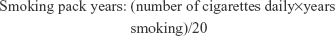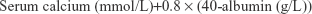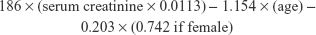Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 13
Vitamin D status is associated with muscle strength and quality of life in patients with COPD: a seasonal prospective observation study
Authors Carson EL, Pourshahidi LK , Madigan SM, Baldrick FR, Kelly MG , Laird E , Healy M, Strain JJ , Mulhern MS
Received 28 February 2018
Accepted for publication 2 June 2018
Published 28 August 2018 Volume 2018:13 Pages 2613—2622
DOI https://doi.org/10.2147/COPD.S166919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Emma L Carson,1 L Kirsty Pourshahidi,1 Sharon M Madigan,2 Francina R Baldrick,1 Martin G Kelly,3 Eamon Laird,4 Martin Healy,5 JJ Strain,1 Maria S Mulhern1
1Nutrition Innovation Centre for Food and Health (NICHE), School of Biomedical Sciences, University of Ulster, Coleraine, Co. Londonderry, UK; 2Respiratory Dietitian, Pulmonary Rehabilitation Team, Belfast Health and Social Care Trust, Belfast, UK; 3Respiratory Team, Altnagelvin Hospital, Western Health and Social Care Trust, Londonderry, UK; 4School of Medicine, Trinity College, Dublin, Ireland; 5Department of Biochemistry, Central Pathology Laboratory, St James Hospital, Dublin, Ireland
Background: Owing to hospitalization, reduced functional capacity and consequently, less sunlight exposure, suboptimal vitamin D status (25-hydroxyvitamin D [25(OH)D]<50 nmol/L) is prevalent among COPD patients.
Objective: This study aimed to investigate seasonal changes in vitamin D status and any associated changes in fat-free mass (FFM), muscle strength and quality of life (QoL) in COPD patients.
Patients and methods: COPD patients living in Northern Ireland (n=51) completed study visits at the end of winter (March/April) and at the end of summer (September/October), corresponding to the nadir and peak of vitamin D status, respectively. At both time points, serum concentration of 25(OH)D was quantified by liquid chromatography-tandem mass spectrometry, FFM (kg) was measured using bioelectrical impedance and muscle strength (kg) was measured using handgrip dynamometry. QoL was assessed using the validated St George’s Respiratory Questionnaire.
Results: Mean±SD 25(OH)D concentration was significantly higher at the end of summer compared to the end of winter (52.5±30.5 nmol/L vs 33.7±28.4 nmol/L, P<0.001); and housebound patients had significantly lower 25(OH)D concentration compared to nonhousebound patients at the end of summer (42.9±4.2 vs 57.2±9.9 nmol/L; P<0.001). Muscle strength (at both time points) and QoL (end of summer only) were positively predicted by 25(OH)D concentration, independent of age, sex and smoking status.
Conclusion: This study highlights the need for health policies to include a recommendation for year-round vitamin D supplementation in housebound COPD patients, and wintertime supplementation in nonhousebound patients, to maintain optimal 25(OH)D concentrations to protect musculoskeletal health. Furthermore, an optimal vitamin D status may have potential benefits for QoL in these patients.
Keywords: vitamin D, COPD, muscle strength, quality of life, seasonal, 25(OH)D
Plain language summary
Why was the study done? To establish if there was seasonal variation in blood levels of vitamin D in COPD patients. Current patient recommendations suggest supplementation during the winter period when vitamin D is at its lowest. The researchers also wanted to establish if there were any links between vitamin D, muscle health and quality of life (QoL) in this population.
What did the researchers do and find? Data were collected from a group of COPD patients living in Northern Ireland. They found that vitamin D levels were low in the majority of patients. Vitamin D was lower in the winter vs summer and in housebound patients vs those who were not housebound. Patients with higher vitamin D levels had better muscle strength and better QoL compared to those with lower levels.
What do these results mean? These results suggest that there is a need for year-round vitamin D supplementation in COPD patients, especially those who do not regularly get outdoors. Ensuring better vitamin D levels may be beneficial for muscle strength and QoL in COPD patients. Revised policies and education are needed for this patient group to help maintain optimal vitamin D levels.
Introduction
COPD is the fourth leading cause of mortality globally1 with an estimated prevalence of 251 million cases in 2016. COPD is characterized by progressive and persistent airflow limitation accompanied by an enhanced chronic inflammatory response and is primarily caused by smoking and environmental exposure to smoke.2 Increased rates of respiratory infections and inflammatory exacerbations in COPD patients result in more frequent hospital stays and more time spent indoors. As a result, decreased sunlight exposure, along with a poor dietary intake, glucocorticoid medication, aging, lower vitamin D storage from muscle or fat and renal dysfunction, places this patient group at high risk of vitamin D deficiency (25-hydroxyvitamin D [25(OH)D] concentrations <25 nmol/L).3–5 One study has reported vitamin D insufficiency (25(OH)D <50 nmol/L) in 58% of COPD patients, even during summer months6 with others reporting vitamin D insufficiency in up to 79% of moderate to severely affected COPD patients within Northern Ireland.7
Vitamin D deficiency, in turn, has been associated with increased rates of exacerbations and hospitalization in COPD patients.8,9 Recent meta-analyses have concluded that vitamin D deficiency is directly associated with COPD disease severity and vitamin D supplementation may prevent exacerbations.10–12 It is purported that this association is mediated through the immunomodulatory effects of vitamin D13 by shifting the inflammatory balance from a pro-inflammatory T-helper cell 1 profile toward a more anti-inflammatory T-helper cell 2 profile.14,15 Therefore, achieving optimal vitamin D status might not only help prevent comorbidities, such as osteoporosis in COPD patients,16 but may also help prevent respiratory infections and exacerbations,9 which have been shown to be predictive of a higher mortality risk in this patient group.17
Furthermore, COPD patients present with a severe reduction in physical performance and capacity for physical activity, mostly owing to impaired respiratory and muscle functions,18,19 resulting in a significant deterioration in quality of life (QoL).20 Patients with moderate to severe COPD often present with muscle wastage and severe muscle weakness, which can manifest as cachexia or sarcopenia, both of which have been associated with increased mortality risk and disease severity.21,22 Cachexia in COPD patients is typically diagnosed through low body mass index (BMI <18.5 kg/m2) and a low fat-free mass index (FFMI),23 while sarcopenia can be defined as low FFM with a normal or elevated BMI.24 Although somewhat disputed,25 the vitamin D receptor (VDR) has been detected in skeletal muscle cells26 and low vitamin D status has been associated with a higher risk of sarcopenia and impaired muscle function in older individuals.27 Nonetheless, the majority of studies investigating the potential benefit of optimal vitamin D in COPD patients have focused on respiratory outcomes, with a dearth of studies considering its impact on the maintenance of muscle mass and function. One recent study reported that vitamin D deficient COPD patients had significantly lower handgrip strength and knee flexor muscle strength compared to vitamin D sufficient patients,28 whereas other studies have produced variable results.29–32
Seasonal variations in respiratory infections and exacerbations have been reported in COPD patients33,34 and are comparable to the seasonal variation in vitamin D status.35 Nonetheless, seasonality is a poorly considered aspect of most vitamin D studies in COPD populations. Therefore, the primary aim of this study was to investigate the seasonality of vitamin D status in COPD patients. Secondary aims were to investigate if muscle strength and QoL were predicted by vitamin D status.
Patients and methods
Patients
Individuals with diagnosed stable COPD were recruited from the Belfast and Western Health and Social Care Trusts within Northern Ireland (53–55°N), at community dietician home visits and pulmonary rehabilitation clinics. Exclusion criteria were: <18 year old, pregnancy or having a diagnosis of lung cancer. Patients were given a verbal outline of the study by clinicians, along with a short written information sheet. Following this visit, those patients who expressed interest in the study provided consent for their details to be passed onto a researcher. Interested patients were then contacted via telephone and/or visited by a researcher at their home, where they received a full verbal explanation, full participant information sheet and given at least 48 hours to consider participating in the study. Full written informed consent was obtained from those who participated. This study was approved by The Office for Research Ethics Committee Northern Ireland (12/NI/0183) and was conducted according to the Declaration of Helsinki.
Study design
A researcher visited patients twice at their home over the course of the study: the first at the end of winter (March/April) and the second at the end of summer (September/October), corresponding to the nadir and peak of vitamin D status, respectively. This study took place over two years: 39 patients completed the study between March and October 2013 and the remaining 12 patients completed the study between March and October 2014. Using data from a previous study investigating seasonal differences in vitamin D status in older adults in this population,36 it was calculated that to achieve a power of 80% and a level of significance of 5% (one sided), and allowing for a 40% dropout rate, a minimum of 44 patients should be recruited to see a significant seasonal difference in 25(OH)D concentrations.
Questionnaires
Health and lifestyle questionnaires were completed at both time points for information on smoking status, sun exposure habits and medical history. Patients self-defined themselves as being housebound or nonhousebound according to whether they spent the majority of their waking hours at home or not. This definition was confirmed by the researcher using responses from the sun exposure habits section of the health and lifestyle questionnaire which estimated time spent outdoors during April to September. Daily number of cigarettes and number of years smoking were used to calculate “smoking pack years” as:
|
A validated St George’s Respiratory Questionnaire (SGRQ) was also completed at both time points to assess QoL.37 SGRQ scores were out of 100, with 100% indicating the most lifestyle limitations or poorest QoL.
Anthropometry and muscle strength
Height (m) was measured at the first time point using a portable stadiometer (Seca, Hamburg, Germany). Weight (kg), BMI, (kg/m2) and FFM (kg) were measured at both time points by bioelectrical impedance analysis, using portable Tanita scales (Tanita Corporation, Tokyo, Japan). FFMI (kg/m2) was calculated by correcting FFM for height (FFM (kg)/height (m2)). Muscle strength (kg) was measured at both time points using a Smedley handgrip dynamometer (Stoelting, IL, USA). Three repetitions of a maximal isometric contraction were performed on the nondominant arm, with 30 seconds rest between repetitions. Average of the three repetitions was calculated as overall grip strength and patients who were unable to complete all three repetitions had the average of two repetitions recorded.
Biochemical analysis
Nonfasting 10 mL blood samples were collected by venipuncture into blood collection tubes by a fully trained phlebotomist at both time points and were processed to serum/plasma within 4 hours of collection. Aliquots were stored at −80°C until required for batch analysis at the end of the study. Total serum 25(OH)D (sum of 25(OH)D2+25(OH)D3) concentrations were quantified using liquid chromatography-tandem mass spectrometry (LC-MS/MS, API 4000, AB SCIEX, Foster City, CA, USA) following sample preparation according to kit manufacturer guidelines from Chromsystems Instruments & Chemicals GmbH, Munich, Germany (MassChrom® 25-OH-Vitamin D3/D2), at St James’s Hospital, Dublin. This laboratory participates in the vitamin D External Quality Assessment Scheme (DEQAS). Vitamin D insufficiency is classified as 25(OH)D concentrations <50 nmol/L. Plasma intact parathyroid hormone (PTH) concentrations were measured using a manual enzyme-linked immunoassay at Ulster University, with a commercially available kit (MD Bioproducts, Division of Biosciences Inc., Oakdale, MN, USA). All samples were measured in duplicate and the mean of the two replicates calculated, with precision ensured via bi-level quality controls. Serum calcium (Ca+), creatinine, albumin and high sensitivity C-reactive protein (CRP) concentrations were measured using an ILab™ 600 Chemistry Systems auto-analyzer. The normal reference range for CRP was <10 mg/L. Albumin-adjusted Ca+ concentration was calculated using serum albumin and calcium concentrations:
|
Renal function was assessed by calculating estimated glomerular filtration rate (eGFR, mL/min/1.73 m2), using the age- and sex-specific Modification of Diet in Renal Disease formula:38
|
Statistical analysis
Statistical analyses were carried out using SPSS® for Windows™ Version 22.0 (IBM Corporation, Armonk, NY, USA). All variables were tested for normality using Kolmogorov–Smirnov and those data, which were not normally distributed, were log transformed to achieve near normal distribution. Data are presented as mean±SD, unless otherwise stated. Differences in means between the end of winter and end of summer were assessed for vitamin D, muscle and SGRQ outcome variables, using paired t-tests. Spearman’s correlation analysis was used to assess correlations between serum 25(OH)D and CRP concentration, muscle outcomes and SGRQ scores at each time point. Stepwise linear regressions were performed to investigate serum 25(OH)D concentration as a predictor of muscular outcomes – FFM (kg), FFMI (kg/m2), muscle strength (kg) – and SGRQ scores at both time points. Linear regression models included age, sex and smoking status (active, previous or never) as covariates. FFM (kg) was an additional covariate in muscle strength models only. A P-value <0.05 was considered statistically significant throughout.
Results
A total of 51 COPD patients (n=28 males, n=23 females), with a mean±SD age of 68.7±7.2 years, completed both seasonal time points of the study: the end of winter and end of summer. Table 1 represents the patient characteristics, anthropometry, muscle mass, muscle strength and biochemical parameters at each seasonal time point. There were no significant differences in any of these variables between the two years of recruitment or between the two Health and Social Care Trust areas (data not shown). At the end of winter, 31%, 67% and 2% actively, previously and never smoked, respectively, whereas, at the end of summer, the proportion of active smokers significantly increased to 41%. There were no significant differences in body weight (kg), BMI (kg/m2), FFM (absolute kg or %), FFMI (kg/m2) or muscle strength between the two seasonal time points. Mean values for BMI at both time points were within the overweight BMI category (25.0–29.9 kg/m2).
At the end of summer 25(OH)D concentrations were significantly higher compared with the end of winter (52.5±30.5 vs 33.7±28.4 nmol/L). A total of 75% and 47% of patients were classified as vitamin D deficient/insufficient (25(OH)D<50 nmol/L) at the end of winter and summer, respectively. Nonhousebound (n=35) patients had significantly higher mean±SD 25(OH)D concentration compared with housebound patients (n=16) at the end of summer (57.2±9.9 vs 42.9±4.2 nmol/L, P=0.029), but there was no significant difference at the end of winter (Figure 1). Nonetheless, both housebound and nonhousebound patients had a significantly higher 25(OH)D concentration at the end of summer compared with at the end of winter (housebound: 42.9 vs 31.1 nmol/L, P=0.006 and nonhousebound: 57.2 vs 35.2 nmol/L, P<0.001).
While there were statistically significant seasonal differences in albumin-adjusted Ca+ and creatinine concentrations and eGFR, these differences were not considered clinically significant and reflect the associated seasonal changes in 25(OH)D concentrations. There was no significant seasonal variation in PTH or CRP concentration and no significant difference in QoL (SGRQ scores) between the two seasonal time points.
Absolute FFM (kg), FFMI and CRP concentrations were not significantly correlated with serum 25(OH)D concentration at any time point (Table 2). Muscle strength was significantly positively, and SGRQ score, negatively associated with serum 25(OH)D concentration at the end of summer, but not at the end of winter. Stepwise linear regression analyses (Table 3) showed that serum 25(OH)D concentration was not a significant predictor of absolute FFM (kg) or FFMI at either time point but was a significant positive predictor of muscle strength at both time points. Higher serum 25(OH)D concentration significantly predicted a better QoL (lower SGRQ score) at the end of summer, but not the end of winter, after adjusting for age, sex and smoking status. Additional analyses presented in Table 4, show that at the end of summer only, vitamin D insufficient (25(OH)D <50 nmol/L) patients had significantly lower muscle strength and QoL (higher SGRQ score) compared to vitamin D sufficient patients (≥50 nmol/L) and are significantly more likely to be housebound.
  | Table 3 Stepwise linear regression models to investigate 25(OH)D concentration as a predictor of FFM, muscle strength and quality of life in COPD patients at the end of winter and at the end of summer |
Discussion
This is the first prospective study to report seasonal variability in vitamin D status in COPD patients within Northern Ireland, as well as significantly lower vitamin D status in COPD patients who are housebound compared to those who are nonhousebound. In this patient group, serum 25(OH)D concentration also positively predicted muscle strength at both the end of winter and end of summer, and QoL (according to SGRQ scores) at the end of summer. Moreover, those patients who did not achieve a sufficient vitamin D status at the end of summer, when vitamin D status is expected to be at its highest, had significantly lower muscle strength and a lower QoL compared to those whose 25(OH)D concentration reached ≥50 nmol/L.
In this COPD patient cohort, the seasonal variation in 25(OH)D concentrations observed mirror those recently reported in the general healthy older Irish population in The Irish Longitudinal Study on Aging (TILDA),35 although at concentrations ~10–20 nmol/L lower than the healthy cohort. The current findings are comparable to those from other European COPD cohorts.4,32 In contrast, a cross-sectional observation study of COPD patients in Northern Ireland reported no difference in vitamin D status across season of sampling. The study, however, found a higher year-round prevalence of vitamin D insufficiency (79% <50 nmol/L)7; results comparable with the proportion of COPD patients in the current study with insufficient vitamin D status at the end of winter (75%).
The current study has shown that nonhousebound COPD patients have a significantly higher vitamin D status, compared with housebound patients at the end of summer, but not at the end of winter. Vitamin D deficiency/insufficiency is prevalent in three quarters of the overall cohort at the end of winter, but remains prevalent in housebound patients, even at the end of summer, when vitamin D is expected to be highest, reflecting the lack of cutaneous vitamin D synthesis at this latitude (53–55°N) during these months (October to March).39 Similar findings have been reported in other housebound and institutionalized populations with severely reduced opportunity for sunlight exposure, such as nursing home patients40 and hospitalized COPD patients.5 These findings highlight a policy requirement for vitamin D supplementation to optimize vitamin D status throughout the year in housebound COPD patients and during winter months in nonhousebound patients.
Although there was a significant seasonal variation in 25(OH)D concentration, this was not reflected by seasonal variations in body composition, according to BMI, FFM or FFMI. In addition, FFM and FFMI were not correlated with 25(OH)D concentration at either seasonal time point. Mean BMI at both seasonal time points was within the overweight category (BMI 25.0–29.9 kg/m2), which has been associated with poor vitamin D status in some studies,41,42 including COPD populations.43 Nonetheless, BMI cannot distinguish between fat mass (FM) and FFM, which is particularly important in COPD patients, who are at risk of sarcopenia22 or sarcopenic obesity (normal BMI but with low FFM and increased FM).44 Despite no significant associations between body composition and 25(OH)D concentration it is encouraging that in a population with a chronic and progressive disease, such as COPD, there is no significant deterioration in FFM over ~6 months.
Compromised muscle strength and physical performance is a common manifestation of vitamin D deficiency in generally healthy older individuals.45,46 The current study has shown that serum 25(OH)D concentration is a positive predictor of muscle strength (as measured by grip strength) at both the end of winter and the end of summer, and this association was stronger at the end of summer, when vitamin D status was higher. Indeed, in the current study, muscle strength was also ~40% higher in patients who reached vitamin D sufficiency at the end of summer compared to those who remained insufficient/deficient, confirming previous observations by others.28 Furthermore, those patients who reached vitamin D sufficiency at the end of summer were also more likely to be nonhousebound patients, reflecting the major source of vitamin D being sun exposure in this cohort. In addition, it is possible, although speculative that those patients who are not housebound may be partaking in more outdoor activities, although physical activity was not assessed as part of this study. Of interest, previous studies have not replicated this association between 25(OH)D concentration and muscle outcomes in COPD patients who are vitamin D replete (25(OH)D ≥50 nmol/L),31 unlike those reported in the current study. Therefore, it is possible that an effect of vitamin D on muscle in COPD patients may only be observed in those who are vitamin D deficient/insufficient at baseline or in patients with particular genetic variations in genes associated with vitamin D metabolism such as FokI and Bsml VDR polymorphisms that have been shown to influence associations between vitamin D and its functions.47 Both of these factors should be considered in future randomized controlled trials in this group.
Genomic and nongenomic mechanisms have been proposed to explain the beneficial effect of vitamin D on muscle and the suggested genomic mechanism, mediated via the VDR, results in proliferation of muscle proteins, predominantly type II muscle fiber proteins.48 This does not seem a plausible mechanism to explain the associations in this study, as we might also expect an increase in FFM owing to increased type II muscle fibers. Rather, the faster intracellular, nongenomic mechanism seems a more appropriate explanation. This mechanism suggests that the active form of vitamin D, 1,25 dihydroxyvitamin D (1,25(OH)2D3) acts upon Ca+ channels on the cell membrane, allowing for an increased flux of intracellular Ca+, improving muscle function and contractility.49 Further investigation is required to determine the exact mechanisms by which vitamin D influences muscle strength in normal populations, as well as those with compromised muscle function, such as those diagnosed with COPD.
Previous literature has suggested the importance of optimal vitamin D status on respiratory function and the frequency of exacerbations in COPD patients,8,12,33,50–54 which may also influence winter time mortality rates.34 Although the current study cannot provide information on respiratory function, disease severity or exacerbation frequency, we do report data on CRP concentrations similar to other stable COPD cohorts55 and which may be a possible mediating factor between vitamin D and respiratory function.52 In the current study, CRP did not differ significantly between the seasons and was not correlated with serum 25(OH)D concentration at either time point, a finding that confirms the findings of others.52 Although higher concentrations of CRP have been associated with impaired lung function, the role of CRP in the etiology of COPD and respiratory function remains poorly understood.56,57 Further research is also warranted to elucidate the relationship between vitamin D and immune-respiratory function in COPD.
Disease severity and exacerbations have been shown to considerably impact upon the QoL in COPD patients.58,59 The current study has shown for the first time that higher serum 25(OH)D concentrations are predictive of lower SGRQ scores and thus a better QoL, independent of age, sex and smoking status in COPD patients. These results are in contrast to a Korean COPD population, where no significant association was found between vitamin D status and QoL at similar 25(OH)D concentration.60 It is noteworthy, however, that in the current study, QoL score was assessed by a subjective self-reported questionnaire and it is possible that higher vitamin D status was a result of a better QoL as patients who reported fewer lifestyle limitations were more likely to spend time outdoors. This finding might also explain why the association was evident at the end of summer, when cutaneous vitamin D synthesis was possible from sunlight exposure, but not at the end of winter.
Although this is an observational study, and the findings might not indicate cause and effect, it analyzes the prospective association between vitamin D status, muscle function, CRP and QoL in a group of COPD patients, with a specific focus on seasonality. Another strength is that serum 25(OH)D concentrations were quantified using the gold standard LC-MS/MS method by a laboratory participating in the DEQAS. One limitation of the current study is the absence of data on respiratory function, disease severity and exacerbations, which may have been significant covariates in the current analyzes. Such clinical markers should be routinely measured and recorded for all patients and this monitoring should be reflected in COPD clinical management guidelines and policy. Dietary vitamin D intake, including supplementation, was also not assessed in this study and may be useful for clinicians to help recommend the best strategy to improve intake and increase status as necessary.
In conclusion, to the authors’ knowledge, this is the first prospective study to demonstrate the seasonality of vitamin D status in COPD patients and importantly noting these differences between housebound and nonhousebound patients. Moreover, specifically at the end of summer, housebound COPD patients had significantly lower vitamin D status compared to nonhousebound patients, and worryingly, a greater proportion remained deficient/insufficient at this time point when vitamin D status should have been at its highest. This finding demonstrates the efficacy of cutaneous vitamin D synthesis from sunlight exposure and highlights the importance of optimizing QoL in this at-risk patient group to facilitate time spent outdoors regularly. Currently vitamin D is not considered as part of the recommended management of COPD by the National Institute for Health and Care Excellence guidelines in the UK, but the recently revised reference nutrient intake of 10 μg/day (400 IU) for the general population should apply to this population and assumes minimal sunshine exposure.61 Findings from the current study may directly impact on policy as they suggest a requirement for year-round vitamin D supplementation particularly for housebound COPD patients and during winter for nonhousebound patients, as a relatively inexpensive and effective means of optimizing vitamin D status and not least maintaining 25(OH)D concentrations to protect musculoskeletal health. The potential positive influence of optimal vitamin D on muscle strength and QoL, as reported in the current study, may in turn lead to improved exercise tolerance, physical function and slower deterioration of respiratory function in COPD patients. Such results should also help inform health care and professional and patient education programs and policy on vitamin D supplementation in this population group with the ultimate aim to ensure adequate vitamin D status in patients with COPD.
Acknowledgments
The authors wish to thank all patients who participated in the study and the respiratory nurses who assisted with sample collection. The study was jointly funded by the Western Health and Social Care Trust Research Office and the Biomedical Sciences Research Institute, Ulster University. The funding sources had no input into the study design, collection, analysis or interpretation of the data and/or production of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20–25. | ||
Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease website. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosismanagement.html. Accessed 5th July 2017. | ||
Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–220. | ||
Persson LJP, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TML. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(6):e38934. | ||
Mekov E, Slavova Y, Tsakova A, et al. Vitamin D deficiency and insufficiency in hospitalized COPD patients. PLoS One. 2015;10(6):e0129080. | ||
Romme EA, Rutten EP, Smeenk FW, Spruit MA, Menheere PP, Wouters EF. Vitamin D status is associated with bone mineral density and functional exercise capacity in patients with chronic obstructive pulmonary disease. Ann Med. 2013;45(1):91–96. | ||
Baldrick FR, Elborn JS, Woodside JV, et al. Vitamin D status in chronic obstructive pulmonary disease. Proc Nutr Soc. 2012;71:E98. | ||
Malinovschi A, Masoero M, Bellocchia M, et al. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res. 2014;15(1):131. | ||
Kokturk N, Baha A, Oh YM, Young Ju J, Jones PW. Vitamin D deficiency: what does it mean for chronic obstructive pulmonary disease (COPD)? A comprehensive review for pulmonologists. Clin Respir J. 2018;12(2):382–397. | ||
Zhu B, Zhu B, Xiao C, Zheng Z. Vitamin D deficiency is associated with the severity of COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1907–1916. | ||
Zhu M, Wang T, Wang C, Ji Y. The association between vitamin D and COPD risk, severity, and exacerbation: an updated systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:2597–2607. | ||
Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120–130. | ||
Heulens N, Korf H, Janssens W. Innate immune modulation in chronic obstructive pulmonary disease: moving closer toward vitamin D therapy. J Pharmacol Exp Ther. 2015;353(2):360–368. | ||
Cantorna MT, Humpal-Winter J, Deluca HF. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;377(1):135–138. | ||
Bemiss CJ, Mahon BD, Henry A, Weaver V, Cantorna MT. Interleukin-2 is one of the targets of 1,25-dihydroxyvitamin D3 in the immune system. Arch Biochem Biophys. 2002;402(2):249–254. | ||
van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Prim Care Respir J. 2010;19(4):326–334. | ||
Garcia-Aymerich J, Serra Pons I, Mannino DM, Maas AK, Miller DP, Davis KJ. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax. 2011;66(7):585–590. | ||
Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(4):637–658. | ||
Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. | ||
Sundh J, Johansson G, Larsson K, et al. Comorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:173–183. | ||
Gologanu D, Ionita D, Gartonea T, Stanescu C, Bogdan MA. Body composition in patients with chronic obstructive pulmonary disease. Maedica. 2014;9(1):25–32. | ||
Jeon YK, Shin MJ, Kim MH, et al. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos Int. 2015;26(10):2423–2429. | ||
von Haehling S, Anker SD, Prevalence ASD. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):261–263. | ||
Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58(11):M1012–M1017. | ||
Wang Y, Deluca HF. Is the vitamin d receptor found in muscle? Endocrinology. 2011;152(2):354–363. | ||
Garcia LA, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)(2)vitamin D(3) enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C(2)C(12) skeletal muscle cells. J Steroid Biochem Mol Biol. 2013;133:1–11. | ||
Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, van Loon LJ, de Groot LC. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr. 2013;67(10):1050–1055. | ||
Yumrutepe T, Aytemur ZA, Baysal O, Taskapan H, Taskapan CM, Hacievliyagil SS. Relationship between vitamin D and lung function, physical performance and balance on patients with stage I–III chronic obstructive pulmonary disease. Rev Assoc Med Bras. 2015;61(2):132–138. | ||
Hornikx M, van Remoortel H, Lehouck A, et al. Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res. 2012;13:84. | ||
Bjerk SM, Edgington BD, Rector TS, Kunisaki KM. Supplemental vitamin D and physical performance in COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2013;8:97–104. | ||
Jackson AS, Shrikrishna D, Kelly JL, et al. Vitamin D and skeletal muscle strength and endurance in COPD. Eur Respir J. 2013;41(2):309–316. | ||
Rafiq R, Prins HJ, Boersma WG, et al. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: a pilot trial. Int J Chron Obstruct Pulmon Dis. 2017;12:2583–2592. | ||
Jenkins CR, Celli B, Anderson JA, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38–45. | ||
Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9:1101–1110. | ||
Laird E, O’Halloran AM, Carey D, et al. The prevalence of vitamin D deficiency and the determinants of 25(OH)D concentration in older Irish adults: Data from The Irish Longitudinal Study on Ageing (TILDA). J Gerontol A Biol Sci Med Sci. 2017;73(4):519–522. | ||
Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S. The National Diet and Nutrition Survey: Results from years 1, 2, 3 and 4 (combined) of the rolling programme (2008/2009 – 2011/2012). London: TSO; 2014. | ||
Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. | ||
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. | ||
Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378. | ||
Verhoeven V, Vanpuyenbroeck K, Lopez-Hartmann M, Wens J, Remmen R. Walk on the sunny side of life – epidemiology of hypovitaminosis D and mental health in elderly nursing home residents. J Nutr Health Aging. 2012;16(4):417–420. | ||
Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90(7):4119–4123. | ||
Pourshahidi LK. Vitamin D and obesity: current perspectives and future directions. Proc Nutr Soc. 2015;74(2):115–124. | ||
Jolliffe DA, James WY, Hooper RL, et al. Prevalence, determinants and clinical correlates of vitamin D deficiency in patients with chronic obstructive pulmonary disease in London, UK. J Steroid Biochem Mol Biol. 2018;175:138–145. | ||
Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33(5):737–748. | ||
Iolascon G, de Sire A, Calafiore D, Moretti A, Gimigliano R, Gimigliano F. Hypovitaminosis D is associated with a reduction in upper and lower limb muscle strength and physical performance in post-menopausal women: a retrospective study. Aging Clin Exp Res. 2015;27 (Suppl 1):23–30. | ||
Salminen M, Saaristo P, Salonoja M, et al. Vitamin D status and physical function in older Finnish people: a one-year follow-up study. Arch Gerontol Geriatr. 2015;61(3):419–424. | ||
Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87(2):385–390. | ||
Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci U S A. 1998;95(26):15603–15607. | ||
Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80(3):1215–1265. | ||
Rabe KF, Fabbri LM, Vogelmeier C, et al. Seasonal distribution of COPD exacerbations in the Prevention of Exacerbations with Tiotropium in COPD Trial. Chest. 2013;143(3):711–719. | ||
Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–114. | ||
Hendryx M, Luo J. A test of vitamin D benefits on respiratory health mediated through inflammatory markers. Chron Respir Dis. 2015;12(1):24–30. | ||
Flexeder C, Thiering E, Koletzko S, et al. Higher serum 25(OH)D concentrations are associated with improved FEV1 and FVC in adolescence. Eur Respir J. 2017;49(4):1601804. | ||
Sluyter JD, Camargo CA, Waayer D, et al. Effect of monthly, high-dose, long-term vitamin D on lung function: a randomized controlled trial. Nutrients. 2017;9(12):E1353. | ||
Aksu F, Çapan N, Aksu K, et al. C-reactive protein levels are raised in stable chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure. J Thorac Dis. 2013;5(4):414–421. | ||
Mendy A, Forno E, Niyonsenga T, Gasana J. Blood biomarkers as predictors of long-term mortality in COPD. Clin Respir J. 2017;11. | ||
Walter RE, Wilk JB, Larson MG, et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008;133(1):19–25. | ||
Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. | ||
Peruzza S, Sergi G, Vianello A, et al. Chronic obstructive pulmonary disease (COPD) in elderly subjects: impact on functional status and quality of life. Respir Med. 2003;97(6):612–617. | ||
Park JH, Park HK, Jung H, Lee SS, Koo HK. Parathyroid hormone as a novel biomarker for chronic obstructive pulmonary disease: Korean National Health and Nutrition Examination Survey. PLoS One. 2015;10(9):e0138482. | ||
SACN. The Scientific Advisory Committee on Nutrition (SACN) Recommendations on Vitamin D. England: Public Health England; 2016. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.