Back to Journals » Clinical Ophthalmology » Volume 10
Visual field defects and changes in macular retinal ganglion cell complex thickness in eyes with intrachoroidal cavitation are similar to those in early glaucoma
Authors Okuma S, Mizoue S , Ohashi Y
Received 9 December 2015
Accepted for publication 23 March 2016
Published 29 June 2016 Volume 2016:10 Pages 1217—1222
DOI https://doi.org/10.2147/OPTH.S102130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shinichi Okuma,1 Shiro Mizoue,2,3 Yuichi Ohashi3
1Department of Ophthalmology, Sumitomo Besshi Hospital, Niihama-shi, 2Department of Ophthalmology, Minami-Matsuyama Hospital, Matsuyama-shi, 3Department of Ophthalmology, Ehime University School of Medicine, Toon-shi, Ehime, Japan
Background/aims: To examine the characteristics of visual field defects and optical coherence tomography (OCT) findings in eyes with intrachoroidal cavitation (ICC) and investigate the similarities between these results and glaucomatous changes.
Methods: We retrospectively analyzed patients diagnosed with ICC based on peripapillary radial cross-sectional scans performed with OCT. Visual field was measured with the Humphrey automated visual field analyzer SITA standard central 24-2 program, and macular ganglion cell complex (GCC) thickness was measured in 9×9 mm areas on OCT. The positive rates for the Anderson criteria, site of visual field defect, and mean GCC thickness in each quadrant were compared; the association between these results and ICC location was assessed.
Results: Fifteen eyes from eleven patients (five males and six females; mean age, 54.6±10.7 years) were selected for investigation. ICC was detected in the inferior temporal side of the optic disc in all studied eyes. The positive rate for the Anderson criteria was 73.3% (11/15 eyes). Visual field defects were most commonly observed in the cluster that corresponded to the superior Bjerrum area (53.3%; 8/15 eyes). GCC thickness was significantly lower in the inferior side, where the ICC was located, than the superior side, where the ICC was absent (P=0.0001). GCC thinning that correlated with ICC was observed in 66.7% (10/15 eyes) of the ICC eyes.
Conclusion: Visual field and GCC findings on OCT in ICC eyes are extremely similar to those observed in superior visual field defect-type early glaucoma, indicating a possible difficulty in distinguishing the two conditions.
Keywords: intrachoroidal cavitation, visual field, ganglion cell complex, glaucoma
Introduction
Recently, structural changes to the external area of the optic disc and the presence of an intrachoroidal sinusoidal structure called the intrachoroidal cavitation (ICC) in the outer area of the peripapillary or myopic conus have been reported, and attracted attention.1–4 Neither of these findings are found in conventional glaucoma, which is defined as a retinal nerve fiber disorder at the lamina cribrosa.
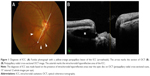
ICC was first reported by Freund et al,1 who observed crescent-shaped yellow–orange lesions near the inferior region of the peripapillary myopic conus in highly myopic eyes. With the optical coherence tomography (OCT) available at the time, this lesion was interpreted as retinal pigment epithelial detachment and was consequently termed “peripapillary detachment of pathologic myopia”. Shimada et al2 conducted an assessment with the Goldmann visual field test and found glaucomatous visual field defects in 71.0% of eyes with peripapillary detachment of pathologic myopia. Later, Toranzo et al3 used a higher resolution OCT to elucidate that this lesion did not have a retinal pigment epithelial detachment but had an intrachoroidal sinusoidal structure, and they subsequently established the name and concept of “ICC”.
In addition, Spaide et al4 reported that inner retinal thinning or disruption is evident at the ICC border area and indicated that this thinning may contribute to visual field defects.
It has been suggested that visual field defects and nerve fiber layer loss that resemble glaucoma may develop due to ICC; however, the only report that examined visual field defects in ICC eyes is the study by Shimada et al2 who used Goldmann perimetry. Consequently, its actual state is not completely understood.
Additionally, if the inner retinal defect at the ICC border area causes visual field defects that are similar to glaucoma, then it is likely that ganglion cell complex (GCC) thinning,5,6 which has been utilized in recent years for glaucoma diagnosis and anticipated for its usefulness in myopia, is detected in correlation to ICC. Nonetheless, there are still no reports on GCC changes in eyes with ICC.
In the present study, to elucidate the characteristics of visual field defects and GCC thickness in eyes with ICC, we used the Humphrey automated visual field analyzer and OCT data to investigate the similarities to glaucomatous changes.
Materials and methods
As the authors had no access to a formal review committee, the study adhered to the tenets of the Declaration of Helsinki, and informed consent was obtained from all patients.
We retrospectively identified all patients who visited Sumitomo Besshi Hospital, Minami-Matsuyama Hospital, or Ehime University Hospital between April 2013 and September 2014 and were diagnosed with ICC by using OCT. We selected patients who completed all of the tests described in the following and for whom the exclusion criteria did not apply.
ICC was diagnosed according to previously reported criteria,3,4 and was defined as a lesion that showed intrachoroidal hyporeflective areas near the optic disc by OCT (Figure 1). OCT examination was performed using an RS-3000 (NIDEK, Aichi, Japan) at all participating facilities. With the papilla at the center, 15° interval radial cross-sectional images (12 whole images per eye) were taken by OCT, and the diagnosis and location identification of ICC were conducted. The peripapillary area was divided into eight 45° sectors from the 12 o’clock position in the temporal direction, and the rate of ICC detection was calculated for each sector. In the peripapillary radial cross-sectional image assessment using OCT, assessments made with images that had a signal strength index of ≤6/10 were excluded from this study.
Visual field testing was performed with the Humphrey automated visual field analyzer SITA standard central 24-2 program (Carl Zeiss Meditec AG, Jena, Germany). To assess the similarities between the obtained visual field and glaucomatous visual field defects, the positive rate for each Anderson criteria (pattern deviation probability plots [PD plots], glaucoma hemifield test, and pattern standard deviation [PSD], P<0.05) and the overall positive rate were examined.
In addition, with regard to the distribution of visual field defects, in order to examine its correlation with ICC location and the similarities with glaucomatous visual field defects, a method described by Garway-Heath et al7 was used. Specifically, each measurement point on the central 24-2 test was divided into six clusters along the course of the nerve fiber, the rate of abnormality was calculated for each cluster, and the correlation with ICC location was confirmed. As a reliability index of the visual field test, patients with ≥20% fixation loss, ≥15% false positive, and ≥33% false negative were excluded from the present study.
GCC thickness was measured using an RS-3000 (NIDEK) at all participating facilities. OCT can analyze the GCC in a 9×9 mm area with the macula as the center, encompassing the optic disc. It can also measure the mean GCC thickness of each area of 9 mm diameter circles with the macula as the center.
To assess the similarities between GCC thickness in eyes with ICC and early glaucoma, the mean GCC thickness was compared between the superior and inferior quadrants and between all four quadrants (superior and inferior regions of the macular temporal region, and superior and inferior regions of the macular nasal region).
In addition, it was confirmed that the GCC thickness was <1 percentile of that of normal eyes according to the database. Furthermore, the presence or absence of vertically asymmetric thinning along the course of the nerve fiber layer and the correlation with ICC location were also confirmed.
The measurement and analysis of GCC thickness was performed by using the long axial length correction function and the long axial length normative database (data on axial length ≥26.0 and <29.0 mm) installed on the OCT. For GCC thickness measurements by OCT, measurements from images that had ≤6/10 signal strength index were excluded.
Eyes with best-corrected visual acuity (logMAR) of >0 or with a history of eye trauma or intraocular surgery were excluded. Additionally, eyes that were diagnosed as having glaucomatous optic neuropathy, eyes with extensive chorioretinal atrophy other than myopic conus, eyes with posterior staphyloma at the inner area of retinal vascular arcades, and eyes with non-ICC ophthalmologic disorders or abnormalities were also excluded.
Statistical analysis was performed with JMP software (SAS Institute Inc., Cary, NC, USA). Student’s t-test was used to compare GCC thickness of the superior and inferior sides. P<0.05 was considered statistically significant.
Results
Fifteen eyes in eleven patients (five males and six females) with mean age ± standard deviation (SD) of 54.6±10.7 years (range, 32–71 years old) were selected for analysis.
For the analyzed eyes, the mean spherical equivalent ± SD was −7.40±2.77 D (range, −3.75 to −12.75 D), and the mean axial length ± SD was 26.81±1.34 mm (range, 24.25–29.12 mm). The mean intraocular pressure ± SD was 14.5±2.4 mmHg (range, 10–18 mmHg). For the Humphrey visual field test, the mean deviation ± SD was −2.53±2.73 dB (range, 0.39 to −8.20 dB), and the mean PSD ± SD was 2.61±1.01 dB (range, 1.28–5.16 dB) (Table 1).
Location of ICC
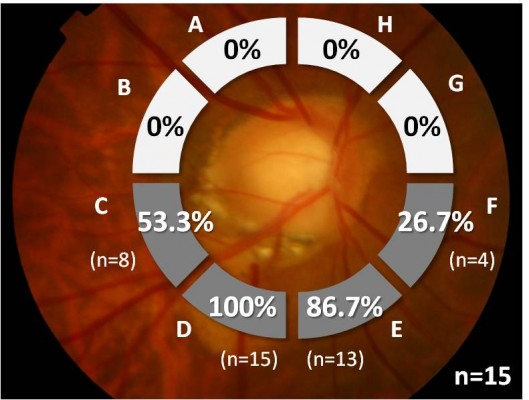
The location of ICC was assessed based on the peripapillary radial cross-sectional OCT images taken at 15° intervals. The peripapillary area was divided into eight 45° sectors (A–H) from the 12 o’clock position toward the temporal side, and the rate of ICC detection was calculated for each sector. When ICC was detected across multiple sectors, overlapping was allowed and each sector was deemed “with ICC”.
ICC was found with the highest frequency (100%; 15/15 eyes) at sector D, corresponding to the inferior temporal side (Figure 2). ICC was not observed in the superior side at all. A horizontal spread of ICC was evident in most eyes, and overlapping was detected in sector C (53.3%; 8/15 eyes) and E (86.7%; 13/15 eyes), which are both adjacent to sector D.
Humphrey visual field
The positive rates for each of the Anderson criteria were assessed. PD plots were positive in 66.7% (10/15) of ICC eyes. Glaucoma hemifield test was outside the normal range in 60.0% (9/15) of ICC eyes. PSD was P<0.05 in 66.7% (10/15) of ICC eyes. The overall positive rate in which at least one of the aforementioned three Anderson criteria was fulfilled was 73.3% (11/15 eyes) (Figure 3A).
In order to examine the correlation to ICC location and the similarities between glaucomatous visual field defects, the measurement points on the central 24-2 test were divided into six clusters along the course of the nerve fiber, and the rate of abnormalities was calculated for each cluster in accordance with the method described by Garway-Heath et al.7 An abnormal cluster was defined as three or more adjacent points along the course of the nerve fiber within the same cluster on the PD plots having P<0.05 with one of these points having P<0.01.
In the assessment by cluster, a markedly greater rate of abnormality (53.3%; 8/15 eyes) was observed in the cluster of the superior Bjerrum area, corresponding to the peripapillary inferior temporal side that showed high rates of ICC compared to other clusters (Figure 3B).
GCC thickness
In the comparison between the superior and inferior quadrants, the mean GCC thickness ± SD was 92.47±8.62 μm on the superior side and 80.07±8.66 μm on the inferior side, indicating a significantly lower thickness in the inferior side, where the ICC was located, compared to the superior side (P=0.0001). The mean GCC thicknesses ± SD of the four quadrants were: 80.30±11.94, 68.48±10.07, 105.62±8.25, and 91.96±8.96 μm at the superior temporal, inferior temporal, superior nasal, and inferior nasal sides, respectively. At both the temporal (P<0.0001) and the nasal sides (P=0.0005), GCC thickness was significantly lower on the inferior side, where ICC was present, compared to the superior side (Figure 4A).
Next, the characteristics of GCC thickness in each ICC eye were assessed, focusing on the correlation with the location of ICC. GCC thinning that correlated with ICC was defined as: GCC thickness <1 percentile of that of a normal eye, distribution of such thinning along the course of the nerve fiber with ICC as the origin, and vertical asymmetry. GCC thinning that correlated with ICC based on the earlier definitions was observed in 66.7% (10/15) of ICC eyes (Figure 4B). The distribution of GCC thinning in ICC was similar to that in early glaucoma.
Discussion
There are three novel findings from the present study that have not been previously reported. First, concerning the characteristics of visual field defects in eyes with ICC, we performed the Humphrey visual field test in all eyes and confirmed the rate of detecting glaucomatous visual field defects as well as the similarities between the two types of visual field defects. Second, we confirmed the correlation between the Humphrey visual field results and ICC location. Third, as a structural validation to visual field defects in ICC eyes, we analyzed GCC thickness for the first time and conducted a comprehensive investigation, including its correlation with ICC location.
To eliminate factors other than ICC that potentially affect visual field and GCC thickness as much as possible, we restricted the study to eyes with logMAR visual acuity of ≤0 and excluded eyes with posterior staphyloma in the arcades, eyes with chorioretinal atrophic changes other than myopic conus, and eyes with obvious glaucomatous optic neuropathy as diagnosed by two glaucoma specialists. The results of our assessment revealed that visual field defects and GCC thinning akin to early glaucoma were evident in ~70% of eyes with ICC. Furthermore, a correlation was suggested between these results and the location of ICC along the course of the nerve fiber.
At the present time, the only report that consistently employed the same visual field test in all ICC eyes is the study by Shimada et al2 in which the authors used the Goldmann visual field test. They investigated 632 highly myopic eyes and reported that, while glaucomatous visual field defects were observed in 71.0% of eyes with ICC, they were only detected in 23.0% of eyes without ICC. Since a large difference in the incidence of glaucomatous visual field defects was observed between eyes with and without ICC, even though both sets of eyes had a similarly high degree of myopia, the authors suggested that ICC may affect glaucomatous visual field defects.
Our present study is the first report in which the Humphrey visual field test was performed in all ICC eyes and the correlation between visual field and ICC location was confirmed. Our identification of visual field defects that fulfill the Anderson criteria (diagnostic criteria of early glaucoma) in 73.3% (11/15) of eyes with ICC was similar to the results reported by Shimada et al.2
In myopia, there are large individual differences in peripapillary deformation and morphology, such as tilted papilla, depending on the extension in axial length. It is difficult to reliably detect early glaucoma under such circumstances. Therefore, there is a possibility that not all glaucoma patients were excluded from this study. However, considering that the prevalence of primary open-angle glaucoma in ≥40-year-old Japanese people is 3.9%,8 and that the hazard ratio is 2.6 in moderate-to-severe myopic eyes;9 our results show that 73.3% of eyes with ICC had glaucoma-like visual field defects and GCC thinning clearly exceeded the percentage that could be caused by undetected glaucoma. These facts suggest an association between ICC and glaucomatous visual field defects and GCC thinning.
If the rate of glaucomatous visual field defects using a similar protocol to the present study in patients without ICC who have similar characteristics such as axial length can be confirmed, and these results can be compared with our findings in ICC eyes, then the association between ICC and glaucomatous visual field defects can be clarified.
While an association between ICC and glaucomatous visual field defects has been suggested, it is a subject of extreme curiosity as to why similar visual field defects occur in glaucoma, which is a retinal nerve fiber disorder at the lamina cribrosa, and in ICC, a lesion that develops outside the optic disc. Regarding this issue, Spaide et al4 used swept-source OCT and enhanced-depth OCT and reported that inner retinal thinning or disruption is observed at the ICC border region, suggesting that this may contribute to the onset of visual field defects. Our study also found inner retinal thinning at the ICC border on peripapillary radial cross-sectional OCT images.
In addition, our analysis of GCC thickness in ICC eyes, which was conducted for the first time, showed a significant thinning of GCC that correlated to the site of ICC in 66.7% of the ICC eyes, and this result supports the hypothesis by Spaide et al.4 Furthermore, we consider that our findings are extremely important as a structural validation of the functional changes of visual field defects.
If inner retinal defects at the ICC border are the cause of visual field defects, then a pathologically different disease concept from glaucoma is suggested, even though the manner in which it develops is extremely similar to that of glaucoma, which is a retinal nerve fiber disorder at the lamina cribrosa that is primarily caused by intraocular pressure.
In addition to the aforementioned inner retinal defects at the ICC border area, Spaide et al4 also identified posterior bowing of the sclera in the region of the ICC. The authors stated that ICC may potentially be formed from such deformation of the sclera. If ICC occurs concomitantly to the deformation of the sclera near the papilla, then fragility in the supporting tissue with the neighboring lamina cribrosa or receding of the lamina cribrosa may also be present. Therefore, it is not possible to definitively conclude that retinal nerve fiber disorder in ICC eyes occurs only at the ICC border. Moreover, even when there is retinal nerve fiber disorder at the ICC border, there is room for consideration before definitively concluding that this is the cause of ICC.
There is biomechanical stress in the area of the ICC, as speculated from the deformation of the sclera observed by Spaide et al4 and this stress leads to ICC in the choroid in the same area and nerve fiber defects in the retina. It is possible that these two conditions coexist similarly to a mirror image rather than being in a causal relationship.
We consider our finding of GCC thinning correlating to ICC supports the association between ICC and retinal nerve fiber disorder, as well as the association between ICC and glaucomatous visual field defects. At the present time, it is unclear whether ICC is the primary cause of these defects. However, we postulate that ICC may be a significant risk factor that suggests retinal nerve fiber disorder and glaucomatous visual field defects that correlate to the same area.
Visual field defects and changes in GCC thickness in the ICC eyes we studied were very similar to those in superior visual field defect-type early glaucoma. Because the pathogenesis of ICC is not completely understood, albeit its elucidation is positively progressing, it is not easy conceptually or clinically to distinguish ICC and glaucomatous optic neuropathy even in the presence of ICC structural abnormalities.
Although the presence or absence of progression over time may become one of the deciding factors, the enlargement in ICC over a long period of time has only been reported by Freund et al1 in a 15-year follow-up of two eyes in one patient. Some of the crucial clinical challenges include whether ICC truly does not change in size over time, and the visual field defects or retinal nerve fiber disorder that occurs in ICC eyes does not progress.
To elucidate the pathogenesis and clinical significance of ICC, prospective studies that include a greater number of patients are desired in the future.
Disclosure
This study was performed without support from any associations or companies. The authors report no conflicts of interest in this work.
References
Freund KB, Ciardella AP, Yannuzzi LA, et al. Peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2003;121(2):197–204. | ||
Shimada N, Ohno-Matsui K, Yoshida T, et al. Characteristics of peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2006;124(1):46–52. | ||
Toranzo J, Cohen SY, Erginay A, Gaudric A. Peripapillary intrachoroidal cavitation in myopia. Am J Ophthalmol. 2005;140(4):731–732. | ||
Spaide RF, Akiba M, Ohno-Matsui K. Evaluation of peripapillary intrachoroidal cavitation with swept source and enhanced depth imaging optical coherence tomography. Retina. 2012;32(6):1037–1044. | ||
Shoji T, Sato H, Ishida M, Takeuchi M, Chihara E. Assessment of glaucomatous changes in subjects with high myopia using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(2):1098–1102. | ||
Yamada H, Hangai M, Nakano N, et al. Asymmetry analysis of macular inner retinal layers for glaucoma diagnosis. Am J Ophthalmol. 2014;158(6):1318–1329. | ||
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–1815. | ||
Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi study. Ophthalmology. 2004;111(9):1641–1648. | ||
Suzuki Y, Iwase A, Araie M, et al. Risk factors for open-angle glaucoma in a Japanese population: the Tajimi study. Ophthalmology. 2006;113(9):1613–1617. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.