Back to Journals » Clinical Ophthalmology » Volume 12
Visual acuity loss associated with excessive “dry macula” in exudative age-related macular degeneration
Authors Takahashi H , Inoue Y , Tan X, Inoda S , Sakamoto S, Arai Y, Yanagi Y, Fujino Y, Kawashima H
Received 19 September 2017
Accepted for publication 5 December 2017
Published 20 February 2018 Volume 2018:12 Pages 369—375
DOI https://doi.org/10.2147/OPTH.S151999
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hidenori Takahashi,1–3 Yuji Inoue,1,2 Xue Tan,2,3 Satoru Inoda,1 Shinichi Sakamoto,1 Yusuke Arai,1 Yasuo Yanagi,4–6 Yujiro Fujino,2,3 Hidetoshi Kawashima1
1Department of Ophthalmology, Jichi Medical University, Shimotsuke, 2Department of Ophthalmology, The University of Tokyo, Bunkyo, 3Department of Ophthalmology, Japan Community Health Care Organization Tokyo Shinjuku Medical Center, Shinjuku, Japan; 4Medical Retina, Singapore National Eye Centre, 5Medical Retina, Singapore Eye Research Institute, 6Eye-ACP, Duke NUS Medical School, National University of Singapore, Singapore
Purpose: To investigate the correlation between visual acuity and central macular thickness (CMT) and choroidal thickness (CCT) in patients with wet age-related macular degeneration (AMD).
Methods: In this retrospective analysis, 14 eyes that received >10 ranibizumab injections (based on pro re nata [PRN] regimen) and maintained initial visual acuity gain were analyzed. The following 5 parameters were measured at the foveal center: CMT (distance from the inner limiting membrane [ILM] to Bruch’s membrane); central retinal thickness (CRT; distance from the ILM to the inner limit of the retinal pigment epithelium or subretinal fluid [SRF]); SRF thickness (SRFT); pigment epithelium detachment thickness (PEDT); and CCT. The correlation between the logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) and the 5 parameters was examined with generalized estimating equations.
Results: CMT, CRT, and CCT were negatively correlated with logMAR BCVA (P=0.031, 0.023, and 0.036, respectively) when only CMT values less than the thickness that maximized visual acuity for each eye were used for the analysis. Each 100-µm reduction in CMT, CRT, or CCT improved logMAR BCVA by -0.1, -0.08, or -0.07, respectively. SRFT and PEDT were not correlated with BCVA. The median CMT that maximized the visual acuity was 230 µm.
Conclusion: Dry macula with CMT <230 µm was associated with temporary decrease in visual acuity in AMD patients whose visual acuity was maintained with PRN regimen.
Keywords: age-related macular degeneration, intravitreal injections, ranibizumab, visual acuity, dry macula
Introduction
Advanced age-related macular degeneration (AMD) is a leading cause of legal blindness in developed countries, and wet AMD (wAMD) is the predominant form of the disease in Asian countries.1 Visual acuity loss due to wAMD is attributed to chronic exudative change and subretinal fibrosis.2 Exudative change and subretinal fibrosis impinge on the function and microstructure of the photoreceptor, unwittingly leading to an irreversible decrease in visual acuity. Intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents has been shown to reduce these changes and lead to improvement in visual acuity.3,4 Large clinical trials such as the CATT (Comparison of AMD Treatments Trials)2 and IVAN (Inhibition of VEGF in Age-related choroidal Neovascularization)5 demonstrated that with repeated injections, visual acuity can be maintained over a period of 2 years. However, VEGF is necessary to maintain the choroidal vascular network6,7 that feeds the outer retina,8 and continued intravitreal anti-VEGF injections once macular “dryness” was achieved may result in an extremely thin retina and a transition to atrophic AMD,9 although there are some disagreements as to whether anti-VEGF therapy is the primary cause of geographic atrophy.
Interestingly, findings from the CATT study suggested that patients in whom thin subretinal fluid (SRF) layer was maintained enjoyed better visual acuity than those with dry macula.10 The Diabetic Retinopathy Clinical Research Network also reported paradoxical changes between central macular thickness (CMT) and visual acuity in some cases of diabetic macular edema.11 Ebneter et al also reported that a thinner macula is not always better in the case of diabetic macular edema.12 These studies suggested the importance of the SRF in supplying nutrients and growth factors that are indispensable for the function of photoreceptor and/or retinal pigment epithelium (RPE)/choriocapillaris. Based on these findings, Arnold et al are currently planning a treat-and-extend prospective study to examine the association between thin SRF layer and treatment outcomes in patients with AMD.13 There are some results to suggest that there is a fluctuation in the patients’ visual acuity during long-term treatment,2 suggesting that visual acuity loss in some AMD patients might be reversible. Thus, this study investigated the association between CMT and visual acuity in AMD patients with fluctuations in visual acuity and CMT during long-term ranibizumab treatment. Our hypothesis is that excessive macular dryness, as indicated by CMT measurements, leads to a temporary decrease in visual acuity.
Patients and methods
This single-center retrospective study was approved by the institutional review board of Japan Community Health Care Organization Tokyo Shinjuku Medical Center. Patients consent to review their medical records was not required by the IRB, because the analyzed dataset does not contain the data that can identify individuals and most of the patients have not already visited the hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and all patients gave informed consent before all procedures. This study adhered to the tenets of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Subjects
Subjects were patients with wAMD who had received 3 monthly injections followed by a pro re nata (PRN)14 regimen based on monthly monitoring. All patients were recommended to return for monitoring every 4 weeks and were treated for fluid on optical coherence tomography (OCT), new or persistent hemorrhage, or decreased best-corrected visual acuity (BCVA) relative to the previous visit if detected. Patients had received intravitreal injections of the anti-VEGF drug, ranibizumab, at the Department of Ophthalmology, Japan Community Health Care Organization Tokyo Shinjuku Medical Center. Intravitreal anti-VEGF injections were administered PRN in all patients according to the CATT study.2
For the analysis, we selected eyes in which visual acuity was maintained and that had moderate variations in CMT over a long observation period. We identified 24 eyes (23 patients) which had received ≥6 injections, among which a total of 10 eyes were excluded for the following reasons: eyes with monthly injections (n=1, it was difficult to fit the curve because the eye had almost same visual acuity and CMT at every visit); subjects with loss of visual acuity due to exudative change, such as CMT >600 μm at some point (n=6); or subjects who had extreme variation in visual acuity due to poor prognosis from cataract or postcataract extraction (n=3) (standard deviation [SD], ≥0.25), leaving 14 patients (14 eyes) for analysis. No patients had a history of verteporfin photodynamic therapy or ocular surgery during the study period.
Parameters assessed
As a routine examination, each patient received BCVA testing and spectral domain OCT (Cirrus HD-OCT Model 4000; Carl Zeiss Meditec AG) at every visit. BCVA was measured as decimal visual acuity and converted to logarithm of the minimum angle of resolution (logMAR) values for statistical analysis. The parameters CMT (defined as distance from the inner limiting membrane [ILM] to Bruch’s membrane), central retinal thickness (CRT; the distance from the ILM to the inner limit of the RPE or SRF), SRF thickness (SRFT), pigment epithelium detachment thickness (PEDT; distance from the inner limit of the RPE to Bruch’s membrane), and central choroidal thickness (CCT; the distance from Bruch’s membrane to choroid/sclera junction) were also measured manually at the foveal center using the OCT caliper function.
Statistical analysis
The relationship of logMAR BCVA and CMT was expressed with a quadratic approximation in representative cases. These indicate that the visual acuity was not necessarily best when CMT was thinnest. The best CMT for each patient was defined as the median CMT from examinations that showed the best visual acuity.
Analysis was performed using generalized estimating equations (GEEs) with patients as clusters, logMAR BCVA as the response variable, and CMT and time from initial examination (days) as explanatory variables. Time from initial examination (days) was added as an explanatory variable to exclude the possibility that decreased visual acuity was due to retinal thinning over the long clinical course. The gee() function of the gee package (ver. 4.13 [01/27/1998]) for the R environment for statistical computing (ver. 3.0.2 [09/25/2013]) was used with the following code: gee(BCVA~CMT+days,id=patient,family=gaussian,corstr=“exchangeable”,data=Dataset). Similarly, GEE analysis was performed with patients as clusters, logMAR BCVA as the response variable, and days and CRT, SRFT, PEDT, or CCT as explanatory variables. Significance was set at P<0.05.
Results
Representative case findings
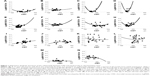
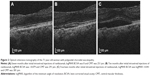
A 71-year-old woman with polypoidal choroidal vasculopathy (PCV) was examined 26 times and received 15 ranibizumab injections over a period of 29 months from initial examination. Figure 1A shows a scattergram of the CMT and logMAR BCVA at the 26 examinations. With a linear coefficient of 0, quadratic approximation gave BCVA =−0.0446+3.29e−6*(CMT −312)^2. The best BCVA in this patient was −0.0792. The best CMT was calculated as the mean CMT from 12 examinations with this visual acuity and was 284 μm. Typical pairs of OCT and visual acuity are shown in Figure 2A–C. When moderate serous retinal detachment (SRD) was present, CMT was 291 μm near the best CMT (Figure 2B). LogMAR BCVA with moderate SRD was better than with no SRD (dry macula), as shown in Figure 2A.
Most of the other cases showed similar results. Results for all cases are shown in Figure 1.
Overall findings
The mean age of 14 patients was 77 (range, 67–92; SD, 7) years. Ten out of 14 eyes had PCV, 3 had type 1 choroidal neovascularization (CNV), and 1 had type 2 CNV. The follow-up observation periods ranged from 21 to 53 (mean, 37; SD, 9) months. Intravitreal injections of ranibizumab were given 6–26 (mean, 16; SD, 5) times. Other demographic data are shown in Table 1. All the 14 eyes were receiving long-term PRN treatment with ranibizumab and had a visual acuity better than 0.55 logMAR units (Figure 3). Visual acuity was worse when CMT increased above the “best” CMT value and when CMT decreased below the “best” CMT value in all cases (Figure 1).
  | Table 1 Patients’ demographic characteristics |
The median of 14 CMTs that maximized each patient’s visual acuity was 230 μm. The data indicated that macular dryness ranged from dry to slightly wet.
GEE analysis using CMT and time from initial examination (days) gave logMAR =0.315−0.00101 CMT +0.0000454 days (P=0.031 and 0.43 for CMT and days, respectively). Each 100 μm reduction in CMT was associated with logMAR BCVA change by −0.1.
Stratification findings
In the stratified analysis, we used CRT, SRFT, PEDT, and CCT. GEE analysis using CRT and days gave BCVA =0.237−0.000832 CRT +0.00004507 days (P=0.023 and 0.47 for CRT and days, respectively). Each 100 μm reduction in CRT reduced logMAR BCVA by −0.08. GEE analysis using SRFT and days gave BCVA =0.0742+0.00119 SRFT +0.0000646 days (P=0.77 and 0.47 for SRFT and days, respectively). GEE analysis using PEDT and days gave BCVA =0.0665+0.000584 PEDT +0.0000587 days (P=0.26 and 0.45 for PEDT and days, respectively). GEE analysis using CCT and days gave BCVA =0.287−0.000651CCT −0.0000101 days (P=0.036 and 0.80 for CCT and days, respectively). Each 100 μm reduction in CCT reduced logMAR BCVA by −0.07. CRT was significantly associated with CMT (r=0.842, P<0.0001), SRFT (r=−0.355, P<0.0001), and PEDT (r=−0.575, P<0.0001), but not significantly associated with CCT (r=0.1133, P=0.14). Stratification analysis therefore revealed a significant association between CRT and BCVA, and CCT and BCVA individually, but not between other factors examined in this study. GEE analysis using CRT, CCT, and days gave BCVA =0.427−0.000802 CRT −0.000637CCT −0.0000316 days (P=0.015, 0.024, and 0.85 for CRT, CCT, and days, respectively).
Discussion
In the current study, we have clearly demonstrated that decreased CMT was associated with decreased visual acuity below a certain level. Recently, several studies were conducted to investigate whether all retinal fluids are harmful and need to be got rid of. A study by Bhavsar and Freund reported that type 1 neovascularization associated with chronic persistent subfoveal SRF despite continuous anti-VEGF therapy might even maintain good long-term visual outcomes.15 Recent studies also indicated that small volumes of SRF during ranibizumab treatment for neovascular AMD ensured well-maintained visual improvement.16,17
Our findings support the previous studies of these effects of SRF on visual function and reflect what we encounter in clinical practice. Most importantly, for those whose visual acuity was maintained over the observation period, excessively dry macula was associated with a temporary decrease in visual acuity. In this study, CRT was significantly correlated with visual acuity, but not SRFT. Hence, it would be rational to consider only excessive dry macular may have the negative effect on visual acuity and that SRF itself may not have the possible effect. The current results, together with previous reports,10 indicate that a plausible explanation for the transient loss of visual acuity is that excessive dryness induced by over-administration of anti-VEGF treatment may cause malnutrition of the outer retinal layer, resulting in decreased visual acuity.9 Needless to say, the outer layer of the retina is nourished from the choroidal side.8 Anti-VEGF treatment is known to cause choroidal thinning18,19 and that may lead to a reduced supply of nutrients via the choroidal vessels through a decreased vascular permeability. In this study, CCT thinning was associated with bad visual acuity independent of CRT. Choroidal ischemia lies at the root of wAMD, inhibiting photoreceptor cell activity and resulting in decreased visual acuity.20 VEGF secretion is regarded as a feedback mechanism in response to ischemia, which generates mild wetness and improves the ischemia, potentially improving visual acuity. Another explanation would be that the fluid contains growth factors that are indispensable for photoreceptor and RPE/choriocapillaris function.
The repeated injections of anti-VEGF are also reported to cause impairment and visual loss due to geographic atrophy.9 However, our analysis clearly demonstrated that days from the baseline visit was not associated with visual acuity. BCVA changed continuously and was not irreversible during the study (Figure 3), suggesting that decreased visual acuity was not due to retinal thinning from gradual accumulation of photoreceptor cell damage caused by many years of PRN treatment for exacerbated exudative changes.
Currently, most physicians in the US employ a treat-and-extend regimen for the management of wAMD, and concerted efforts are being made to achieve macular dryness. However, we showed that the decreased visual acuity might be due to excessive dry macula and may be reversible. Thus, our current study recommends that if CMT is reduced and visual acuity is worse than at the previous visit, injection of anti-VEGF should be postponed to the next visit. This warrants further studies.
This study has some limitations. First, this is the retrospective study design. We included only patients in whom visual acuity was maintained over the long observation period. Thus, our study does not indicate that it is possible to tolerate mild exudative change. Prospective studies are required to obtain results with a higher level of evidence. Second, stratification analysis revealed the significance of retinal and choroidal thickness, but not of SRFT or PEDT. However, in the CATT study, intraretinal fluid was associated with poor visual acuity over a period of 2 years. In contrast, the current study showed that increased CRT was associated with better visual acuity. This difference might be due to differences in analysis and patient selection. Furthermore, we investigated the association between visual acuity and each OCT parameter using data from several time points of the patients. We selected patients whose visual acuity fluctuated during the course of anti-VEGF treatment. Thus, the current results cannot be extrapolated to exudative AMD in general.
Conclusion
This study examined data on CMT and visual acuity in patients with wAMD who were receiving long-term PRN treatment with ranibizumab and had satisfactory visual acuity. The data indicated that macular dryness ranged from dry to slightly wet. GEE analysis of the data using CMT and time from initial examination (days) as explanatory variables and patients as clusters revealed that CMT rather than days was significantly correlated with visual acuity. After correcting for time from initial examination, thereby eliminating the effects of atrophy of the outer layer of the retina due to a long clinical course, the CMT value that was associated with optimal visual acuity was around a moderate 230 μm. If CMT decreases below this value – in other words, if excessive dryness occurs – visual acuity temporarily decreases. Stratification analysis revealed significance for retinal and choroidal thickness, but not for SRFT or PEDT.
Acknowledgments
This work was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT) Promotion of Private Schools (grant number S1311029). The authors thank Natsuko Kakinuma, Hironobu Tampo, Satoko Tominaga, Aya Sato, and Mikiko Takezawa for their participation in this study.
Disclosure
HT reports grants and personal fees from Novartis Pharmaceuticals, personal fees from Bayer Yakuhin, personal fees from Santen Pharmaceutical, personal fees from Kowa Pharmaceutical, and personal fees from Tochigi Prefectural Ophthalmologists Association, outside the submitted work. YI reports personal fees from Novartis Pharmaceuticals, Mitsubishi-Tanabe Pharmaceutical, and Tochigi Prefectural Ophthalmologists Association, outside the submitted work. YY reports personal fees from Novartis Pharmaceuticals, Bayer Healthcare, and Santen Pharmaceutical, outside the submitted work. HK reports personal fees from Santen Pharmaceutical, Mitsubishi-Tanabe Pharmaceutical, and Senju Pharmaceutical, outside the submitted work. XT, SI, SS, YA, and YF report no conflicts of interest in this work.
References
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. | ||
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. | ||
Brown DM, Kaiser PK, Michels M, et al; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. | ||
Chakravarthy U, Harding SP, Rogers CA, et al; IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. | ||
Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155(2):421–428. | ||
Marneros AG, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167(5):1451–1459. | ||
Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch Ophthalmol. 2003;121(4):547–557. | ||
Grunwald JE, Daniel E, Huang J, et al; CATT Research Group. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. | ||
Jaffe GJ, Martin DF, Toth CA, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(9):1860–1870. | ||
Diabetic Retinopathy Clinical Research Network; Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–536. | ||
Ebneter A, Wolf S, Abhishek J, Zinkernagel MS. Retinal layer response to ranibizumab during treatment of diabetic macular edema: thinner is not always better. Retina. 2016;36(7):1314–1323. | ||
Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration – a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016;16:31. | ||
CATT Research Group; Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. | ||
Bhavsar KV, Freund KB. Retention of good visual acuity in eyes with neovascular age-related macular degeneration and chronic refractory subfoveal subretinal fluid. Saudi J Ophthalmol. 2014;28(2):129–133. | ||
Gianniou C, Dirani A, Jang L, Mantel I. Refractory intraretinal or subretinal fluid in neovascular age-related macular degeneration treated with intravitreal ranizubimab: functional and structural outcome. Retina. 2015;35(6):1195–1201. | ||
Wickremasinghe SS, Janakan V, Sandhu SS, Amirul-Islam FM, Abedi F, Guymer RH. Implication of recurrent or retained fluid on optical coherence tomography for visual acuity during active treatment of neovascular age-related macular degeneration with a treat and extend protocol. Retina. 2016;36(7):1331–1339. | ||
Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology. 2012;119(8):1621–1627. | ||
Koizumi H, Kano M, Yamamoto A, et al. Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(4):627–633. | ||
Ciulla TA, Harris A, Martin BJ. Ocular perfusion and age-related macular degeneration. Acta Ophthalmol Scand. 2001;79(2):108–115. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.