Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Visual Performance Following Bilateral Implantation of Refractive Rotationally Asymmetric Bifocal Intraocular Lens (LS-313 MF30) or Apodized Diffractive Bifocal Intraocular Lens (ReSTOR SN6AD1)
Authors Li H , Liu D, Gao H, Sun J, Bai H, Wu X
Received 20 June 2021
Accepted for publication 16 August 2021
Published 1 September 2021 Volume 2021:17 Pages 917—926
DOI https://doi.org/10.2147/TCRM.S325287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Honglei Li,1,2 Dongle Liu,1,2 Han Gao,3 Jiajun Sun,1,2 Huiran Bai,1,2 Xiaoming Wu1,2
1Qingdao Eye Hospital of Shandong First Medical University, Qingdao, People’s Republic of China; 2State Key Laboratory Cultivation Base, Shandong Provincial Key Laboratory of Ophthalmology, Shandong Eye Institute, Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao, People’s Republic of China; 3Department of Ophthalmology, Qingdao Central Hospital, The Second Affiliated Hospital of Medical College of Qingdao University, Qingdao, People’s Republic of China
Correspondence: Xiaoming Wu
Qingdao Eye Hospital of Shandong First Medical University, 5 Yanerdao Road, Qingdao, 26600, People’s Republic of China
Email [email protected]
Purpose: This study compared the clinical outcomes after cataract surgery with implantation of refractive rotationally asymmetric bifocal intraocular lens (IOL) (LS-313 MF30) and apodized diffractive bifocal IOL (ReSTOR SN6AD1).
Methods: This was a prospective, non-randomized, controlled study, where patients diagnosed with age-related cataracts were selected for phacoemulsification combined with bilateral IOL implantation. Based on the type of IOL voluntarily implanted, the patients were divided into two groups, ie, refractive and diffractive groups. In total, 30 cases (60 eyes) were in a refractive group, while 30 cases (60 eyes) were in diffractive group. Three months after surgery, we examined the uncorrected distance visual acuity (UDVA), uncorrected intermediate visual acuity (UIVA), uncorrected near visual acuity (UNVA), defocus curve, objective visual quality, and subjective questionnaire.
Results: Three months after surgery, the UIVA of the refractive group (0.18 ± 0.08) logMAR was better than that of the diffractive group (0.29 ± 0.16) logMAR (P < 0.05). No significant difference in UDVA and UNVA was noted between the two groups. For a 4mm pupil diameter, the intraocular and total eye aberration, higher-order aberration (HOA), coma, spherical aberration, and trefoil in the refractive group were significantly higher than those in diffractive group (P < 0.05). The intraocular modulation transfer function (MTF), intraocular strehl ratio (SR), total eye MTF, and total eye SR in the refractive group were lower than those in diffractive group (P < 0.05). No significant difference in glare incidence, spectacle independence rate, and patient satisfaction was observed between the two groups (P > 0.05). The halos incidence in the refractive group was lower than the diffractive group (P < 0.05).
Conclusion: Both bifocal IOLs obtained satisfactory UDVA and UNVA, with higher patient satisfaction. Unlike the apodized diffractive bifocal IOL, the refractive rotationally asymmetric bifocal IOL yielded slightly better UIVA, lower halos incidence, whereas the apodized diffractive bifocal IOL showed a better objective visual quality.
Keywords: refractive rotationally asymmetric, apodized diffractive, intraocular lens, visual quality
Introduction
With the continuous advancement of phacoemulsification technology, cataract surgery has gradually developed to refractive surgery. Traditional monofocal IOL has only one fixed focus, causing the loss of accommodation after implantation.1 Based on different optical principles, different multifocal intraocular lens (MIOL) are designed, which are primarily divided into refractive MIOL, diffractive MIOL, and diffractive refractive MIOL. MIOL provides a clear vision at different distances and a better near vision than single focus IOL with significant success in clinical practice.2 Nonetheless, the decreased contrast sensitivity of MIOL and the high incidence of photic phenomena including halos and glare potentially influence visual quality and patient satisfaction after MIOL.3
Refractive rotationally asymmetric MIOL is a novel type of MIOL, with a rotational asymmetric design. IOL combines distant vision provided by the larger fan-shaped area above and near vision provided by the smaller +3D sector-shaped refractive surface below with a smooth transition between the two areas. Theoretically, refractive rotationally asymmetric MIOL lacks a concentric ring for refraction or diffraction, and the light passing through the transition region is reflected to the area far away from the optical axis. Therefore, the occurrence of photic phenomena including glare and halos can be minimized.4,5 To our knowledge, the visual performance of refractive rotationally asymmetric MIOL (LS-313 MF30) and apodized diffractive MIOL (ReSTOR SN6AD1) has not been compared.
This paper aims to compare the clinical efficacy of the two types of MIOL to provide clinical guidance for the selection of MIOL.
Patients and Methods
A prospective, non-randomized, controlled study was conducted on patients with age-related cataracts treated in Qingdao Eye Hospital between June 2019 and September 2020. The characteristics of MIOL were comprehensively introduced to the patients. Based on the type of IOL voluntarily implanted, the patients were subdivided into two groups including refractive and diffractive groups. This study followed the principles of the Helsinki Declaration and was approved by the Ethics Committee of Qingdao Eye Hospital. All patients signed the informed consent form to the treatment plan with a clinical trial registration number of ChiCTR1900022818.
The inclusion criteria included (1) axial length (AL) ≥22mm and ≤26mm; (2) preoperative corneal astigmatism ≤1.0D; and (3) 4 mm pupil diameter total HOA < 0.3 μm.
Meanwhile, the exclusion criteria included (1) previous history of ophthalmic surgery; (2) amblyopia; (3) chronic or recurrent uveitis; (4) progressive retinopathy; (5) glaucoma; (6) corneal disease; (7) maculopathy (based on optical coherence tomography).
Intraocular Lens
LS-313 MF30 (Oculentis Co., Germany) adopted a one-piece plate loop bifocal IOL design with a total length of 11.0mm and optical diameter of 6mm. Unlike the previous concentric circular multifocal intraocular lens, its optical region exhibited two sector-shaped regions, ie, the larger region was the far-sighted region while the smaller area was the near-sighted region with additional + 3D, and a transition zone between them. Through the spectroscopic principle, the light was divided into two focal points to see far and near (Figure 1A).
 |
Figure 1 Two models of multifocal intraocular lens. (A) Refractive rotationally asymmetric bifocal intraocular lens (LS-313 MF30); (B) Apodized diffractive bifocal intraocular lens (ReSTOR SN6AD1). |
Restor SN6AD1 (Alcon Co., USA) aspheric apodized diffractive bifocal IOL with +3.0 D near addition power, which uses the principle of Huygens-Fresnel diffraction and refraction had an optical diameter of 6mm. The diameter of the central diffraction region was 3.6mm comprising nine concentric micro-slope rings. The height decreased step by step from the middle to the periphery, the width gradually narrowed, while the periphery gradually changed into a refractive zone. The higher part of the slope ring focused on the light to the near focus. The lower part focused on the light to the far focus and the separation of the far (Figure 1B).
Preoperative Examination
All patients underwent comprehensive preoperative ophthalmological examination, including UDVA, axial length, anterior chamber depth, corneal curvature, measured by optical biometric instrument (OA-2000, Tomey Co., Japan), slit-lamp examination, intraocular pressure, funduscopy, corneal endothelial count, optical coherence tomography, Pentacam (Oculus Co., Germany). The power of IOL was calculated using Barrett Universal II, the target refractive state was emmetropia, while the degree of IOL was in the range of ±0.25D.
Surgical Procedures
The standard phacoemulsification was performed by one experienced operator (WXM) in both groups. The incision was 2.2mm transparent corneal incision, the diameter of the capsulorhexis was 5.0–5.5 mm, phacoemulsification, the residual cortex was removed by I/A, IOL was placed in the capsule bag, and the near-sighted region of LS-313 MF30 was placed below.
Postoperative Examination
Three months after surgery, the UDVA, UIVA, and UNVA of the patients were examined, where the examination distance of UDVA, UIVA, and UNVA was 4m, 80cm, and 40cm, respectively. Refraction, defocus curve, objective visual quality and subjective questionnaire including Visual Functioning Questionnaire-146 were examined and objective visual quality were measured by iTrace (Tracey Company, USA). All the postoperative examinations were performed by the same person and the examiner did not know the type of IOL implanted in the patient.
Data Analysis
SPSS (version 22.0; SPSS, Inc., Chicago, IL) statistical software was used for data analysis. The data between the two groups were compared, where the chi-square test was used for classified data, Kruskal–Wallis test was used for measurement data to check whether the data conformed to a normal distribution, while independent sample t-test was used if normal distribution was satisfied; otherwise, Mann–Whitney U-test was used. P < 0.05 was considered statistically significant.
Results
In total, 30 cases (60 eyes) were implanted with LS-313 MF30, while 30 cases (60 eyes) were implanted with Restor SN6AD1. No intraoperative and postoperative complications were reported. Moreover, no significant difference in age, gender, laterality, preoperative CDVA, corneal curvature, axial length, anterior chamber depth, and power of IOL implantation was noted between the two groups (P > 0.05) (Table 1).
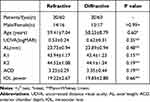 |
Table 1 Preoperative Conditions of the 2 Groups of Eyes |
Visual and Refractive Outcomes
Three months after surgery, no significant difference in sphere, cylinder, and spherical equivalent was found between the two groups, and no significant difference in UDVA, UNVA, and CDVA was observed between the two groups (P > 0.05). The UIVA of the refractive group was 0.18 ±0.08, while that of the diffractive group was 0.29 ±0.16. Notably, the UIVA of the refractive group was better than that of the diffractive group (Table 2). Figure 2 shows the percentage of eyes with a UDVA, UNVA, and UIVA of 0.3 logMAR or better between the two groups. Diffractive group had a limitation in UIVA.
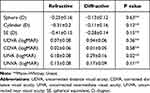 |
Table 2 Postoperative Visual and Refractive Outcomes in the 2 Groups |
Defocus Curve
Three months after surgery, the wave peaks of the eyes in the refractive group appeared at 0D and-3D, stable in the range between 0D and −3D, and slightly decreased in the range from-3.0D to-5.0D. The peaks in the diffractive group appeared at 0D and-3D, and the fluctuation in the range from 0D to-3D in the apodized diffractive group was more apparent than that in the refractive group. The curve decreased rapidly in the range of −3.0D to −5.0D (Figure 3).
 |
Figure 3 Mean defocus curve in the 2 groups of eyes 3 months after cataract surgery. |
Objective Visual Quality
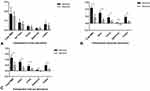
For a 4mm pupil diameter, the intraocular and total eye aberration, HOA, coma, spherical aberration, and trefoil in the refractive group were significantly higher than those in diffractive group (P < 0.05) (Figure 4). The intraocular MTF, intraocular SR, total eye MTF, and total eye SR in the refractive group were lower than those in diffractive group (P < 0.05) (Figure 5) (Table 3).
 |
Table 3 Postoperative SR in the 2 Groups |
Subjective Questionnaire Survey
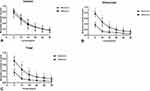
In the refractive group, 36.67% of the eyes showed glare compared to 43.33% in the diffractive group (P=0.79). The halos were present in 36.67% of the eyes with a refractive rotationally asymmetric IOL and 66.67% of the eyes with an apodized diffractive IOL (P=0.04). The halos were more frequent than glare in diffractive groups (Figure 6).
 |
Figure 6 Frequencies of halos and glare cataract surgery. |
In the refractive group, 96.67% reported no need of spectacles for far distance compared to 96.67% in the diffractive group (P=0.99), 63.33% for intermediate distance compared to 40.0% in the diffractive group (P=0.12) and 86.67% for near distance compared to 83.33% in the diffractive group (P=0.99) (Figure 7).
 |
Figure 7 Rates of spectacle independence at far, intermediate and near distance. |
The Visual Functioning Questionnaire-14 for evaluation of the difficulty in performing vision-related activities demonstrated no significant difference in any parameter between the two groups (P > 0.05) (Table 4). 93.33% of the patients with refractive rotationally asymmetric IOL were satisfied, compared to 90% of the patients with apodized diffractive IOL (P =0.99).
 |
Table 4 National Eye Institute Visual Functioning Questionnaire-14 |
Discussion
The primary purpose of MIOL is to obtain ideal clinical efficacy after cataract surgery, achieving satisfactory distance, intermediate, and near vision. At present, two types of MIOL are used clinically, ie, rotational symmetrical MIOL and rotational asymmetric MIOL. Rotational symmetric MIOL has been widely investigated and evaluated, however, the resulting visual interference limits its application.7,8 For instance, patients with diffractive MIOL implantation might have many types of photic phenomena, including decreased contrast sensitivity, glare, or halos.9 By comparing the clinical visual effects of different models of MIOL, it may be helpful for ophthalmologist to select the appropriate IOL according to the needs of the patients. Herein, subjective and objective visual quality were compared between refractive rotationally asymmetric bifocal IOL (LS-313 MF30) and apodized diffractive bifocal IOL (ReSTOR SN6AD1). We found that refractive rotationally asymmetric bifocal IOL had better UIVA, lower halos incidence, whereas the apodized diffractive bifocal IOL showed a better objective visual quality.
Refractive group showed slightly better UIVA than diffractive group. LogMAR value of the refractive group (0.18 ±0.08) was significantly lower than that of the diffractive group (0.29 ±0.16), confirming that the refractive rotationally asymmetric bifocal IOL provides a better UIVA than the apodized diffractive bifocal IOL. The results were consistent with the previous findings of rotationally asymmetric and diffractive MIOL.10–12 Wang et al10 reported that rotationally asymmetricMIOL (SBL-3) provided better UIVA and wider range of intermediate vision than apodized diffractive MIOL (SN6AD1). Alio et al11 also found that refractive MIOL (Lentis Mplus LS-312) provided better intermediate vision than diffractive MIOL (Acri.Lisa 366D). Although there was a statistical difference between the two groups in UIVA, the slight advantage of average 0.11 logMAR value (approximately 1 logMAR line) was limited in refractive group. In our study, the spectacle independence rate, especially in intermediate distance, did not differ between the two groups. This illustrated that the slightly better UIVA in refractive group might not have a meaningful effect on the spectacle independence rate in intermediate distance. Perhaps, only patients who had a higher demand of intermediate visual acuity might benefit more from refractive bifocal IOL compared with diffractive bifocal IOL.
Defocus curve is an effective method to evaluate the whole visual acuity of MIOL, showing a visual acuity of different defocus levels, with the result being equivalent to the visual acuity of different viewing distances. The mid-range visual acuity in the refractive group was better than diffractive group, as confirmed by the results of the defocus curve. Both groups provided two peaks of vision of-3D and 0.0D, with a slight decrease between 0.0D and-3D. This decrease was not apparent on the defocus curve of the refractive group; hence, the defocus curve effect of the refractive group was better than that of the diffractive group. This was consistent with previous studies reporting that refractive rotationally asymmetric bifocal IOL exhibits a satisfactory visual range of intermediate visual acuity.12–14
Apodized diffractive bifocal IOL showed a better objective visual quality. The intraocular and total eye aberration, HOAs, coma, spherical aberration, and trefoil in the refractive group under 4mm pupil diameter were higher than those in the diffractive group. This was similar to a previous study where the HOAs and coma of rotationally asymmetric MIOL were higher than those of diffractive MIOL.11,12 This might be related to the asymmetric design of rotationally asymmetric MIOL because of a gradual transition between the two regions from far vision to near.14 Higher coma harms vision because of visual interference, which might reduce the objective optical quality of refractive rotationally asymmetric MIOL.15 However, the increased intraocular aberration of rotationally asymmetric MIOL potentially extended the focal depth, ie, its advantage in UIVA compared with diffractive bifocal IOL.10,16 It was believed that the presence of this optical defect allowed an extended depth of focus that would grant adequate vision at various distances. We also reported significant differences in 5c/d, 10c/d, 15c/d, 20c/d, 25c/d, and 30c/d in intraocular and total eye MTF between the two groups. The value of MTF represents the contrast ratio of the retinal image to the actual object in different spatial frequencies, and the higher the value, the better the contrast of the image. We found that the value of apodized diffractive MIOL MTF was better than that of refractive rotationally asymmetric MIOL. Previous studies showed that asymmetric MIOL exhibited a better contrast sensitivity than rotationally symmetrical diffractive MIOL.11,17 Nevertheless, the introduction of intraocular aberration potentially reduces the retinal image quality of rotationally asymmetric MIOL.10 Similarly, Nio et al18 discovered that HOA increases the depth of focus and decreases the MTF value at higher spatial frequency. Therefore, we concluded that the increase of intraocular aberration might decrease the MTF value. Besides, we evaluated SR as a parameter to compare the objective visual quality between MIOLs provided by iTrace. SR is a parameter used to estimate the overall optical quality, defined as the peak intensity ratio of the image formed by the aberration optical system to the intensity of the aberration-free system. The higher the value, the better the visual quality.19 The SR value showed that the objective visual quality of the refractive group was better than that of the diffractive group, consistent with previous research findings.10
De Vries et al8 reported that 38.2% of dissatisfied patients after MIOL implantation complained primarily of optical phenomena-glare and halos. Therefore, the phenomenon of optical interference after operation is a primary concern of ophthalmologists. There was a trend toward a lower incidence of halos perception in eyes with the refractive rotationally asymmetric MIOL (P=0.04). This trend may be related to the rotational asymmetric design in optical performance with the refractive rotationally asymmetric MIOL. Just as Montés-Micó et al9 reported, patients with diffractive MIOL implantation might have more photic phenomena, including glare, or halos. This difference may also be related to the concentric rings design in optical performance with the diffractive IOL.20–22 Although refractive rotationally asymmetric bifocal IOL might have less photic phenomena, its objective visual quality was not as good as that of diffractive bifocal IOL. It was reported that because of the vertical asymmetric optical geometry of refractive rotationally asymmetric MIOL, the direct application of conventional wavefront sensors cannot precisely evaluate the aberrations.23 Objective aberration measurements may be inaccurate in refractive group. This suggested that subjective feelings of patients were an indispensable part of our evaluation of visual performance of the two MIOLs. A topic that should be addressed in future studies with larger samples.
No significant difference in UDVA and UNVA was found between the two groups, corroborating with previous findings.11,12 More than 83.33% of patients in both groups reported spectacle independence at far and near distance. In general, most of the patients in both groups revealed an extremely high spectacle independence rate and satisfaction, corroborating with the previous research findings.5,12,13,22,24,25 We administered a Visual Functioning Questionnaire-14 to assess postoperative patient satisfaction. This questionnaire evaluated the patient’s ability to perform daily activities. We have not found significant differences in any parameter. Although 40.0% spectacle independence for intermediate distance and 45% eyes of 0.3 logMAR or better for UIVA was relatively lower and a higher incidence of halos perception in diffractive group, the Visual Functioning Questionnaire-14 parameters between the 2 groups exhibited no significant difference, especially when intermediate distance vision was required for fine handwork, cooking, and card games. The level of overall satisfaction was high. The reason may be that the slightly better intermediate visual acuity in refractive group was not enough to affect postoperative patient satisfaction between the 2 groups. This may also be a reflection of adequate preoperative communication, careful patient selection, and less expense.
Previous studies showed that because of the process of neuroadaptation, difficulties with photopic phenomena might decrease over time.26,27 The evaluation of these parameters requires a larger study population (at least 50 cases in each group) and a longer follow-up period (≥6 months). In future studies, we will continue to follow up the study to expand the sample size and extend the follow-up time. Additionally, because we hope to offer patients a personalized choice prior to surgery, patients were assigned to different groups according to their requirement for intermediate vision and the price of IOLs. The lack of randomization could have affected the generalizability of the findings. In future studies, it is necessary to confirm this finding providing a more accurate assessment.
In conclusion, to our knowledge, this was the first study to compare the visual acuity, optical quality, and satisfaction of patients between a refractive rotationally asymmetric bifocal IOL (LS-313 MF30) and an apodized diffractive bifocal IOL (ReSTOR SN6AD1). Both IOLs could effectively restore visual function after cataract surgery upto 3 months. However, eyes with LS-313 MF30 showed better UDVA, UIVA and lower halos incidence, and eyes with ReSTOR SN6AD1 showed significantly lower HOAs. Therefore, the rotationally asymmetric bifocal IOL seems to be a promising alternative for MIOL implantation because it provides a wide range of visual acuity and a more physiologic defocus curve. When patients choose an IOL, it is necessary to fully inform them of the advantages and disadvantages of both IOL models to improve postoperative satisfaction.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Hayashi K, Hayashi H, Nakao F, Hayashi F. Aging changes in apparent accommodation in eyes with a monofocal intraocular lens. Am J Ophthalmol. 2003;135(4):432–436. doi:10.1016/S0002-9394(02)02091-3
2. Greenstein S, Pineda R
3. Alio JL, Plaza-Puche AB, Fernandez-Buenaga R, Pikkel J, Maldonado M. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017;62(5):611–634. doi:10.1016/j.survophthal.2017.03.005
4. Alio JL, Pinero DP, Plaza-Puche AB, Chan MJ. Visual outcomes and optical performance of a monofocal intraocular lens and a new-generation multifocal intraocular lens. J Cataract Refract Surg. 2011;37:241–250. doi:10.1016/j.jcrs.2010.08.043
5. Venter JA, Pelouskova M, Collins BM, Schallhorn SC, Hannan SJ. Visual outcomes and patient satisfaction in 9366 eyes using a refractive segmented multifocal intraocular lens. J Cataract Refract Surg. 2013;39(10):1477–1484. doi:10.1016/j.jcrs.2013.03.035
6. Steinberg EP, Tielsch JM, Schein OD, et al. The VF-14: an index of functional impairment in patients with cataract. Arch Ophthalmol. 1994;112:630–638. doi:10.1001/archopht.1994.01090170074026
7. Alfonso JF, Puchades C, Fernandez-Vega L, Montes-Mico R, Valcarcel B, Ferrer-Blasco T. Visual acuity comparison of 2 models of bifocal aspheric intraocular lenses. J Cataract Refract Surg. 2009;35(4):672–676. doi:10.1016/j.jcrs.2008.11.061
8. de Vries NE, Webers CA, Touwslager WR, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:859–865. doi:10.1016/j.jcrs.2010.11.032
9. Montes-Mico R, Alio JL. Distance and near contrast sensitivity function after multifocal intraocular lens implantation. J Cataract Refract Surg. 2003;29(4):703–711. doi:10.1016/S0886-3350(02)01648-6
10. Wang X, Tu H, Wang Y. Comparative analysis of visual performance and optical quality with a rotationally asymmetric multifocal intraocular lens and an apodized diffractive multifocal intraocular lens. J Ophthalmol. 2020;2020:7923045. doi:10.1155/2020/7923045
11. Alio JL, Plaza-Puche AB, Javaloy J, Ayala MJ, Moreno LJ, Pinero DP. Comparison of a new refractive multifocal intraocular lens with an inferior segmental near add and a diffractive multifocal intraocular lens. Ophthalmology. 2012;119(3):555–563. doi:10.1016/j.ophtha.2011.08.036
12. Ye L, Chen T, Hu Z, Yang Q, Su Q, Li J. Comparison of the visual performance between oculentis MF30 and Tecnis ZMB00 multifocal intraocular lenses. Ann Transl Med. 2021;9(2):144. doi:10.21037/atm-20-7777
13. McNeely RN, Pazo E, Spence A, et al. Visual quality and performance comparison between 2 refractive rotationally asymmetric multifocal intraocular lenses. J Cataract Refract Surg. 2017;43:1020–1026. doi:10.1016/j.jcrs.2017.05.039
14. Ramon ML, Pinero DP, Perez-Cambrodi RJ. Correlation of visual performance with quality of life and intraocular aberrometric profile in patients implanted with rotationally asymmetric multifocal IOLs. J Refract Surg. 2012;28(2):93–99. doi:10.3928/1081597X-20111213-02
15. Applegate RA, Sarver EJ, Khemsara V. Are all aberrations equal? J Refract Surg. 2002;18(5):S556–S562. doi:10.3928/1081-597X-20020901-12
16. Venter JA, Barclay D, Pelouskova M, Bull CEL. Initial experience with a new refractive rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2014;30(11):770–776. doi:10.3928/1081597X-20141021-09
17. Alio JL, Plaza-Puche AB, Javaloy J, Ayala MJ. Comparison of the visual and intraocular optical performance of a refractive multifocal IOL with rotational asymmetry and an apodized diffractive multifocal IOL. J Refract Surg. 2012;28:100–105. doi:10.3928/1081597X-20120110-01
18. Nio YK, Jansonius NM, Fidler V, Geraghty E, Norrby S, Kooijman AC. Spherical and irregular aberrations are important for the optimal performance of the human eye. Ophthalmic Physiol Opt. 2002;22(2):103–112. doi:10.1046/j.1475-1313.2002.00019.x
19. Diaz-Douton F, Benito A, Pujol J, Arjona M, Guell JL, Artal P. Comparison of the retinal image quality with a Hartmann-Shack wavefront sensor and a double-pass instrument. Invest Ophthalmol Vis Sci. 2006;47:1710–1716. doi:10.1167/iovs.05-1049
20. Bohm M, Hemkeppler E, Herzog M, et al. Comparison of a panfocal and trifocal diffractive intraocular lens after femtosecond laser-assisted lens surgery. J Cataract Refract Surg. 2018;44:1454–1462. doi:10.1016/j.jcrs.2018.07.060
21. Mendicute J, Kapp A, Levy P, et al. Evaluation of visual outcomes and patient satisfaction after implantation of a diffractive trifocal intraocular lens. J Cataract Refract Surg. 2016;42(2):203–210. doi:10.1016/j.jcrs.2015.11.037
22. Bilbao-Calabuig R, Llovet-Rausell A, Ortega-Usobiaga J, et al. Visual outcomes following bilateral implantation of two diffractive trifocal intraocular lenses in 10 084 eyes. Am J Ophthalmol. 2017;179:55–66. doi:10.1016/j.ajo.2017.04.013
23. Akondi V, Perez-Merino P, Martinez-Enriquez E, et al. Evaluation of the true wavefront aberrations in eyes implanted with a rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2017;33(4):257–265. doi:10.3928/1081597X-20161206-03
24. Vryghem JC, Heireman H. Visual performance after the implantation of a new trifocal intraocular lens. Clin Ophthalmol. 2013;7:1957–1965. doi:10.2147/OPTH.S44415
25. Ang R, Martinez G, Cruz E, Tiongson A, Dela cruz A. Prospective evaluation of visual outcomes with three presbyopia-correcting intraocular lenses following cataract surgery. Clin Ophthalmol. 2013;7:1811–1823. doi:10.2147/OPTH.S49848
26. Mester U, Vaterrodt T, Goes F, et al. Impact of personality characteristics on patient satisfaction after multifocal intraocular lens implantation: results from the “happy patient study”. J Refract Surg. 2014;30(10):674–678. doi:10.3928/1081597X-20140903-05
27. Goes FJ. Refractive lens exchange with the diffractive multifocal Tecnis ZM900 intraocular lens. J Refract Surg. 2008;24:243–250.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.