Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 12
Vitamin D Pretreatment Attenuates Ciprofloxacin-Induced Antibacterial Activity
Authors Masadeh MM, Alzoubi KH , Al-Taani BM, Masadeh MM, Aburashed ZO , Alrabadi N
Received 9 July 2020
Accepted for publication 28 August 2020
Published 12 October 2020 Volume 2020:12 Pages 171—176
DOI https://doi.org/10.2147/CPAA.S268330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Majed M Masadeh,1 Karem H Alzoubi,2 Bashar M Al-Taani,1 Majd M Masadeh,1 Zainah O Aburashed,1,2 Nasr Alrabadi3
1Department of Pharmaceutical Technology, Jordan University of Science and Technology, Irbid 22110, Jordan; 2Department of Clinical Pharmacy, Jordan University of Science and Technology, Irbid 22110, Jordan; 3Department of Pharmacology, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan
Correspondence: Majed M Masadeh
Department of Pharmaceutical Technology, Jordan University of Science and Technology, Irbid 22110, Jordan
Email [email protected]
Background: Ciprofloxacin is an antimicrobial that is commonly used to treat several types of infections. It exerts its antimicrobial activity through interfering with bacterial DNA replication and transcription, leading to increase oxidative stress and eventually bacterial death. Vitamin D, on the other hand, has been found to have DNA protective and antioxidant effects. In the current study, the possible interactive effect of vitamin D on ciprofloxacin-induced cytotoxicity was investigated in various standard bacterial strains.
Methods: The bacterial strains that were used include Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Staphylococcus epidermidis, Acinetobacter baumannii, Proteus mirabilis, and Klebsiella pneumoniae. The antibacterial effect of ciprofloxacin with and without vitamin D treatment of the bacteria was assessed using disc diffusion method and by measuring the minimum inhibitory concentration (MIC) and zones of inhibition of bacterial growth. Moreover, reactive oxygen species (ROS) generation after pretreatment of E. Coli cells with ciprofloxacin and/or vitamin D was measured as a function of as a function of hydrogen peroxide generation.
Results: Ciprofloxacin demonstrated a potent antibacterial effect against the tested strains of bacteria. Moreover, pretreatment with vitamin D resulted in protecting the bacteria from the cytotoxicity of ciprofloxacin, this was indicated by the significantly smaller zones of inhibition and higher MIC values compared to ciprofloxacin alone as well as reduced ciprofloxacin-induced ROS generation after treatment with vitamin D.
Conclusion: Results revealed the possible reduction in the activity of ciprofloxacin when used in combination with vitamin D. This could be explained by the ability of vitamin D to reduce oxidative stress in the bacterial cells.
Keywords: fluoroquinolones, vitamins, MIC, zones of inhibition, antimicrobial susceptibility, oxidative stress
Introduction
Fluoroquinolones is a group of broad spectrum antimicrobials.1,2 They exert an excellent activity against gram negative and atypical bacteria with good coverage on gram positive and anaerobic bacteria.1,2 The exact mechanism of action for this group of antimicrobials is not fully understood, but it is thought to be primarily, via inhibiting DNA replication through interfering with the bacterial DNA gyrase/topoisomerase II enzyme, leading to prevent DNA supercoiling and duplication.3,4 Fluoroquinolones are bactericidal and exhibit bacterial killing in a concentration-dependent manner.5 The prototype of quinolones is nalidixic acid that was commonly used to treat urinary tract infections because it gets concentrated in the urine.1,2 However, its use has been reduced because of side effects and high resistance towards it.6 Of the fluoroquinolones, ciprofloxacin is the most potent fluoroquinolone against gram-negative bacteria (mainly the Enterobacteriaceae species such as Escherichia coli, Salmonella, Shigella, and Neisseria).5,7 Since its introduction, ciprofloxacin was found to be effective for a variety of bacterial infections including urinary tract infections (both complicated and uncomplicated), intra-abdominal infections, skin and bone infections, gynecological infections and sinusitis.1,8 Ciprofloxacin is one of the few oral antimicrobials that is used to treat pseudomonas aeruginosa.5
Microbial studies have suggested that some antimicrobials including fluoroquinolones may exert their effect through increasing oxidative stress in the bacteria.9–12 Oxidative stress occurs when there is an imbalance between the production of harmful molecules known as free radicals (including the reactive oxygen species) and their defense mechanisms known as antioxidants.13 Ciprofloxacin increases oxidative stress in the host cells through increasing the production of reactive oxygen species (ROS).10,12 The increase in oxidative stress induced by fluoroquinolones can also explain some of their toxicities on humans including photosensitivity and tendonitis.14,15
Vitamin D (calcitriol or 1.25-dihydroxyvitamin D3) is a lipid soluble vitamin.16 Its beneficial effect on maintaining calcium homeostasis and bone mineralization is well known.17 However, within the last two decades, extensive studies have focused on the possible non-classic protective effects of vitamin D.17–19 Being a potent antioxidant is one of these non-calcemic effects of this vitamin.19 Given that ciprofloxacin induces bacterial damage through increasing oxidative stress,12,20 several studies have been conducted to evaluate a possible effect of antioxidants on ciprofloxacin. These studies have delineated that the use of antioxidants including vitamin E, vitamin C, tempol and melatonin attenuate the antimicrobial effect of ciprofloxacin through interfering with its main mechanism of action.21,22 The results of these studies have revealed a possible attenuating effect of antioxidants on ciprofloxacin. However, no study have evaluated the effect of vitamin D on the antimicrobial activity of ciprofloxacin. Now, given the fact that vitamin D supplements are widely used to treat or prevent vitamin D deficiency/insufficiency, it is likely for vitamin D supplements to interact with antimicrobials such as ciprofloxacin, when the later are used to treat human infections. Therefore, the aim of the present investigation was to evaluate the possible mitigating effect of vitamin D as an antioxidant on the antimicrobial functions of ciprofloxacin.
Materials and Methods
Chemicals
Ciprofloxacin was provided as a gift from Al-HIKMA pharmaceuticals (Amman, Jordan). Vitamin D was obtained as Vitamin D3 solution from Sigma-Aldrich (CAS Number 67–97-0, St. Louis, MI, USA).
Microbial Culture and Growth Conditions
The antibacterial effect of ciprofloxacin with vitamin D was studied on seven reference bacterial; Escherichia coli ATCC 35,218, Staphylococcus aureus ATCC29213, Pseudomonas aeruginosa ATCC 9027, Staphylococcus epidermidis ATCC 12,228, Acinetobacter baumannii ATCC 17,978, Proteus mirabilis ATCC 12,459 and Klebsiella pneumoniae ATCC 13,883. The organisms were stored at −70 °C in trypticase-soy broth and 20% glycerol (Becton Dickinson, East Rutherford, NJ, USA). Samples were thawed when they were ready for batch susceptibility. Minimum inhibitory concentrations (MICs) were determined in accordance with the Clinical and Laboratory Standards Institute.23
Antimicrobial Susceptibility Test
Serial 2 fold dilutions were added to molten BBL Muller-Hinton Gold II agar from BBL Microbiology Systems. After slight cooling and drying of the plates, a steer replicator was used to place aliquots containing approximately 5 × 104 colony forming units per 50 µL for each tested bacterial strain. The plates were incubated at 37 °C and read after 24 hours of incubation. Ciprofloxacin solutions (100 µg/mL) were prepared on the day of use according to the manufacturer’s recommendations. Ciprofloxacin was dissolved in water, whereas vitamin D was dissolved in ethanol. In each experiment, ciprofloxacin was added either alone (the positive control) or in combination with a final concentration of 100 µM of vitamin D to agar right before they were added to plates for the 24 hour incubation period.24,25 Results (the mean of 3 independent experiments) were recorded by measuring the zones of growth inhibition surrounding the antimicrobial-containing discs. A zone of growth inhibition of 15 mm or more was selected to represent the bacterial susceptibility of the compound. This was based on the breakpoint indicated in the tables of the Clinical and Laboratory Standards Institute guidelines to determine susceptibility and resistance.23
Determination of Minimum Inhibitory Concentration (MIC)
The MIC was determined by serial dilution method as discussed previously.21 Stock solution of ciprofloxacin and vitamin D were passed through a pyrogenic filter to sterilize the solutions, which were, then, serially diluted to their final concentrations. Thereafter, they were added to plates containing 100 µL of molten BBL Muller-Hinton Gold II agar (Becton Dickinson, Franklin Lakes, NJ, USA). Next, plates were slightly cooled and dried. Then, aliquots containing 20 µL inoculum (about 5 × 104 colony forming units per drop of different bacterial strains) were placed in each plate using a steer replicator. Plates were read after an 18-hour incubation period at 37 °C. The growth of the microorganisms was determined by turbidity. Clear wells indicated absence of bacterial growth. MIC was defined as the lowest concentration at which no growth, a faint haze or fewer than three discrete colonies were detected. Plates were read in duplicate, and the highest MIC values were recorded.
Measurement of ROS Generation
ROS generation was measured as a function of hydrogen peroxide generation. E. coli bacterial cells were cultured using nutrient broth (Hi-media, M002, Mumbai, India) and were treated with ciprofloxacin (100 µg/mL) for variable points of time. E. coli bacterial cells were then incubated with the fluorescent probe 2’,7’-dichlorofluorescein diacetate for 30 minutes (DCF-DA, Sigma Aldrich). Fluorescence DCF-DA intensity was determined using a FACS flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), with an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
Statistical Analysis
Analysis was performed using GraphPad Prism software (version 4.0, GraphPad software, La Jolla, CA). One-way ANOVA followed by Tukey’s post-test was used to determine if there was any statistically significant difference. P values <0.05 were considered statistically significant.
Results
In this study, the possible attenuating effect of vitamin D on the antimicrobial activity of ciprofloxacin has been investigated using several reference bacterial strains, namely, E. coli, S. aureus, P. aeruginosa, S. epidermidis, A. baumannii, P. mirabilis and K. pneumoniae. Results shown in Table 1 revealed that ciprofloxacin induced a significant antimicrobial activity against the reference bacteria except for K. pneumoniae. When bacteria were treated with the combination of ciprofloxacin and vitamin D, the diameters of the zones of inhibition for all tested bacteria were significantly smaller compared to those obtained when bacteria were treated with ciprofloxacin alone. Among the different tested strains, E.coli was the most sensitive bacteria to ciprofloxacin compared to K. pneumoniae, which was the least sensitive (Table 1).
 |
Table 1 Comparison of the Zones of Inhibition (mm) of Ciprofloxacin Alone and Ciprofloxacin in the Presence of Vitamin D Against the Standard Bacterial Strains |
After that, minimum inhibitory concentrations (MICs) of ciprofloxacin alone or in combination with vitamin D were assessed. As shown in Table 2, treatment of different bacterial strains with vitamin D significantly inhibited the antimicrobial effect of ciprofloxacin. This can be denoted by the significantly higher MIC values for the combination compared to ciprofloxacin alone (Table 2).
 |
Table 2 Comparison Between the MICs (µg/ml) of Ciprofloxacin in the Presence of Vitamin D Against Standard Bacterial Strains |
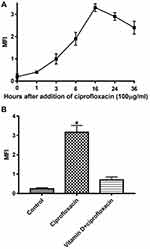
Previous studies from this lab showed that ROS generation induced and manipulated the antibacterial activity of ciprofloxacin.21,22,26 To study this possibility, E. coli cells were treated with ciprofloxacin for various time points. Ciprofloxacin induced an increase in ROS generation of treated cells as indicate generation of DCFH-DA at 16 hours (Figure 1A). Pretreatment E. coli cells with vitamin D at 100 µM for 16 hours reduced ciprofloxacin-induced ROS generation by 71.9% (Figure 1B). Similarly, E.coli cells pretreatment with vitamin D significantly reduced cytotoxicity induced by ciprofloxacin (Tables 1 and 2).
Discussion
In this study, we revealed that the antimicrobial effect of fluoroquinolones, namely, ciprofloxacin was inhibited when bacteria were pretreated with vitamin D. Results obtained in this study were based upon using various strains of reference bacteria. These results could be of importance for patients receiving vitamin D while taking ciprofloxacin for bacterial infections.
Results obtained in the current study showed that ciprofloxacin possess an antimicrobial activity against a wide panel of bacterial strain including E.coli, S. Aureus, P. aeruginosa, S. epidermidis, A. baumannii, and P. mirabilis. These results are in accordance with many previous studies that have shown susceptibility of these bacterial strains to ciprofloxacin.5,21 Moreover, the formation of reactive oxygen species inside the bacteria has been suggested as one of the mechanisms through which ciprofloxacin exerts its antimicrobial effect,12,20,21 as several studies revealed protection of the bacteria from the antimicrobial effect of ciprofloxacin when these bacteria are pretreated with antioxidants, namely, vitamin C, vitamin D, vitamin B12, tempol, melatonin and pentoxifylline.21,22,26 In concordance, current results showed that the cytotoxicity of ciprofloxacin against bacterial cells was associated with a time-dependent ROS generation. This generation of ROS was attenuated via treatment of bacterial cells with vitamin D, which possesses well documented antioxidant activity.27,28
In the current study, combining ciprofloxacin with vitamin D resulted in inhibiting the antibacterial activity of ciprofloxacin against the reference bacterial strains. To our knowledge, this is the first study to report such effect. These results could reveal that the concomitant administration of vitamin D and ciprofloxacin might negatively affect the therapeutic potential of ciprofloxacin through possibly, interfering with one of its antibacterial mechanisms.
The exact mechanism of this interaction between ciprofloxacin and vitamin D is not fully known. Fluoroquinolones, including ciprofloxacin, exert their bactericidal effect through mainly, interfering with bacterial DNA gyrase, type II topoisomerase,29 leading to excess production of ROS in the bacterial cells and eventually, cell death.30,31 The results of the current study further emphasize the role of ROS in the antimicrobial effect of ciprofloxacin.
Furthermore, results of this study revealed the high sensitivity of E.coli against ciprofloxacin, compared to other bacterial species tested such as A. baumannii and K. pneumoniae, which have low to intermediate sensitivity. This was manifested by the significantly smaller diameters of zone of inhibition of ciprofloxacin for A. baumannii and K. pneumoniae compared to large zones of inhibition against E.coli. This higher sensitivity of E. coli could be due to the abundance of outer membrane proteins –porins – as compared to other investigated bacterial strain in the current study. Porins were shown to be related to increased sensitivity to E. coli.32 In correlation with this, the MIC values for A. baumannii, for example, was several folds higher than those for E.coli. To this end, the possibility of this interaction between ciprofloxacin and vitamin D exists, future studies are still needed for better understanding of the exact mechanisms of this interaction.
In this study, the possible reducing effect for vitamin D on the antimicrobial activity of ciprofloxacin was assessed based only on few parameters (the zones of inhibition, MIC values, and ROS generation). Our future studies will use other several parameters for better understanding of this possible interaction. In this study, we used limited number of bacterial strains. Our future studies will be directed toward the use of more standard bacterial strains. In vivo studies concerned with the effect of this combination in patients receiving this combination are also warranted.
In conclusion, the antibacterial activity of ciprofloxacin was inhibited when combined with vitamin D. If further proved in clinical studies, this interaction could be significant where patients who are on a vitamin D supplement may be not be treated with ciprofloxacin.
Funding
The authors would like to acknowledge Jordan University of Science & Technology, Irbid; Jordan, for their financial support (grant number 239/2018).
Disclosure
The authors reported no potential conflicts of interest for this work.
References
1. LeBel M. Ciprofloxacin: chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacotherapy. 1988;8(1):3–33.
2. Blondeau JM. Expanded activity and utility of the new fluoroquinolones: a review. Clin Ther. 1999;21(1):3–42. doi:10.1016/S0149-2918(00)88266-1
3. Oliphant CM, Green GM. Quinolones: a comprehensive review. Am Fam Physician. 2002;65(3):455–464.
4. Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22(4):664–689. doi:10.1128/CMR.00016-09
5. Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A. Ciprofloxacin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35(4):373–447. doi:10.2165/00003495-198835040-00003
6. Pandey A, Aggarwal N, Adholeya A, Kochar M. Resurrection of nalidixic acid: evaluation of water-based nanoformulations as potential nanomedicine. Nanoscale Res Lett. 2018;13(1):298. doi:10.1186/s11671-018-2718-8
7. Paton JH, Reeves DS. Fluoroquinolone antibiotics: microbiology, pharmacokinetics and clinical use. Drugs. 1988;36(2):193–228. doi:10.2165/00003495-198836020-00004
8. Reis ACC, Santos S, Souza SCD, Saldanha MG, Pitanga TN, Oliveira RR. Ciprofloxacin resistance pattern among bacteria isolated from patients with community-acquired urinary tract infection. Rev Inst Med Trop Sao Paulo. 2016;58:53. doi:10.1590/S1678-9946201658053
9. Masadeh MM, Alzoubi KH, Khabour OF, Al-Azzam SI. Ciprofloxacin-induced antibacterial activity is attenuated by phosphodiesterase inhibitors. Curr Ther Res Clin Exp. 2014;77:14–17. doi:10.1016/j.curtheres.2014.11.001
10. Becerra MC, Eraso AJ, Albesa I. Comparison of oxidative stress induced by ciprofloxacin and pyoverdin in bacteria and in leukocytes to evaluate toxicity. J Biol Chem Lumin. 2003;18(6):334–340. doi:10.1002/bio.742
11. Becerra MC, Paez PL, Larovere LE, Albesa I. Lipids and DNA oxidation in Staphylococcus aureus as a consequence of oxidative stress generated by ciprofloxacin. Mol Cell Biochem. 2006;285(1–2):29–34. doi:10.1007/s11010-005-9051-0
12. Albesa I, Becerra MC, Battan PC, Paez PL. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun. 2004;317(2):605–609. doi:10.1016/j.bbrc.2004.03.085
13. Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287–299. doi:10.1016/j.bpobgyn.2010.10.016
14. Martinez LJ, Sik RH, Chignell CF. Fluoroquinolone antimicrobials: singlet oxygen, superoxide and phototoxicity. Photochem Photobiol. 1998;67(4):399–403. doi:10.1111/j.1751-1097.1998.tb05217.x
15. Pouzaud F, Bernard-Beaubois K, Thevenin M, Warnet JM, Hayem G, Rat P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: involvement of oxidative stress. J Pharmacol Exp Ther. 2004;308(1):394–402. doi:10.1124/jpet.103.057984
16. Albahrani AA, Greaves RF. Fat-soluble vitamins: clinical indications and current challenges for chromatographic measurement. Clin Biochem Rev. 2016;37(1):27–47.
17. Tagliaferri S, Porri D, De Giuseppe R, Manuelli M. The controversial role of vitamin D as an antioxidant: results from randomised controlled trials. Nutr Res Rev. 2019;32(1):99–105.
18. Tran B, Armstrong BK, Ebeling PR, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. 2013;99(1):156–161. doi:10.3945/ajcn.113.063271
19. Mokhtari Z, Hekmatdoost A, Nourian M. Antioxidant efficacy of vitamin D. J Parathyr Dis. 2017;5(1):11–16.
20. Becerra MC, Albesa I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem Biophys Res Commun. 2002;297(4):1003–1007. doi:10.1016/S0006-291X(02)02331-8
21. Masadeh MM, Mhaidat NM, Alzoubi KH, Al-Azzam SI, Shaweesh AI. Ciprofloxacin-induced antibacterial activity is reversed by vitamin E and vitamin C. Curr Microbiol. 2012;64(5):457–462. doi:10.1007/s00284-012-0094-7
22. Masadeh MM, Alzoubi KH, Al-Azzam SI, Khabour OF, Al-Buhairan AM. Ciprofloxacin-induced antibacterial activity is atteneuated by pretreatment with antioxidant agents. Pathogens. 2016;5(1):28. doi:10.3390/pathogens5010028
23. (CLSI) CaLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically; Approved Standard.
24. Saito M, Sasaki T, Matsuoka H. Vitamin B12 promotes Cx40 and HCN4 gene expression at an early stage of cardiomyocyte differentiation. Exp Anim. 2009;58(1):57–60. doi:10.1538/expanim.58.57
25. Solovieva ME, Solovyev VV, Kudryavtsev AA, Trizna YA, Akatov VS. Vitamin B12b enhances the cytotoxicity of dithiothreitol. Free Radic Biol Med. 2008;44(10):1846–1856. doi:10.1016/j.freeradbiomed.2008.02.002
26. Masadeh M, Alzoubi K, Al-Azzam S. Flouroquinolones-induced antibacterial activity atteneuation by pretreatment with vitamin B12. Int J Pharmacol. 2015;11(1):67–71. doi:10.3923/ijp.2015.67.71
27. Dzik K, Skrobot W, Flis DJ, et al. Vitamin D supplementation attenuates oxidative stress in paraspinal skeletal muscles in patients with low back pain. Eur J Appl Physiol. 2018;118(1):143–151. doi:10.1007/s00421-017-3755-1
28. Jamilian M, Mirhosseini N, Eslahi M, et al. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth. 2019;19(1):107. doi:10.1186/s12884-019-2258-y
29. Gootz TD, Barrett JF, Sutcliffe JA. Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob Agents Chemother. 1990;34(1):8–12. doi:10.1128/AAC.34.1.8
30. Gürbay A, Hincal F. Ciprofloxacin-induced glutathione redox status alterations in rat tissues. Drug Chem Toxicol. 2004;27(3):233–242. doi:10.1081/DCT-120037504
31. Goswami M, Mangoli SH, Jawali N. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob Agents Chemother. 2006;50(3):949–954. doi:10.1128/AAC.50.3.949-954.2006
32. Veiga-Crespo P, Fusté E, Vinuesa T, Viñas M, Villa TG. Synergism between outer membrane proteins and antimicrobials. Antimicrob Agents Chemother. 2011;55(5):2206–2211. doi:10.1128/AAC.01786-10
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.