Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Visceral Leishmaniasis Associated with HIV Coinfection in Pará, Brazil
Authors Camargo Júnior RNC , Sarmento Gomes JS, Corrêa Carvalho MC, Chalkidis HDM , Silva WCd, Sousa da Silva J, Silva de Castro SR, Lima Neto RC, Moutinho VHP
Received 4 December 2022
Accepted for publication 1 April 2023
Published 25 May 2023 Volume 2023:15 Pages 247—255
DOI https://doi.org/10.2147/HIV.S400189
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Raimundo Nonato Colares Camargo Júnior,1,* Jaciara Simone Sarmento Gomes,2,* Mônica Cristina Corrêa Carvalho,2,* Hipócrates de Menezes Chalkidis,2,* Welligton Conceição da Silva,1,* Juliana Sousa da Silva,3,* Samia Rubielle Silva de Castro,4,* Raul Cunha Lima Neto,4,* Victor Hugo Pereira Moutinho4,*
1Animal Science Graduate Program, Federal University of Pará (UFPA), Belém, Pará, Brazil; 2Department of Biological and Health Sciences, Amazon University Center (Unama), Santarém, Pará, Brazil; 3Postgraduate Program in Natural Resources of the Amazon, Federal University of Western Pará, Santarém, Pará, Brazil; 4Institute of Biodiversity and Forests, Federal University of Western Pará, Santarém, Pará, Brazil
*These authors contributed equally to this work
Correspondence: Raimundo Nonato Colares Camargo Júnior, Federal University of Pará (UFPA), R. Augusto Corrêa, 01 - Guamá, Belém, Pará, 66075-110, Brazil, Tel +55 015 93 98124-9425, Fax +55 015 93 98124-9425, Email [email protected]
Introduction: Human visceral leishmaniasis (VL) is a zoonosis of great importance to public health due to its epidemiological diversity, with emphasis on the possibility of aggravation by coinfection with the human immunodeficiency virus (HIV).
Objective: The aim was to study the epidemiological characteristics of VL cases associated with HIV coinfection in Pará. Methods. Reported cases of VL from January 2006 to December 2016 were investigated. A descriptive epidemiological method related to age, gender, area of residence and coinfection with HIV was used. To calculate variance and test equity, the F-test (Fisher) was performed. To observe the influence of one aspect on another, the chi-square was used to verify if there was dependence or independence between the variables.
Results: A total of 1171 cases of VL were reported during the study period. There was an annual mean of LV of 94.9, with a statistical difference (p< 0.05) between age groups, with the highest number of cases being observed in children aged 1 to 4 years (27.16%). Males and the urban area had a higher number of cases. There were 57 cases of VL/HIV coinfection, with emphasis on the year 2013 and the municipality of Santarém, which had the highest number of cases. During the ten years studied, there was a correlation between coinfection VL/ HIV, with significant differences between patients with and without HIV who contracted VL (p< 0.001).
Conclusion: The data reveal the endemic nature of VL in the region, with a high percentage of infection in children living in urban areas. Although the studied region is not identified as a predominant area of HIV cases, this study showed a high annual average (10.3) of cases of VL/HIV coinfection being the first time that cases of VL/HIV coinfection were reported in the Mesoregion of the Lower Amazon and Southwest Pará.
Keywords: age group, male, children, analytical epidemiology
Introduction
Human visceral leishmaniasis (VL) known as calazar1,2 or kala-azar3,4 (“black fever”), is a chronic zoonosis,5 of great importance for public health6 due to its epidemiological diversity,7 and in the absence of adequate treatment, results in fatal cases for humans.8 VL has high mortality, especially in untreated people and malnourished children, is also seen developing in individuals with acquired immunodeficiency virus (HIV) infection.
It is estimated that around 350 million people worldwide live in areas at risk for leishmaniasis, having been diagnosed in 88 countries, of which 72 are developing.9 Approximately 94%of the world’s cases of calazar are concentrated in the South Asian region, South and Central America.10 In Brazil, the disease is endemic, there has been an expansion, becoming a growing public health problem in rapid geographical expansion.11–13
Regardless of the care in the management of vectors and tanks, the disease is in vertiginous growth, so it characterizes a threat to individuals and worries the health authorities. Although there have been resources in certain routines for the precise treatment of visceral leishmaniasis, the country has been notifying an increase in mortality in numerous regions, one of the main factors contributing to the increase of this lethality is the late diagnosis.14,15
So many socioeconomic aspects, such as environmental aspects, influence the distribution of VL, and hinder disease control strategies.16 Environmental transformations have occurred intensely, and for this reason, knowledge of the epidemiological profile and understanding of the local evolution of the disease are necessary to guide more effective actions aimed at surveillance, prevention, and control of this disease.17,18
Cases of leishmaniasis associated with coinfection with HIV are worrisome, as they involve complications and usually present in the most aggressive forms of the disease.19 In addition, impaired immune function in patients with VL coinfected with HIV may favor the reactivation of latent Leishmania infection, cause a worse therapeutic response, and increase the risk of relapse after treatment.20,21
The first report of VL/HIV coinfection occurred in southern Europe and currently coinfection is documented in 45 countries, highlighting the high rates reported in Brazil, Ethiopia and the state of Bihar in India.22–24 Recent studies reveal VL/HIV coinfection in adult males,25,26 pregnant women,27–29 and childrens.30,31 Given the above, the objective of this work was to describe relevant epidemiological aspects of human visceral leishmaniasis in the Lower Amazon and Southwest Paraense - Pará, Brazil.
Materials and Methods
Location and Series Analyzed
A study of cases of Visceral Leishmaniasis in Humans (VL) was conducted in nineteen municipalities in Pará (Figure 1). Of these, 13 are part of the Lower Amazon mesoregion, namely: Santarém, Alenquer, Almerim, Belterra, Curuá, Juruti, Mojuí dos Campos, Monte Alegre, Óbidos, Oriximiná, Prainha, Placas and Terra Santa. The other 6 municipalities studied belong to the Southwest Paraense mesoregion, that is: Aveiro, Itaituba, Jacareacanga, Novo Progresso, Rurópolis and Trairão (Figure 2). The occurrence of cases was verified within a ten-year historical series, from January 2006 to December 2016, in which the notification of 1171 cases in humans with an annual average of 97.5 cases was analyzed.
 |
Figure 1 Location of the studied area. Notes: Adapted from Silva WCd, Santos FB, Costa JWS et al. Epidemiological characteristics of pregnant patients accidented by fish in the Brazilian Amazon. International Journal of Development Research. 2022/01/30 2022;12(1):53,379–53,381. Copyright © 2022, Welligton Conceição da Silva et al. This is an open access article distributed under the Creative Commons Attribution License.58 |
 |
Figure 2 Mesoregions from Pará state. Notes: Adapted from Andrade FWC, Pinto TI, Moreira LdS et al. The Legal Roundwood Market in the Amazon and Its Impact on Deforestation in the Region between 2009–2015. Forests. 2022;13(4):558. © 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).59 |
Data Collection
The data from this research were obtained from the Notifiable Diseases Information System (SINAN),32,33 provided by the Endemic Division, the 9th Regional Health Center, the State Department of Public Health - Endemic Division/9°CRS/SESPA. Data were collected from June 1 to 20, 2017.
Variables Studied
The descriptive epidemiological method was used, based on the study of variables related to age, gender, residence zone and confirmed cases of VL coinfected with HIV.
The variables studied were: age, with age groups divided as follows: group 1: less than one year old, group 2: from one to four years old, group 3: from five to nine years old, group 4: ten to fourteen years old, group 5: fifteen to nineteen years old, group 6: twenty to thirty-four years old, group 7: thirty-five to forty-nine years old, group 8: fifty to sixty-four years old, group 9: sixty-five to seventy-nine years old, group 10: eighty years old or more.
As for the gender variable, both female and male were considered. Finally, for the residence zone variable, rural and urban areas were considered. The variable coinfection with the HIV virus admitted only yes or no values.
Statistical Analysis
Descriptive statistics were performed to obtain the means and standard deviation. To calculate variance and test equity, the F-test (Fisher) was performed, useful for using data when the size of your samples is small. To observe the influence of one aspect on another, qui-quadrado was used to verify whether there was dependence or independence between the variables, which were tabulated and analyzed and thus obtained if the results were obtained.
Ethical Aspects
The data used in this study were free, secondary and the names of the different patients involved in the study were not mentioned, respecting, therefore, the ethics of the study in Resolution No. 510 of the National Health Council of April 7, 2016, and it is not necessary to approve the Ethics Committee in the Research Committee.
Results
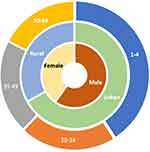
In cases of human VL, 1171 cases were reported in 19 municipalities in the Mesoregion of Western Pará. There was a statistical difference (p<0.05) between age groups (Figure 3), and the highest number of cases was observed in children aged 1 to 4 years old (27.16%), later in individuals aged between 20 and 34 years old (13.92%), followed by people aged between 35 and 49 years old (13.66%), and by elderly purposes aged between 50 and 64 years old (11.53%).
Regarding the occurrence of the disease according to the area of residence, many of the affected cases are resident in urban areas (66.52%) and the rural area was with the minority (32.96%) of the reported cases (Figure 3).
It was also observed in the cases studied that the most relevant was male (59.94%), accompanied by (40.06%) belonging to females (Figure 3).
During the ten years studied, a correlation was observed between HIV coinfection and visceral leishmaniasis. According to the data obtained, there were significant differences between patients with and without HIV who contracted Leishmaniasis (p<0.001).
We reported 57 cases of VL/HIV coinfection during the study period, with an average of 10.3 cases per year reported, especially in 2013 and 2010, with the highest (13) and lower number (1), respectively.
In this study, only 8 municipalities had cases of VL/HIV coinfection. Santarém presented the highest number of cases (41), followed by Itaituba (6) and Novo Progresso (3) (Table 1), being the first time that cases of VL/HIV coinfection were reported in the Mesoregion of the Lower Amazon and Southwest Pará.
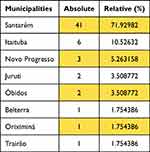 |
Table 1 Absolute and Relative Values of HIV and VL Cases in Municipalities with Positive Cases |
Discussion
For VL, there is no distinction of susceptibility between age, sex, and race. However, children and the elderly are more predisposing to VL infection corresponding to the state of relative cellular immunological prematurity.34 In relation to the age group with the greatest involvement, VL refers especially to children, especially malnourished children.35
In this study, a higher prevalence was found between 1 and 4 years of age. The disease in its traditional form affects individuals of all ages, 80% of the cases cataloged occur in children under 10 years. In some urban areas there is a predisposition to change in the ordering of cases by age group, with incidence of high rates also in the group of young adults.36 A situation observed in the study in question, which among adults describes a higher frequency in the age group between 20 and 34 years.
The preference for childhood is justified, the fact that malnutrition is frequent in groups of people with low socioeconomic status and in whom the disease is frequent to another factor to collaborate in this genesis.37
Visceral leishmaniasis mainly affected male people, which was also evidenced in Bahia,38 this may be related to increased exposure to environments in which natural transmission of infection occurs.39 However, there are genetic and hormonal factors related to the greater susceptibility of males to infection, but it is not properly explained.40
In the present study, the urban area presented a higher record of occurrence of VL, which shows that in the municipalities studied the urban profile of the disease prevailed, although there is also occurrence in the rural area. VL is undergoing changes in the epidemiological pattern of transmission in some regions of Brazil,41 changing from a profile that was initially characterized as rural,42 for a profile characterized as urbanized.43 There is overcoming the paradigm of being VL a typically rural disease.44
A recent study shows that there is no change in the epidemiological pattern in the process of transmission of the disease,45 the change has been happening in the geographic expansion of VL, to an area designated as an urban area, which has environments very similar to the rural environment,46–48 providing a better adaptation of the vector to the place.
Brazil today challenges the expansion and urbanization of VL with human cases and many positive dogs in several regions, which are large and medium-sized. The transmission cycle that used to take place in a wild and rural environment, today intensifies in urban centers.49
The highest risk of this infection occurs due to instability as poor sanitary infrastructure, breeding of animals that act as deposit of the parasite, climatic conditions favorable to vector multiplication, disordered development in urban areas with accumulation of organic matter and diligence of the first indications of this pathology.50,51
A correlation was noted between cases of VL and HIV. Acquired immunodeficiency syndrome (AIDS) is a relevant cause of immunodeficiency worldwide and HIV contamination significantly intensifies the possible complications of VL coinfection. Regardless of whether it is a mandatory notification disease, the information presented is based on passive perception of cases. The number of individuals exposed to infection or asymptomatic infected in some areas is much higher than the number of cases detected.52
The occurrence of subclinical infections in urban areas is the result, among other factors, of the greater exposure of man to infectious bites of sandfly already adapted to the environment, which indicates a serious problem associated with areas of greater HIV circulation; can turn VL into opportunistic parasites.53
VL exhibits different management in HIV coinfection, which is mainly reflected in the irregular response to treatment and alteration of the diagnostic pattern. The increasing number of Leishmania/HIV cases in urban areas of Brazil has been verified, leading to the emergence of a new form of propagation by the sharing of contaminated needles, with risk of propagation of the artificial anthroponotic cycle.54
VL can change the follow-up of HIV disease and immunodepression caused by this virus, simplifying the progression of leishmaniasis. The opinion of the set of clinical occurrences of VL in HIV-infected individuals indicates that there is no definite description of symptoms that can be unquestionably aggregated with coinfection.55
There are few studies of the epidemiological aspects of VL/HIV coinfection cases in Brazil56,57 and there are no studies developed in the studied region, making this study the pioneer in the research of cases of VL/HIV coinfection in the studied region and produced worrying results regarding the concomitant occurrence of the two infections. Although the occurrence of AIDS or aids has not been evaluated, the annual mean VL/HIV coinfection of approximately 10 patients leads to the need for further investigations into the epidemiology of these coinfection cases.
Conclusion
This is the first study to report cases of VL/HIV coinfection in the Mesoregion of the Lower Amazon and Southwest Pará, with emphasis on the occurrence of coinfection in the municipalities of Santarém, Itaituba, Novo Progresso, Juruti, Óbidos, Belterra, Oriximiná, Trairão.
VL was endemic in this study, affecting mainly boys from 1 to 4 years old living in the urban area. Although the studied region is not identified as the predominant area of AIDS cases, this study showed cases of coinfection in individuals with HIV.
Studies conducted in other countries indicate higher lethality for VL-HIV coinfected patients when compared to those with VL. This alone should make the obligation of the HIV test to patients with VL routine with a view to the early diagnosis of coinfection and, consequently, the probable reduction of lethality.
However, in this research, many epidemiological records in the SINAN evaluated did not contain concrete information in the variable “HIV coinfection”, which implied the white/ignored attribution to this field of notification, which by the results presented here refers to a probable underreporting of cases of coinfection.
Among the limitations encountered while carrying out this research, the neglect of adequate investigation of the possibility of VL/HIV coinfection in cases of VL was poignant, as well as the lack of filling out this information in many epidemiological forms.
Acknowledgments
The authors would like to thank the Federal University of Western Pará for the financial support.
Funding
This study was funded by the Federal University of West Pará (UFOPA) by Public Notice of 03/2022 (Opinion No. 00048/2022/PFE/PFUFOPA/PGF/AGU present in Electronic Process No. 23204.5677/2022-67) and also support for the publication fee of the Dean of Research, Graduate Studies and Technological Innovation (PROPPIT/UFPA).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alves AA, Rodrigues NS, Martins RB, Rocha BC, Moraes RA. Abordagem Do Paciente Com Calazar: Uma Revisão De Literatura. Doenças Infecciosas E Parasitárias No Contexto Brasileiro. 2021;2:71–80.
2. Lemos MDA, Sousa O. Perfil da leishmaniose visceral no Brasil: uma revisão bibliográfica. Facit Bus Tech J. 2019;9(1):93–114.
3. Brito GDR, Marreiros ADS, Araujo IFDB, Da Silva Júnior RG, Sene IDS, Braz DC. Severe Kala-azar and seric level of IL-6: case reports. J Health Biol Sci. 2020;8(1):1. doi:10.12662/2317-3076jhbs.v8i1.3035.p1-4.2020
4. Rahman KM, Olsen A, Harley D, et al. Early diagnosis of kala-azar in Bangladesh: findings from a population based mixed methods research informing the post-elimination era. Parasitol Int. 2021;85:102421. doi:10.1016/j.parint.2021.102421
5. Marcolino Silva D, Passarella Teixeira AI, Sierra Romero GA. Socioeconomic Status of Guardians as a Risk Factor for Canine Visceral Leishmaniasis: a Cohort Study in an Endemic Area of the Federal District, Brazil. Am J Trop Med Hyg. 2023;108(2):328–334. doi:10.4269/ajtmh.21-1170
6. Camargo Júnior RNC, Cunha T, da Silva Lima DJ, da Silva WC. Aspectos importantes na epidemiologia de cães com Leishmaniose Visceral Canina em Santarém-Pará, Pará, Brasil. Caderno de Ciências Agrárias. 2022;14:1–5. doi:10.35699/2447-6218.2022.41354
7. Correa-Cárdenas CA, Pérez J, Patino LH, et al. Distribution, treatment outcome and genetic diversity of Leishmania species in military personnel from Colombia with cutaneous leishmaniasis. BMC Infect Dis. 2020;20(1):938. doi:10.1186/s12879-020-05529-y
8. Bispo AJB, Almeida MLD, de Almeida RP, Bispo Neto J, de Oliveira Brito AV, França CM. Pulmonary involvement in human visceral leishmaniasis: clinical and tomographic evaluation. PLoS One. 2020;15(1):e0228176. doi:10.1371/journal.pone.0228176
9. Debash H, Bisetegn H, Nigatie M, Abeje G, Feleke DG. Epidemiological, clinical and hematological profiles of visceral leishmaniasis among patients visiting Tefera Hailu Memorial Hospital, Northeast Ethiopia: a 4 year retrospective study. Sci Rep. 2023;13(1):931. doi:10.1038/s41598-023-28139-5
10. Bi K, Chen Y, Zhao S, Kuang Y, John Wu C-H. Current Visceral Leishmaniasis Research: a Research Review to Inspire Future Study. Biomed Res Int. 2018;2018:9872095. doi:10.1155/2018/9872095
11. RMFd A, Cardoso DT, Teixeira-Neto RG, et al. Space-time analysis of the incidence of human visceral leishmaniasis (VL) and prevalence of canine VL in a municipality of southeastern Brazil: identification of priority areas for surveillance and control. Acta Trop. 2019;197:105052. doi:10.1016/j.actatropica.2019.105052
12. Cruz C, Barbosa DS, Oliveira VC, Cardoso DT, Guimarães NS, Carneiro M. Factors associated with human visceral leishmaniasis cases during urban epidemics in Brazil: a systematic review. Parasitology. 2021;148(6):639–647. doi:10.1017/S0031182021000019
13. Ávila IR, de Araújo GR, Barbosa DS, Bezerra JMT. Occurrence of human visceral leishmaniasis in the Central-West region of Brazil: a systematic review. Acta Trop. 2023;237:106707. doi:10.1016/j.actatropica.2022.106707
14. Reis L, Balieiro A, Fonseca FR, Gonçalves MJF. Changes in the epidemiology of visceral leishmaniasis in Brazil from 2001 to 2014. Rev Soc Bras Med Trop. 2017;50(5):638–645. doi:10.1590/0037-8682-0243-2017
15. Selvapandiyan A, Croft SL, Rijal S, Nakhasi HL, Ganguly NK. Innovations for the elimination and control of visceral leishmaniasis. PLoS Negl Trop Dis. 2019;13(9):e0007616. doi:10.1371/journal.pntd.0007616
16. Grifferty G, Shirley H, McGloin J, Kahn J, Orriols A, Wamai R. Vulnerabilities to and the Socioeconomic and Psychosocial Impacts of the Leishmaniases: a Review. Res Rep Trop Med. 2021;12:135–151. doi:10.2147/rrtm.s278138
17. Gianchecchi E, Montomoli E. The enemy at home: leishmaniasis in the Mediterranean basin, Italy on the focus. Expert Rev Anti Infect Ther. 2020;18(6):563–577. doi:10.1080/14787210.2020.1751611
18. Marinho DS, Casas CNPR, Pereira C, Leite IC. Health Economic Evaluations of Visceral Leishmaniasis Treatments: a Systematic Review. PLoS Negl Trop Dis. 2015;9(2):e0003527. doi:10.1371/journal.pntd.0003527
19. Coelho AAS, de Loureiro EVS, da Silva ACJ, et al. Historical analysis of leishmaniasis cases in the transamazonian region: from 2009 to 2019. Rev Eletrônica Acervo Saúde. 2021;13(11):e9163–e9163. doi:10.25248/reas.e9163.2021
20. Wamai RG, Kahn J, McGloin J, Ziaggi G. Visceral leishmaniasis: a global overview. J Glob Health Sci. 2020;2(1). doi:10.35500/jghs.2020.2.e3
21. Desjeux P. Global control and Leishmania HIV co-infection. Clin Dermatol. 1999;17(3):317–325. doi:10.1016/S0738-081X(99)00050-4
22. WHO. Visceral leishmaniasis and HIV coinfection: WHO publishes new guideline with region-specific treatment recommendations. Available from: https://www.who.int/news/item/08-06-2022-visceral-leishmaniasis-and-HIV-coinfection-WHO-publishes-new-guideline-with-region-specific-treatment-recommendations.
23. Paredes R, Munoz J, Diaz I, Domingo P, Gurgui M, Clotet B. Leishmaniasis in HIV infection. J Postgrad Med. 2003;49(1):39–49. doi:10.4103/0022-3859.929
24. Alvar J, Vélez ID, Bern C, et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One. 2012;7(5):e35671. doi:10.1371/journal.pone.0035671
25. Leite de Sousa-Gomes M, Romero GAS, Werneck GL. Visceral leishmaniasis and HIV/AIDS in Brazil: are we aware enough? PLoS Negl Trop Dis. 2017;11(9):e0005772. doi:10.1371/journal.pntd.0005772
26. Machado CAL, Sevá ADP, Silva A, Horta MC. Epidemiological profile and lethality of visceral leishmaniasis/human immunodeficiency virus co-infection in an endemic area in Northeast Brazil. Rev Soc Bras Med Trop. 2021;54:e0795. doi:10.1590/0037-8682-0795-2020
27. Leishmaniases WHOECotCot, World Health O. Control of the Leishmaniases: Report of a Meeting of the WHO Expert Commitee on the Control of Leishmaniases, Geneva, 22-26 March 2010. Geneva: World Health Organization; 2010.
28. Monge-Maillo B, López-Vélez R. Treatment Options for Visceral Leishmaniasis and HIV Coinfection. AIDS Rev. 2016;18(1):32–43.
29. Mahajan R, Das P, Isaakidis P, et al. Combination Treatment for Visceral Leishmaniasis Patients Coinfected with Human Immunodeficiency Virus in India. Clin Infect Dis. 2015;61(8):1255–1262. doi:10.1093/cid/civ530
30. Rey LC, Martins CV, Ribeiro HB, Lima AA. American visceral leishmaniasis (kala-azar) in hospitalized children from an endemic area. J Pediatr. 2005;81(1):73–78. doi:10.2223/JPED.1286
31. Sundar S, Agarwal D. Visceral Leishmaniasis-Optimum Treatment Options in Children. Pediatr Infect Dis J. 2018;37(5):492–494. doi:10.1097/INF.0000000000001885
32. Laguardia J, Domingues CMA, Carvalho C, Lauerman CR, Macário E, Glatt R. Sistema de informação de agravos de notificação em saúde (Sinan): desafios no desenvolvimento de um sistema de informação em saúde. Epidemiologia e Serviços de Saúde. 2004;13(3):135–146. doi:10.5123/S1679-49742004000300002
33. Melo MADS, Dela Coleta MF, Dela Coleta JA, et al. Percepção dos profissionais de saúde sobre os fatores associados à subnotificação no Sistema Nacional de Agravos de Notificação (Sinan). Rev de Admin em Saúde. 2018;18:71. doi:10.23973/ras.71.104
34. Freire ML, Machado de Assis T, Oliveira E, et al. Performance of serological tests available in Brazil for the diagnosis of human visceral leishmaniasis. PLoS Negl Trop Dis. 2019;13(7):e0007484. doi:10.1371/journal.pntd.0007484
35. Lima ID, Lima ALM, Mendes-Aguiar CDO, et al. Changing demographics of visceral leishmaniasis in northeast Brazil: lessons for the future. PLoS Negl Trop Dis. 2018;12(3):e0006164. doi:10.1371/journal.pntd.0006164
36. Gontijo CMF, Melo MN. Leishmaniose visceral no Brasil: quadro atual, desafios e perspectivas. Revista Brasileira de Epidemiologia. 2004;7(3):338–349. doi:10.1590/s1415-790x2004000300011
37. Nunes BEBR, Leal TC, Paiva J, et al. Social determinants of mortality due to visceral leishmaniasis in Brazil (2001-2015): an ecological study. Rev Soc Bras Med Trop. 2019:53. doi:10.1590/0037-8682-0262-2019
38. Leal Silva P, de Lima Alves T, Natal Teixeira P. Epidemiologia Da Leishmaniose Visceral Em Um Município Da Bahia. Revista Saúdecom. 2017;13(3). doi:10.22481/rsc.v13i3.463
39. Dahal P, Singh-Phulgenda S, Olliaro PL, Guerin PJ. Gender disparity in cases enrolled in clinical trials of visceral leishmaniasis: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(3):e0009204. doi:10.1371/journal.pntd.0009204
40. Guerra-Silveira F, Abad-Franch F. Sex Bias in Infectious Disease Epidemiology: patterns and Processes. PLoS One. 2013;8(4):e62390. doi:10.1371/journal.pone.0062390
41. Santos GM. Aspectos epidemiológicos e clínicos da leishmaniose visceral no estado do Piauí, Brasil. Ciência Desenvolvimento-Revista Eletrônica da FAINOR. 2017;10(2):142–153.
42. Rocha M, Matos-Rocha T, Ribeiro C, Abreu S. Aspectos epidemiológicos da leishmaniose visceral canina e humana no estado de Alagoas, Nordeste, Brasil. Br J Biol. 2018;78(4):609–614. doi:10.1590/1519-6984.166622
43. Farias FTG, Furtado Júnior F, Alves ASC, Pereira LE, Carvalho D, Sousa M. Perfil epidemiológico de pacientes diagnosticados com leishmaniose visceral humana no Brasil. Revi Ciência e Desenvolvimento. 2019;12(3):485–501. doi:10.11602/1984-4271.2019.12.3.1
44. Cavalcante FRA, Cavalcante KKDS, Florencio CMGD, Moreno JDO, Correia FGS, Alencar CH. Human visceral leishmaniasis: epidemiological, temporal and spacial aspects in Northeast Brazil, 2003-2017. Rev do Ins de Med Trop de São Paulo. 2020;62. doi:10.1590/s1678-9946202062012
45. Carvalho AG, Kuhn ALM, Dias JVL, Luz JGG. Epidemiological patterns related to deaths caused by visceral leishmaniasis in the southern Amazon region of Brazil. Trans R Soc Trop Med Hyg. 2022. doi:10.1093/trstmh/trac110
46. Cruz CDSS, Cardoso DT, Ferreira Júnior CL, Barbosa DS, Carneiro M. Spatial and spatiotemporal patterns of human visceral leishmaniasis in an endemic southeastern area in countryside Brazil. Rev Soc Bras Med Trop. 2022;55. doi:10.1590/0037-8682-0702-2021
47. Machado CAL, Sevá ADP, Silva AAFAE, Horta MC. Epidemiological profile and lethality of visceral leishmaniasis/human immunodeficiency virus co-infection in an endemic area in Northeast Brazil. Rev Soc Bras Med Trop. 2021;54.
48. Graepp-Fontoura I, Barbosa DS, Fontoura VM, et al. Visceral leishmaniasis and HIV coinfection in Brazil: epidemiological profile and spatial patterns. Trans R Soc Trop Med Hyg. 2022;116:163–172. doi:10.1093/trstmh/trac093
49. Braz BMDA, Silva RBS, Lins SC, Silva DRX, Ramalho WM, Melo MAD. Demographic and spatial study of visceral leishmaniasis in the state of Alagoas, Brazil, during 2007-2018. Rev Soc Bras Med Trop. 2021;54. doi:10.1590/0037-8682-0610-2020
50. Sevá A, Mao L, Galvis-Ovallos F, Tucker Lima JM, Valle D. Risk analysis and prediction of visceral leishmaniasis dispersion in São Paulo State, Brazil. PLoS Negl Trop Dis. 2017;11(2):e0005353. doi:10.1371/journal.pntd.0005353
51. Silva L, Aquino DM, Bezerra JMT, et al. Fatores associados à leishmaniose visceral na área endêmica de Codó, estado do Maranhão, Brasil. Rev de Epidemiologia e Controle de Infecção. 2016;6(2):74–80. doi:10.17058/reci.v6i2.6419
52. Souza EC, Braga KL, Silva T, Silva M. Apresentação clínica da leishmaniose visceral em pacientes portadores do HIV: análise dos Fatores Relacionados ao Aparecimento da Doença/ Clinical presentation of visceral leishmaniasis in patients with HIV: analysis of disease-related factors. Br J Health Rev. 2020;3(2):1766–1777. doi:10.34119/bjhrv3n2-037
53. Távora LGF, Nogueira MB, Gomes ST. Visceral Leishmaniasis/HIV co-infection in northeast Brazil: evaluation of outcome. Br J Infect Dis. 2015;19(6):651–656. doi:10.1016/j.bjid.2015.07.004
54. Lindoso J, Moreira C, Cunha M, Queiroz IT. Visceral leishmaniasis and HIV coinfection: current perspectives. HIV/AIDS Res Palliative Care. 2018;10:193–201. doi:10.2147/hiv.s143929
55. Lindoso JAL, Alves Cunha M, Queiroz I, Valente Moreira CH. Leishmaniasis–HIV coinfection: current challenges. HIV/AIDS Res Palliative Care. 2016;8:147–156. doi:10.2147/hiv.s93789
56. Sousa-Gomes M, Maia-Elkhoury ANS, Pelissari DM. Coinfecção Leishmania-HIV no Brasil: aspectos epidemiológicos, clínicos e laboratoriais. Epidemiologia e Serviços de Saúde. 2011;20(4):519–526. doi:10.5123/S1679-49742011000400011
57. Costa RKED, Holanda EC, Andrade SMD, Nascimento MDSVD, Soares LF, Oliveira EHD. Coinfecção Leishmaniose visceral e Vírus da Imunodeficiência Humana: perfil epidemiológico dos casos notificados em São Luís-Maranhão, Brasil. Res Soc Dev. 2021;10(4):e2310413317. doi:10.33448/rsd-v10i4.13317
58. Silva W, Santos FB, Costa JWS, et al. Epidemiological characteristics of pregnant patients accidented by fish in the Brazilian Amazon. research article. Int J Dev Res. 2022;12(1):53379–53381. doi:10.37118/ijdr.23756.01.2022
59. Andrade FWC, Pinto TI, Moreira L, et al. The Legal Roundwood Market in the Amazon and Its Impact on Deforestation in the Region between 20092015. Forests. 2022;13(4):558. doi:10.3390/f13040558
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.