Back to Journals » Clinical Ophthalmology » Volume 14
Visibility of the Retina Through an Air-Filled Anterior Chamber During Simultaneous Vitrectomy and Descemet’s Stripping Automated Endothelial Keratoplasty
Authors Yokogawa H , Kobayashi A, Mori N, Nishino T, Sugiyama K
Received 14 May 2020
Accepted for publication 19 June 2020
Published 24 July 2020 Volume 2020:14 Pages 2119—2123
DOI https://doi.org/10.2147/OPTH.S262403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hideaki Yokogawa, Akira Kobayashi, Natsuko Mori, Tsubasa Nishino, Kazuhisa Sugiyama
Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
Correspondence: Hideaki Yokogawa
Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, 13-1 Takara-machi, Kanazawa-shi, Ishikawa-ken 920-8641, Japan
Tel +81-76-265-2403
Fax +81-76-222-9660
Email [email protected]
Purpose: To describe the visibility of the retina through an air-filled anterior chamber during simultaneous pars plana vitrectomy (PPV) and Descemet’s stripping automated endothelial keratoplasty (DSAEK), and to discuss the technical challenges of a fluid-air exchange under such conditions.
Methods: Six eyes from 6 patients with coexisting bullous keratopathy and posterior segment problems such as vitreous opacity, retained silicon oil, intraocular lens subluxation, or aphakia underwent simultaneous PPV and DSAEK. In all cases, after completion of 25-gauge PPV with/without flanged intrascleral intraocular lens fixation, DSAEK donor tissue was inserted into the anterior chamber. Air was then injected into the anterior chamber to attach the donor graft to the back surface of the host cornea. At this point, the visibility of the retina was evaluated using endoillumination and a wide-angle viewing system.
Results: In all cases, visibility of the retina through an air-filled anterior chamber, with the DSAEK donor attached, was sufficient to perform a fluid-air exchange.
Conclusion: Our clinical observations of such rare but clinically important conditions indicate that simultaneous PPV and DSAEK is possible with fair visualization of the posterior segment including the retina.
Keywords: Descemet’s stripping automated endothelial keratoplasty, pars plana vitrectomy
Introduction
In recent years, Descemet’s stripping automated endothelial keratoplasty (DSAEK) has been commonly performed for endothelial dysfunction.1–4 Among the eyes in need of DSAEK, there may be complex cases with aphakia, intraocular lens (IOL) luxation, and iris defects caused by trauma or multiple surgeries. Simultaneous surgery, “vitreo-corneal surgery” involving simultaneous DSAEK, flanged intrascleral IOL fixation, and pars plana vitrectomy (PPV) is an ideal approach for such eyes.5–7 In comparison with staged surgery, simultaneous surgery can provide rapid visual rehabilitation, a reduction in patient costs, and the ability to utilize the same incision.
However, PPV has the potential risk of intraoperative iatrogenic retinal detachment which may require gas tamponade of the retina.8 Importantly, no one knows whether it is possible to achieve tamponade for both an anterior graft and the posterior retina at the same time. The purpose of the current study is to determine the visibility of the retina through an air-filled anterior chamber during simultaneous PPV and DSAEK, and to discuss the technical challenges of such a fluid-air exchange under these rare circumstances.
Methods
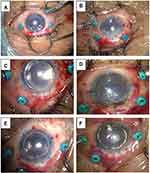
The present study has followed the tenets of the Declaration of Helsinki and written informed consent was obtained by all subjects. An approval from the Ethical Committee of Kanazawa University Graduate School of Medical Science was also obtained (approval number 2019–029 (3063)). Between October 2018 and March 2020, 6 consecutive eyes from 6 patients (mean age, 61.3 ± 21.2 years; 2 females, 4 males) with coexisting bullous keratopathy and posterior segment problems such as vitreous opacity, retained silicon oil, IOL subluxation, or aphakia underwent simultaneous PPV and DSAEK (Table 1). In all cases, after completion of 25-gauge PPV with/without flanged intrascleral IOL fixation, DSAEK donor tissue was inserted into the anterior chamber using NS Endo-Inserter (Hoya Surgical Optics, Tokyo, Japan) from the 4.6 mm width superior sclerocorneal incision. During the delivery, pars plana infusion flow was temporarily stopped, and a balanced salt solution flow of the inserter pushed the graft into the anterior chamber. Only in Case 3, a 9-0 polypropylene suture pulled the graft into the anterior chamber (lifeline suture technique).6 Air was injected into the anterior chamber to attach the donor graft to the back surface of the host cornea (Figure 1). At this point, visibility of the retina was evaluated using endoillumination and the RESIGHT® 700 wide-angle viewing system (Carl Zeiss Meditec, Germany). At the end of surgery, the anterior chamber was kept full of air, and the 25-gauge pars plana trocars were removed. The patient was instructed to lie on his or her back for 1 h.
 |
Table 1 Clinical Characteristics and Outcomes in 6 Patients Who Underwent Simultaneous PPV and DSAEK |
Results
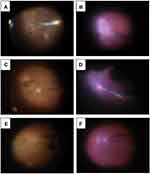
In all cases, after air injection to press the DSAEK graft against the back surface of the host cornea, using endoillumination and a wide-angle viewing system visibility of the retina was sufficient to safely control the cannula near the optic disc. This demonstrates that a fluid-air exchange maneuver is possible (Figure 2).
Discussion
In the current study, we demonstrated that the use of endoillumination and a wide-angle viewing system enabled sufficient visibility of the retina through an air-filled anterior chamber with a DSAEK donor attached, such that we were able to control the needle near the optic disc. Retinal image quality was determined based on multiple factors such as corneal transparency, pupil size, ocular media, and the viewing system. The RESIGHT® 700 wide-angle viewing system enabled a panoramic view even in eyes with host-graft stromal edema and an air-filled anterior chamber. These were the first observations made of such a rare but clinically important condition. This observation supports the technical feasibility of the fluid-air exchange maneuver for achieving tamponade of both an anterior graft and the posterior retina.
Among the patients that require DSAEK, there may be complex eyes due to multiple surgeries or trauma. Complex eyes often have posterior segment problems including aphakia, luxation/subluxation of IOL, vitreous opacity, or retained silicone oil. In these eyes, careful preoperative evaluation of the entire eye is essential. Typically, when staged surgery was chosen, pretreatment surgery for PPV with iris-IOL diaphragm reconstruction was usually performed first. Visual recovery is then postponed until secondary DSAEK is performed in a few months. However, if staged surgery is possible, it is likely that simultaneous surgery is also possible. In other words, candidates for staged surgery are also candidates for simultaneous surgery, unless very active retinal diseases are present. The biggest advantage of simultaneous surgery is the rapid visual rehabilitation without pretreatment surgery.
Although modern small-gauge PPV has become a very safe procedure, the potential risk remains for intraoperative iatrogenic retinal detachment, which may require tamponade of the retina. The present study described the management of such rare complications when performing simultaneous surgery. The only concern regarding the presence of gas in both the anterior and posterior segments is that a long duration gas-endothelium touch or iris-endothelium touch may cause endothelial damage.
The limitation of the present study was the small sample size and a lack of quantitative analysis. In addition, our surgical approach may require two surgeons at the same time, both a cornea specialist and a vitreoretinal specialist.
In the future, there is possibility of employing simultaneous PPV and Descemet membrane endothelial keratoplasty (DMEK). Visibility of the retina through an air-filled anterior chamber with a dye-stained DMEK graft is unknown. Thus far, DMEK graft unfolding requires a stable iris-lens platform, and to plan the staged procedure is safe.
In conclusion, there may be complex bullous keratopathy after multiple surgeries or trauma, and these cases might be candidates for simultaneous PPV and DSAEK. Our clinical observations under rare but clinically important situations indicate that simultaneous PPV and DSAEK is possible with sufficient visualization of the posterior segment that includes the retina. Moreover, rapid visual rehabilitation is expected with simultaneous surgery. Future studies are needed to fully understand the indications, risks, and benefits of simultaneous PPV and DSAEK.
Disclosure
The authors have no funding nor conflicts of interest to disclose for this work.
References
1. Price MO, Gorovoy M, Benetz BA, et al. Descemet’s stripping automated endothelial keratoplasty outcomes compared with penetrating keratoplasty from the Cornea Donor Study. Ophthalmology. 2010;117:438–444. doi:10.1016/j.ophtha.2009.07.036
2. Yokogawa H, Kobayashi A, Saito Y, et al. Rationale for performing penetrating keratoplasty rather than DSAEK in patients with bullous keratopathy in Japan. Ophthalmic Surg Lasers Imaging. 2012;43(6):446–451. doi:10.3928/15428877-20120726-04
3. Park CY, Lee JK, Gore PK, et al. Keratoplasty in the United States: a 10-year review from 2005 through 2014. Ophthalmology. 2015;122(12):2432–2442. doi:10.1016/j.ophtha.2015.08.017
4. Nishino T, Kobayashi A, Yokogawa H, et al. Changing indications and surgical techniques for keratoplasty during a 16-year period (2003–2018) at a tertiary referral hospital in Japan. Clin Ophthalmol. 2019;13:1499–1509. doi:10.2147/OPTH.S214515
5. Yokogawa H, Kobayashi A, Okuda T, et al. Combined keratoplasty, pars plana vitrectomy, and flanged intrascleral intraocular lens fixation to restore vision in complex eyes with coexisting anterior and posterior segment problems. Cornea. 2018;37 Suppl 1:S78–S85. doi:10.1097/ICO.0000000000001716
6. Yokogawa H, Kobayashi A, Mori N, et al. Clinical evaluation of the “lifeline suture” technique for DSAEK in cases without posterior capsule using a novel donor insertion device (NS Endo-Inserter). Cornea. 2020;39(4):523–526.
7. Sane M, Shaikh N, Shaikh S. Combined DSEK and transconjunctival pars plana vitrectomy. Case Rep Ophthalmol Med. 2016;2016:9728035.
8. Yoon YH, Sheu SJ, Terasaki H. Primary vitrectomy in rhegmatogenous retinal detachment. In: Ryan SJ, Schachat AP, Wilkinson CP, editors. Retina.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.