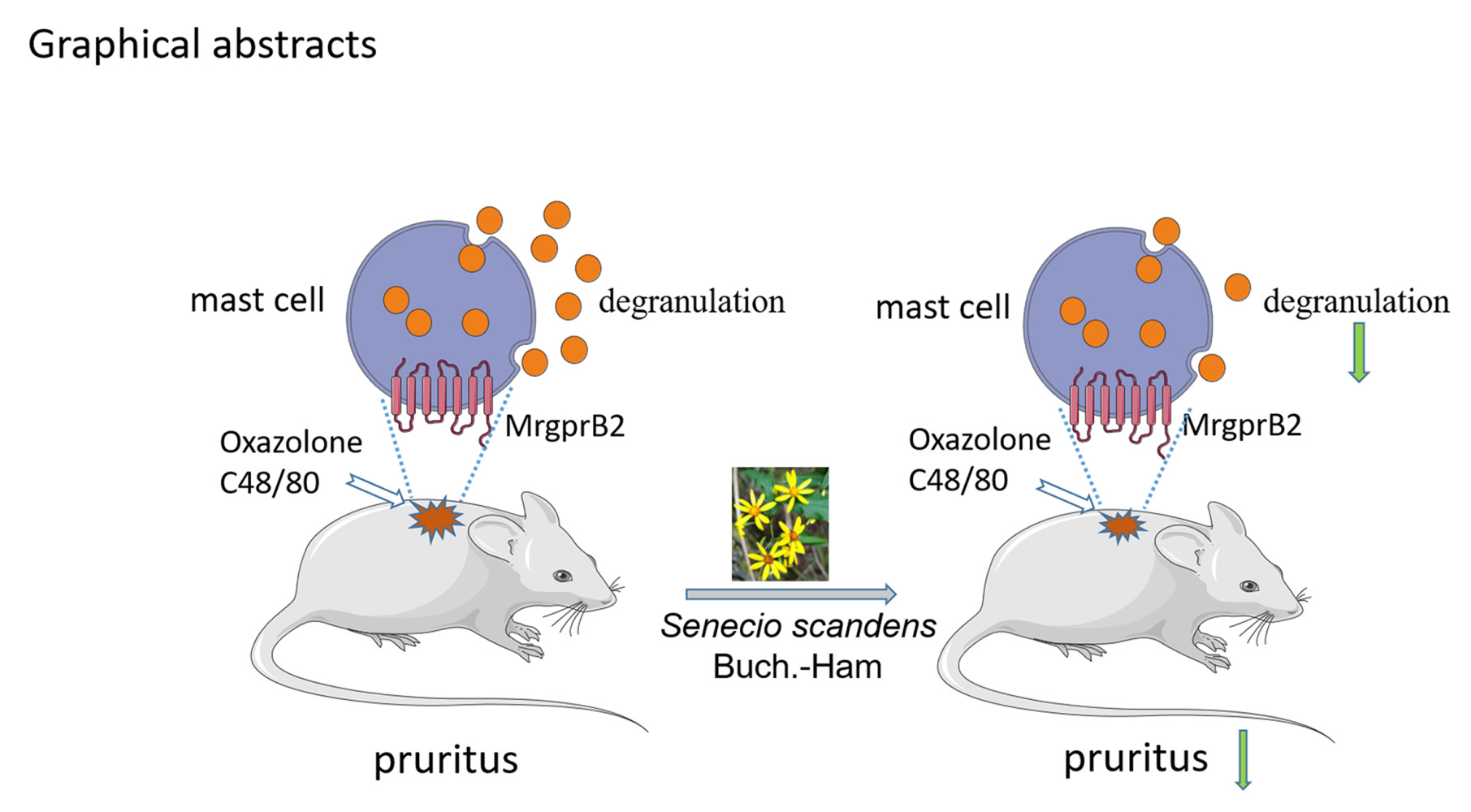
Background: Senecio scandens Buch.-Ham (S. scandens) belongs to the Compositae family. As a Traditional Chinese medicine, S. scandens has been used in China to treat conjunctivitis, mastitis and vaginitis, it also has the function of antibacterial and relieving itching.
Methods: Water extract of S. scandens (WSS) was prepared and its quality was controlled by HPLC. The antipruritic effects of WSS were evaluated by itch behavioral experiments. The oxazolone and compound 48/80 were induced to mice scratch behavior, scratch was recorded 30 min after sensitization. The relationship between the antipruritic mechanism and MrgprB2 on mast cell was studied by using mast cell-deficient Kit (W-sh) “Sash” mice and MrgprB2−/− mice. The mast cells were observed by toluidine blue staining. In vitro, the effects of WSS on MrgprB2 were studied by calcium imaging; The whole-cell patch clamp method recorded the MrgprB2 mediate voltage-dependent currents in mast cells.
Results: The content of rutin (0.012%) and hyperin (0.014%) in the WSS were determined. WSS could ameliorate the pruritus induced by Oxazolone (inhibition was 41.19%, p = 0.004) and compound 48/80 (inhibition was 50.29%, p = 0.001). Meanwhile, WSS could reduce the number of mast cells in mice skin tissue with allergic contact dermatitis (ACD) (p = 0.002) or compound 48/80 (p = 0.013). In addition, WSS could inhibit the calcium influx (1 mg/mL: p = 0.001, 3 mg/mL: p < 0.0001) and the voltage-dependent currents induced by activation of MrgprB2 on mast cell. WSS also attenuated the calcium influx induced by compound 48/80 in HEK293 cells overexpressing MrgprB2/X2.
Conclusion: These results showed that WSS could ameliorate pruritus by inhibiting MrgprB2 receptor on mast cells.
Keywords: Senecio scandens Buch.-Ham, pruritus, mast cell, MrgprB2 receptor
Graphical Abstract:
Introduction
The S. scandens is used in Chinese medicine as a treatment for antimicrobial, antioxidant and anti-inflammatory medicine.1 Studies have shown that the S. scandens water extract could be commonly used to treat amniotic keratitis.2 And could effectively alleviate the inflammation of keratoconjunctivitis in rats with allergic conjunctivitis via the NLRP3/Caspase-1/IL-1β pathway.3 Petroleum ether extract of S. scandens participated in process of anti-inflammation response by inhibiting the expression of some pro-inflammatory mediators such as NO, IL-1β and IL-6, and the activation of the NF-kB and MAPK signaling pathways.4 S. scandens flavonoid could alleviate inflammation by inhibiting the production and release of inflammatory factor prostatin E2 (PGE2) and the infiltration of white blood cell.5 Three novel polysaccharides (SP2-1, SP2-2 and SP3-2) from S. scandens have recently displayed prominent antioxidant activity and potential immune response;6 however, the antipruritic mechanism of S. scandens needs to be further studied.
Pruritus is caused by a desire or reflex to scratch.7 About 25% of people experience itching during their lifetime,8 this common symptom has a serious impact on their way of life (such as working and sleeping).9,10 The skin immune diseases have pruritus symptoms, such as allergic dermatitis (AD), allergic contact dermatitis (ACD), and urticarial.11 Since conventional anti-pruritus therapies, such as antihistamines, exert only limited effects, a drug of a treatment option for itch is urgently needed.12,13 Mast cell as sentinels, regulate innate and adaptive the immune responses and have a significant impact on disease.14 Currently, skin mast cells have been indicated to play a role of pruritus in autoimmune bullous disease.15 Mast cell activation by MrgprB2 mediates the neurogenic inflammation-evoked itch in mice, and promoted ACD-associated pruritus in mice.16 Activation of MrgprB2 receptors on mast cells, could lead to mast cell degranulation and release of histamines, tryptase, and serotonin, which are involved in pruritus. Therefore, in this study, we explored whether the antipruritic mechanism of S. scandens was related to the activity of MrgprB2.
Materials and Methods
Materials
Ethics Statement
The Experimental Animal Ethics Committee of Nanjing University of Chinese Medicine approved the development of this animal experiment. All animal experiments conformed to the Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Nanjing University of Chinese Medicine (Ethics licence ACU190601, 20,190,605).
Animals
C57BL/6J mice (WT), purchased from Charles River (Beijing, China), sash mice (C-kit mutant genetically mast cell-deficient Kit (W-sh) /Kit (W-sh)) and MrgprB2−/− mice were donated by Johns Hopkins University. Male mice and 18–22 g for experimental animals were housed at constant humidity (40–60%) and temperature (22 ± 2°C).
Reagents
DMEM culture medium, Fetal bovine serum, Penicillin, Streptomycin and Trypsin (All purchased from Gibco, USA); Fibronectin (Sigma, USA), Stem cell growth factor (Sigma, USA), Percoll (Sigma, USA), Oxazolone (Sigma, USA), Compound 48/80 (Sigma, USA). Rutin and hyperin (Yuanye, Shanghai). H&E kit (Solarbio, Beijing), Toluidine Blue (Aladdin, Shanghai).
Methods
Water Extract of S. scandens (WSS)
S. scandens (Suzhou Tianling Chinese Herbal Medicine Co. Ltd. voucher no: 210722, dry and aboveground parts) were purchased from the Taizhou Hospital of Traditional Chinese Medicine, Jiangsu, and authenticated by Gongxi Chen. The preparation method of the water extract S. scandens: first, we weighed 30 g of S. scandens in ultrapure water with a volume of 600 mL; then we boiled them at 100 °C for 30 min to collect the extract. And the materials were boiled again in 600 mL of ultrapure water with 100 °C for 30 min, then we mixed the two extracts, boiled and evaporated them to 25 mL (equivalent to 1.2 g (S. scandens)/mL). We filtered the extract through a 0.22 µm filter and stored it at −20 °C.
Behavior Tests
Pruritus behavior test in ACD: Acetone and olive oil were mixed in a ratio of 4:1, and oxazolone was added to prepare solutions containing 3% and 1% oxazolone for presensitization and sensitization, respectively. C57BL/6J, Sash and MrgprB2−/− mice were randomly divided into Vehicle group, ACD group, and ACD + WSS group, respectively. According to the human-mouse dose conversion formula, the dose of WSS was 3.9 g/kg/d (The optimal concentration of S. scandens was screened in Supplementary Figure 1), and the administration method was gavage. Four days before sensitization, presensitization was performed by adding 40 μL of 3% oxazolone solution to the hairless area of the abdomen of mice. The baseline of scratch behavior was recorded at day 0 for 30 min. Then, mice were sensitized with 1% oxazolone solution 40 μL in the middle of the hairless area of the back, and stimulated once every other day. Mice in the WSS group were given WSS 1 hour before sensitization every day. Scratch behavior was recorded 30 min after sensitization (Supplementary Figure 2).
Behavior test in compound 48/80-induced pruritus: The scratch behavior baseline of mice was recorded 1.5 h before modeling, and the WSS group was given S. scandens 1 h before modeling. We dissolved 50 μg compound 48/80 in 0.9% NaCl 25 μL and injected subcutaneously into the neck. Scratching behavior was observed for 30 min (Supplementary Figure 3).
Each group number of mice was not less than 6 in all experiments. The frequency of hind foot lift was used to evaluate scratching behavior. All experiments were conducted from August 2021 to the end of August 2022.
Histopathological Analysis
After the itching test, the skin in the model area (about 1 cm2) was removed and fixed with 4% paraformaldehyde (4°C, 12 h) and 30% sucrose solution (4°C, 12 h). After optimal cutting temperature compound (OCT) embedding, the tissue sections were prepared with a thickness of 15 microns. We used 1% toluidine blue staining to identify mast cells and hematoxylin-eosin (H&E) staining was used to evaluate skin inflammation.
Intracellular Calcium Measurement
Perfusion buffer configuration (1 L): (7.25 g NaCl, 222 mg KCl, 187 mg CaCl2, 121 mg MgCl2·6H2O, 3.6032 g glucose, 2.383 g HEPES, 6.846 g sucrose, 112.8 mg NaHCO3, PH was 7.4 by NaOH). Mast cells were treated with mixture (1 μL /mL fluo-4 AM and 0.02% Pluronic F-127 dissolve in buffer) for 30 min at 25 °C. The intracellular calcium ions were detected in anechoic chamber by 488-nm excitation.17 For HEK293 cells, the intracellular calcium ions were detected by 340 and 380 nm excitation.
Whole-Cell Currents Recording
The electrode resistance was 4–6 MΩ, and 5 μg/mL compound 48/80 as agonist. The currents signal was collected by Multiclamp 700 B amplifier (Molecular Devices, Inc, San Jose, USA), and the currents signal was digitized by Digidata 1440 A (Molecular Devices, Inc, San Jose, USA). Whole-cell currents were recorded and stored in computer. We used Clampex 10.3 for currents numerical analysis.
High Performance Liquid Chromatography (HPLC) Analysis
A mixed standard stock solution containing two reference substances of rutin and hyperin were prepared by dissolving them in methanol and their concentrations were as follows: 0.2 mg/mL rutin, 0.1 mg/mL hyperin. The mixed standard stock solution was then diluted with methanol to a series of appropriate concentrations for construction of the calibration curves.
Analyses were performed by using a reversed-phase HPLC system with a C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase consisted of 0.1% formic acid (A) and acetonitrile (B) in a ratio specified by the following binary gradient with linear interpolation: 1–3 min, 5%B; 3–6 min, 5–15%B; 6–15 min, 15–20%B; 15–17 min, 20–70%B; 17–17.5 min, 70–5%B; 17.5–23 min, 5%B; The flow rate: 0.8 mL/min; temperature: 30°C; detection wave length: 360 nm; and sample volume: 1 μL.
Statistical Analysis
All results values are exported as mean ± standard error (SEM) from GraphPad Prism 8.0 software, two-tailed Student’s t-test, or one-way ANOVA analysis for comparing different groups.
Results
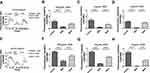
Rutin and Hyperin Were Detected in WSS
To identify the quality and authenticity of S. scandens, the content of rutin and hyperin in the WSS were determined by HPLC.18 After calculation, the content of rutin was 0.012% and the content of hyperin was 0.014% (Figure 1A and B).
 |
Figure 1 High-performance liquid chromatography of WSS. (A) High-performance liquid chromatography rutin and hyperin (Reference substances). (B) High-performance liquid chromatography of WSS. |
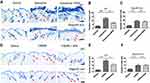
WSS Could Ameliorate Pruritus Mediated by MrgprB2 Receptor
Our experimental results showed that WSS is not relieve the skin damage (Figure 2A); it could significantly inhibit the scratching behavior of ACD model in C57BL/6J mice (Figure 2B, the inhibition was 41.19%, P = 0.004). In addition, results also showed that compared with WT mice, the inhibition ratio of WSS on the scratching behavior in sash (Figure 2C, the inhibition was 27.96%, P = 0.033) or MrgprB2−/− mice (Figure 2D, the inhibition was 12.83%, P = 0.046) was reduced. These results suggest that the mechanism of WSS alleviating pruritis induced by allergic contact dermatitis may be related to the MrgprB2 receptor on mast cells.
To verify the mechanism of WSS anti-pruritus was related to mast cell and MrgprB2 receptor, we examined the effect of WSS on scratching behavior induced by compound 48/80. Our results showed that mast cell stabilizer (ketotifen) could reduce the scratching bouts (Figure 2E, P < 0.0001), and WSS could reduce the scratching bouts induced by compound 48/80 (Figure 2E, inhibition was 50.29%, P = 0.001). In addition, WSS could not reduce the scratching bouts induced by compound 48/80 in sash mice (Figure 2F, P = 0.991) or in MrgprB2−/− mice (Figure 2G, P = 0.672). Therefore, we believed that the anti-pruritus mechanism of WSS may be related to MrgprB2 receptor.
The Infiltration of Mast Cell Was Inhibited by WSS
Toluidine blue staining results showed that WSS could reduce the number of mast cells in mice skin tissue with ACD (Figure 3A and B, P = 0.002). However, WSS had no significant inhibitory effect in MrgprB2−/− mice (Figure 3C, P = 0.613). Meanwhile, WSS could reduce the number of mast cells in C57BL/6J mice induced by compound 48/80 (Figure 3D and E, P = 0.013). However, WSS did not show significant inhibitory effect in MrgprB2−/− mice (Figure 3F, P = 0.975). In addition, HE staining showed that WSS did not improve inflammatory cell infiltration in C57BL/6J mice (Supplementary Figure 4).
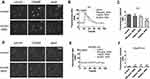
WSS Inhibited MrgprB2-Mediated [Ca2+]i Elevation
Next, we verified the effect of WSS on MrgprB2-mediated [Ca2+]i influx (Figure 4A). The results showed that WSS inhibited [Ca2+]i influx of mast cells, and depended on concentration (Figure 4B–C, 300 ng/mL: P = 0.13, 1 mg/mL: P = 0.001, 3 mg/mL: P < 0.0001). Meanwhile, WSS had no effect on the [Ca2+]i influx in mast cells from MrgprB2−/− mice (Figure 4D–F, 300 ng/mL: P = 0.382, 1 mg/mL: P = 0.961, 3 mg/mL: P = 0.572). In addition, WSS inhibited [Ca2+]i influx of mast cells, which had nothing to do with the cytotoxicity (Supplementary Figure 5).
WSS Inhibited the Generation of Currents Mediated by MrgprB2 Receptor
The MrgprB2 receptor mediated whole-cell currents were evoked by compound 48/80 at different voltage commands. The whole-cell currents (baseline) are shown in Figure 5A; compound 48/80 (5 µg/mL) has increased voltage dependent currents (Figure 5B). Currents increased from −41.66 ± 4.11 pA to −209.0 ± 53.89 pA at −130 mV; Currents increased from 67.29 ± 7.71 pA to 1053 ± 176.4 pA at +130 mV (Figure 5C, n=16, P = 0.0007). 1 mg/mL WSS has inhibited the currents increased by compound 48/80 (Figure 5D and E). Currents increased from 118.9 ± 13.24 pA to 306.2 ± 77.17 PA at +130 mV (Figure 5F, n=10, P = 0.024); WSS has decreased current amplitude of variation (Figure 5G, P = 0.004). Furthermore, compound 48/80 has no effect on currents in mast cells come from MrgprB2−/− mice (Figure 5H, n=6, P = 0.893) (Figure 5I, n=6, P = 0.552).
WSS Inhibited the Activation of MrgprB2/X2 Receptor
In addition, we also found that WSS could reduce the calcium influx in HEK-293 cells over-expressing MrgprB2 receptor (Figure 6A–D,) or MrgprX2 receptor (Figure 6E–H,) induced by compound 48/80 in a concentration-dependent manner. These results indicate that WSS could inhibit the function of MrgprB2/X2 receptor. MrgprB2: At the 300 µg/mL, 1 mg/mL, 3 mg/mL WSS, the inhibition was 53.47%, 68.92%, 77.42%; MrgprX2: At the 300 µg/mL, 1 mg/mL, 3 mg/mL WSS, the inhibition was 62.05%, 69.64%, 85.11%.
Discussion
Our experiment results show that WSS could ameliorate MrgprB2-mediated pruritus in vivo. Meanwhile, WSS also inhibited MrgprB2 receptor activity in vitro by intracellular calcium measurement and whole-cell currents recording experiments.
Pruritus are major characteristic of skin pathologies mainly including allergic contact dermatitis, but the underlying molecular mechanisms are no clear.19 ACDs are common skin disorders that are characterized by crusting and pruritus.20 Allergic contact dermatitis affects up to 20% of adults and children in America.21 Although itch is the major symptom of dermatitis, which often leads to excessive scratching, tissue damage, the mechanisms that drive strong pruritus in ACD remain largely unknown.22 Mast cells are believed to play an important role in ACD pathogenesis.23 A large number of studies have shown that Traditional Chinese Medicine can relieve itching in ACD by inhibiting mast cell degranulation. For example, ethanol extract of Sanguisorbae Radix inhibits mast cell degranulation.24 Root bark of Dictamnus dasycarpus ACD via decreasing degranulation of mast cells by inhibiting p38 MAPK pathway.25 Astragalus membranaceus and Nepeta Tenuifolia could inhibit skin hyperplasia and mast cell infiltration and ACD.26 In our experiments, we found that WSS alleviated the pruritus in the ACD model and the pruritus induced by compound 48/80, a mast cell degranulation agent.27 In addition, our results show that compared with C57BL/6J mice, pruritus induced by ACD is significantly reduced in mast cell-deficient Kit (W-sh) /Kit (W-sh) sash mice (sash) (Supplementary Figure 6, P < 0.05). The antipruritic effect of WSS was decreased in Sash mice. These results indicated that the anti-pruritus mechanism of WSS is related to mast cells.
MrgprB2 receptor is mainly expressed on connective tissue-type mast cells in the skin and lungs, and can be activated by a broad range of cationic ligands, including basic secretagogues such as compound 48/80, substance P (SP), numerous FDA-approved drugs, and endogenous protein fragments.28 MrgprB2 has been implicated in allergic and chronic inflammatory diseases, such as allergic contact dermatitis,16 imiquimod-related dermatitis.29 In addition, our results show that compared with C57BL/6J mice, pruritus induced by ACD is significantly reduced in MrgprB2−/− mice (Supplementary Figure 6, P < 0.05). The number of mast cells was used as the index of pruritus in mice skin tissue with ACD.16 Compared with C57BL/6J mice, the number of mast cells in MrgprB2−/− mice induced by compound 48/80 were significantly reduced (Supplementary Figure 6, P < 0.01); The antipruritic effect of WSS was decreased in MrgprB2−/− mice. These results indicated that the anti-pruritus mechanism of WSS is related to MrgprB2 receptor.
Furthermore, we also found that the calcium flux induced by compound 48/80 in mouse peritoneal mast cells or MrgprB2/X2 over expressing HEK293 cells could be inhibited by WSS. These results suggest that the antipruritic mechanism of WSS is by inhibiting the activity of MrgprB2. Therefore, we believe that the antipruritic effect of WSS depends on the MrgprB2 receptor. S. scandens contained numerous valuable compounds, such as flavonoids, alkaloids and phenolic acids. Flavonoids mainly include quercetin, kaempferol, luteolin, hyperin, rutin, isorhamnetin and emodin, etc.1 Quercetin, Kaempferol and luteolin could inhibit Mrgprx2-induced Ca2+ fluctuations.30–32 We speculated that the material basis of WSS inhibition of the MrgprB2 receptor was related to quercetin, kamanol and luteolin; however, we need further investigate which active component in WSS can inhibit MrgprB2 receptor.
Conclusion
In conclusion, our study revealed that the water extract of S. scandens (WSS) could ameliorate pruritus, and that the antipruritic mechanism of WSS is by inhibiting the activity of MrgprB2 receptor in mast cells.
Abbreviations
ACD, Allergic contact dermatitis; HEK293, Human Embryonic Kidney 293; H&E, Hematoxylin-eosin staining; HPLC, High performance liquid chromatography; MrgprB2, Mas-related G-protein-coupled receptor B2; Senecio scandens Buch.-Ham, S. scandens; WSS, Water extract of S. scandens.
Acknowledgments
The authors thank Prof. Xinzhong Dong in Johns Hopkins University for kindly providing MrgprB2 mutant mice (MrgprB2−/−).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was financially supported by the National Natural Science Foundation of China (No. 31771163) for ZX Tang, the key project of science and technology development plan of traditional Chinese medicine in Jiangsu Province (No. ZD202001) for ZX Tang, and Innovative Project of postgraduate education in Jiangsu Province (No. KYCX21_1745) for F Ye.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Wang D, Huang L, Chen S. Senecio scandens Buch.-Ham.: a review on its ethnopharmacology, phytochemistry pharmacology, and toxicity. J Ethnopharmacol. 2013;149:1–23. doi:10.1016/j.jep.2013.05.048
2. Ma J. Wild Chinese herb Senecio scandens Buch.-Ham treats sheep eye keratitis. Chin J Trad Vet Sci. 2018;35:96.
3. Zou H, Hu H, Liu D, Li H, Hu Z, Li T. Effect of Senecio scandens Buch.-Ham on inflammation of cornea and conjunctiva in rats with allergic conjunctivitis via NLRP3/Caspase-1/IL-1β Pathway. Trad Chin Drug Res Clin Pharmacol. 2019;30:1346–1351.
4. Yang H, Shen J, Liu Q, Peng Y. Anti-inflammatory effect of petroleum ether from Senecio scandens Buch.-Ham on LPS-stimulated RAW264.7 cells. J Huazhong Agric Univ. 2020;39:187–191.
5. Zhang W, Chen H, Zhang W, Cao H. Study of the anti-inflammatory effect of total flavonoids from Senecio scandens Buch.-Ham. Lishizhen Med Mater Med Res. 2008;34:605–607.
6. Yu J, Hu M, Wang Y, et al. Extraction, partial characterization and bioactivity of polysaccharides from Senecio scandens Buch.-Ham. Int J Biol Macromol. 2018;109:535–543. doi:10.1016/j.ijbiomac.2017.12.119
7. Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi:10.1038/nrn1950
8. Matterne U, Apfelbacher CJ, Vogelgsang L, Loerbroks A, Weisshaar E. Incidence and determinants of chronic pruritus: a population-based cohort study. Acta Derm Venereol. 2013;93:532–537. doi:10.2340/00015555-1572
9. Leader B, Carr CW, Chen SC. Pruritus epidemiology and quality of life. Handb Exp Pharmacol. 2015;226:15–38.
10. Bathe A, Weisshaar E, Matterne U. Chronic pruritus--more than a symptom: a qualitative investigation into patients’ subjective illness perceptions. J Adv Nurs. 2013;69:316–326. doi:10.1111/j.1365-2648.2012.06009.x
11. Abreu D, Kim BS. Innate immune regulation of dermatitis. Immunol Allergy Clin North Am. 2021;41:347–359. doi:10.1016/j.iac.2021.04.011
12. Church MK. Allergy, histamine and antihistamines. Handb Exp Pharmacol. 2017;241:321–331.
13. Komiya E, Tominaga M, Kamata Y, Suga Y, Takamori K. Molecular and cellular mechanisms of itch in psoriasis. Int J Mol Sci. 2020;21:8406.
14. Abraham SN, St JA. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–452. doi:10.1038/nri2782
15. Xia Q, Liu T, Wang J, et al. Mast cells and thymic stromal lymphopoietin (TSLP) expression positively correlates with pruritus intensity in dermatitis herpetiformis. Eur J Dermatol. 2020;30:499–504. doi:10.1684/ejd.2020.3881
16. Meixiong J, Anderson M, Limjunyawong N, et al. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity. 2019;50:1163–1171. doi:10.1016/j.immuni.2019.03.013
17. Jiang Y, Ye F, Du Y, Zong Y, Tang Z. P2X7R in mast cells is a potential target for salicylic acid and aspirin in treatment of inflammatory pain. J Inflamm Res. 2021;14:2913–2931. doi:10.2147/JIR.S313348
18. Wu X, Wang Y, Chen Y, Zhu Y, Xu Y. Simultaneous determination of three components in Senecio cannabifolius var. integrifolius by accelerated solvent extraction-HPLC. Chinese Pharm J Analysis. 2016;36:1555–1562.
19. Voisin T, Perner C, Messou MA, et al. The CysLT2R receptor mediates leukotriene C4-driven acute and chronic itch. Proc Natl Acad Sci U S A. 2021;118:e2022087118.
20. Leonard A, Guttman-Yassky E. The unique molecular signatures of contact dermatitis and implications for treatment. Clin Rev Allergy Immunol. 2019;56:1–8. doi:10.1007/s12016-018-8685-0
21. Brown C, Yu J. Pediatric allergic contact dermatitis. Immunol Allergy Clin North Am. 2021;41:393–408. doi:10.1016/j.iac.2021.04.004
22. Li F, Wang C, Hu D, et al. mMrgprA3/mMrgprC11/hMrgprX1: potential therapeutic targets for allergic contact dermatitis-induced pruritus in mice and humans. Contact Dermatitis. 2022;86:286–294. doi:10.1111/cod.14051
23. Gimenez-Rivera VA, Siebenhaar F, Zimmermann C, et al. Mast cells limit the exacerbation of chronic allergic contact dermatitis in response to repeated allergen exposure. J Immunol. 2016;197:4240–4246. doi:10.4049/jimmunol.1600236
24. Yang JH, Yoo JM, Cho WK, Ma JY. Ethanol extract of sanguisorbae radix inhibits mast cell degranulation and suppresses 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions. Mediators Inflamm. 2016;2016:2947390. doi:10.1155/2016/2947390
25. Kim H, Kim M, Kim H, et al. Anti-inflammatory activities of Dictamnus dasycarpus Turcz., root bark on allergic contact dermatitis induced by dinitrofluorobenzene in mice. J Ethnopharmacol. 2013;149:471–477. doi:10.1016/j.jep.2013.06.055
26. Jo SY, Kim MH, Lee H, Lee SH, Yang WM. Ameliorative and synergic effects of Derma-H, a new herbal formula, on allergic contact dermatitis. Front Pharmacol. 2020;11:1019. doi:10.3389/fphar.2020.01019
27. Santos FA, Rao VS. Possible role of mast cells in cineole-induced scratching behavior in mice. Food Chem Toxicol. 2002;40:1453–1457. doi:10.1016/S0278-6915(02)00088-1
28. McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi:10.1038/nature14022
29. Hao Y, Peng B, Che D, et al. Imiquimod-related dermatitis is mainly mediated by mast cell degranulation via Mas-related G-protein coupled receptor B2. Int Immunopharmacol. 2020;81:106258. doi:10.1016/j.intimp.2020.106258
30. Ding Y, Che D, Li C, et al. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCgamma-IP3R related Ca(2+) fluctuations. Int Immunopharmacol. 2019;66:185–197. doi:10.1016/j.intimp.2018.11.025
31. Cao J, Wang Y, Hu S, et al. Kaempferol ameliorates secretagogue-induced pseudo-allergic reactions via inhibiting intracellular calcium fluctuation. J Pharm Pharmacol. 2020;72:1221–1231. doi:10.1111/jphp.13312
32. Hao Y, Che D, Yu Y, et al. Luteolin inhibits FcepsilonRIota- and MRGPRX2-mediated mast cell activation by regulating calcium signaling pathways. Phytother Res. 2022;36:2197–2206. doi:10.1002/ptr.7447
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.