Back to Journals » Infection and Drug Resistance » Volume 13
Virological Treatment Failure Among Adult HIV/AIDS Patients from Selected Hospitals of North Shoa Zone, Amhara Region, Ethiopia
Authors Derseh B , Shewayerga B, Dagnew Mekuria A , Admasu Basha E
Received 8 September 2020
Accepted for publication 28 November 2020
Published 10 December 2020 Volume 2020:13 Pages 4417—4425
DOI https://doi.org/10.2147/IDR.S280966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Behailu Tariku Derseh,1 Belay Shewayerga,1 Abinet Dagnew Mekuria,1 Elyas Admasu Basha2
1Department of Public Health, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia; 2Department of Nursing, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia
Correspondence: Behailu Tariku Derseh
Debre Berhan University, P.O. Box: 445, Debre Berhan, Ethiopia
Tel +251 911071777
Email [email protected]
Purpose: The study aimed at assessing the magnitude of virological treatment failure and associated factors among HIV reactive adults at selected hospitals.
Patients and Methods: A facility-based cross-sectional study was conducted among 498 study participants who started their first-line HAART from August 2015 to December 2018. Data were collected from patients’ charts and face-to-face interviews using a structured questionnaire. The bivariable analysis was executed to select candidate variables at a p-value of less than 0.2. Multivariable logistic regression (forward, stepwise, and conditional) analysis was used to find factors associated with virological failure at a significant level of 5%. A model adequacy check was done by Hosmer and Lemeshow test (p = 0.57).
Results: More than half 290 (58.2%) of the study participants were women. The median (IQR) age at ART initiation was 40 (15) years. The median (IQR) duration when a virological failure occurred from the initiation of ART treatment was 96 (72) months. The prevalence of virological treatment failure was 10.24% (95% CI: 7.57%, 12.91%). Poor ART drug adherence (AOR = 4.54; 95% CI: 2.09, 9.87), CD4 count less than 250 cell/μL (AOR = 24.88; 95% CI: 11.73, 52.81) and poor quality of life (QoL) (AOR = 2.65; 95% CI: 1.12, 6.25) were independent predictors of virological treatment failure.
Conclusion: The magnitude of virological ART treatment failure in this study was relatively high. Poor ART drug adherence, patients’ having lower CD4 count and poorer quality of life were predictors of treatment failure. Thus, intervention programs that enrich patients’ health-related quality of life should be implemented. Moreover, counseling that supplements the importance of drug adherence and reduction of risks that lower CD4 counts should be given emphasis which in turn helps to prevent first-line ART treatment failure.
Keywords: adherence, first-line ART, Ethiopia, quality of life
Introduction
Globally, HIV/AIDS continues to be a major global public health problem.1,2 In 2016 around 1 million people died with AIDS-related illnesses compared to 1.9 million in 2005 and 1.5 million in 2010.3,4 In Ethiopia, 420 000, 59% [47–73%] people were accessing antiretroviral therapy in 2016, among this 399,000, 61% [49–75%] adults aged 15 years and older living with HIV had access to treatment.3,5 In Ethiopia HIV is dominated by HIV-1 (subtype C). Different studies have shown that there are two distinct HIV subtype C strains in Ethiopia, labeled C and C’. Moreover, based on phylogenetic relationship, there are also ten distinct subtype C clades (termed C1-C10).6–8
The global expansion of antiretroviral therapy has been the primary contributor to a 48% decline in deaths from AIDS-related causes, from a peak of 1.9 million [1.7 million–2.2 million] in 2005 to 1.0 million [830 000–1 2 million] in 2016.9,10 With this scale-up of ART coverage, an increasing proportion of people initiating ART are likely to be infected with a virus that is resistant to one or more WHO-recommended first-line ARV drugs.9
According to the WHO definition, ART failure is defined as clinically (New or recurrent clinical event), immunologically (CD4 count falls to the baseline) and virological failure (viral load above 1000 copies/mL based on two consecutive viral load measurements after 3 months with adherence support), after 6 months of Effective treatment.11 WHO’s Report on HIV drug resistance (HIVDR) in 2017 demonstrates a steady increase in the prevalence of HIVDR in people initiating first-line ART since 2001, most notably in Southern and Eastern Africa. The prevalence of HIVDR in people initiating first-line ART was 6.8% in 2010, and estimates from recent nationally representative surveys show levels of HIVDR above 10%.3 Currently, WHO recommended first-line ART includes Tenofovir (TDF), Efavirenz (EFV) combined with Lamivudine (3TC), and Zidovudine (AZT) alternatives for TDF and Nevirapine (NVP), Dolutegravir (DTG) as alternatives for EFV.11
The treatment of people with these first-line antiretroviral (ARV) drugs will inevitably be accompanied by the emergence and transmission of drug-resistant virus. HIVDR limits treatment options and may necessitate a switch to more expensive regimens (2nd line ART) which is associated with greater long-term toxicity. Moreover, significant population-levels of treatment failure may lead to an increase in HIV/AIDS-related morbidity and mortality.11,12
Antiretroviral Therapy (ART) service began in August 2003 with payment and free ART launched in January 2005. Ethiopia has already adopted major strategies of WHO guidance to meet the third 90 targets (90% of people on treatment are virally suppressed). In 2016 a total of 420,000 people living with HIV were put on anti-retroviral treatment and virological suppression rates were 51% at the national level.5,13 Currently, patients on HAART are monitored with viral load, immunological and clinical assessment among this viral load monitoring is the gold standard method to diagnose ART failure. Though WHO’s recommendation to “treat all” living with HIV immediately after confirming HIV diagnosis that helps to reduce morbidity levels and premature death and the continued expansion of ART coverage, there were an increasing proportion of people on ART had HIV drug resistance (HIVDR); like Pretreatment HIV drug resistance (PDR) and acquired HIV drug resistance (ADR) to one or more of first-line ARV drugs.13,14
The human cost of HIVDR cannot be underestimated. HIV drug resistance is associated with increased mortality and reduced effectiveness of treatment regimens. So preventing, monitoring, and responding to HIVDR is therefore critical to maintaining current achievements, improving patient outcomes, and guarantee the long-term sustainability of care and treatment programs.9 Studies have been conducted from different countries to identify the magnitude and factors associated with first-line ART a failure like in low-income and middle-income countries, a systematic review and meta-regression analysis, which indicate that the prevalence estimate of first-line ART resistance in 2016 was 11% in southern Africa, 10.1% in eastern Africa, 7.2% in western and central Africa, and 9.4% in Latin America and the Caribbean.15 A study conducted in the Tigray region in 2017 virological and immunological failure in the study area were 11.5% and 6.5%, respectively,16,17 29% in Colombia,18 74% in Sweden (Honduran),19 28% in China.20 However, in our study settings, studies that assess the association between virological failure and health-related quality of life among ARV clients were scarce. Therefore, this study was aimed to assess the magnitude and predictors of virological treatment failure among adult people with first-line ART.
Methods
Study Area and Setting
Currently, there are eight public hospitals, one private hospital, and more than ten Health center that providing ART services in the North Shoa zone. Among these eight public hospitals, four district hospitals started ART services recently. The study was conducted in high caseload health institutions; Debre Berhan referral hospital and Mehal Meda District hospital, both in north Shoa zone, Amhara region, Ethiopia. According to the monthly report of Debre Berhan hospital, as of December 2018, in Debre Berhan referral hospital ever enrolled HIV positive clients to chronic HIV care starting from September 2005–December 2018 is 5951, patients ever started on Highly Active Antiretroviral Therapy (HAART) is 3834 and total current on ART is 1712 among this who’s age >18 years is 1651 (F=1067, M=584) (Debre Berhan Referal Hospital, unpublished report, 2018). In Mehal Meda Hospital the number of patients ever enrolled in HIV chronic care starting from September 2005–December 2018, is 1005, patients ever started on Highly Active Antiretroviral Therapy (HAART) is 767 and the total current on ART is 448 among this whose age >18 years is 411 (F=246, M=165) (Mehal-Meda District Hospital, unpublished report, 2018).
Study Design and Period
The facility-based cross-sectional study design was conducted among HIV-positive patients on HAART for >6 months to test the size of first-line ART failure and its associated factors from January 28/2019 - March 22/2019.
Source and Study Population
The source population was all human immunodeficiency virus (HIV) infected clients aged ≥18yrs who were on first-line Antiretroviral Therapy (ART) regimen in Debre Berhan Referral Hospital and Mehal Meda District Hospital. The study population was all HIV/AIDS clients who enrolled in the HAART program, who meet the inclusion criteria, and available during the study period to get service from the ART unit. The inclusion criteria used in this study were HIV/AIDS patients on first-line antiretroviral therapy, adult patients aged above 18 years, and patients whose viral load were measured at six and nine months. However, patients on second-line antiretroviral therapy were excluded from the study.
Sample Size Determination and Sampling Procedure
The sample size is determined based on a single population proportion formula. The proportion of first-line Anti-retroviral Treatment Failure is taken from a study done in Ethiopia.17 Where, p = 0.115, confidence interval of 95% (Zα/2, critical value 1.96), with a marginal error of 4% and 10% non-response rate. The sample size (n) is calculated as; n = (Z2α/2)*P (1-P)/d2 = (1.96)2*(0.115 (1–0.115)/(0.04×0.04)2) = 244 by considering the 10% non-response rate, and multiplying it by a design effect of 2, the total sample size estimated was 538.
A cluster sampling technique was used to select hospitals. There are eight public hospitals in North Shoa Zone. Out of eight ART-site public hospitals, four hospitals were selected by the number of cases they have and late initiation of ART. Then, Debre Berhan referral hospital and Mehal Meda district hospital were selected by a simple random sampling technique from four hospitals.
Finally, a consecutive sampling technique was used to select 538 study participants from two HIV care center clinics, by allocating the total sample size proportionally based on the number of clients/patients they have.
Operational Definition
Adherence: the extent to which a person’s activities, taking medications, following corresponds with accepting instructions from a health care provider.21
Good adherence: if a client used greater than or equal to 95% adherence, that is, missing only 1 out of 30 doses or missing 2 from the 60 doses.11
Fair adherence: if a client used 85–94% adherence, meaning, missing 2–4 doses out of 30 doses or 4 to 9 doses from 60 doses.11
Poor adherence: if a client used less than 85% adherence, that is, missing ≥5 doses out of 30 doses or more than 10 doses from 60 doses.11
Virological failure: is considered in this study, if a patient has a virological failure. That is the viral load above 1000 copies/mL based on two consecutive viral load measurements in 3 months, with adherence support following the first viral load test.11
Immunological failure: CD4 count at or below 250 cells/mm3 following clinical failure or persistent CD4 levels below 100 cells/mm3.11
Clinical failure: New or recurrent clinical event indicating severe immunodeficiency after 6 months of effective treatment.11
Data Collection Tool and Procedure
Data collection was conducted by using a structured questionnaire and structured checklist to collect data from patient follow-up form, ART register, and the electronic database for the ART program. Relevant clinical data such as CD4 count, clinical stage, HAART regimen, and drug adherence status were extracted from participants’ medical charts. Data on supply disruptions of HIV-commodities like ART regimens were interviewed by the clients and from existing registers. Besides, all selected sampled clients who come to ART follow-up clinics were interviewed to collect socio-demographic information and drug adherence level. The questionnaire was prepared in the English language and translated into Amharic and back to English to confirm the consistency questionnaire and checklist. Data were collected by two health information technology (HIT) staff that has a diploma and experience in managing ART data. Two data collectors and two supervisors (BSC nurses) were trained for half a day about the aims of the study, the contents of the tool, and how to collect the data before the data collection. The health-related Quality of Life (HRQoL) of the clients was measured to quantify multidimensional components of health perceived by clients in the past two weeks before data collection and includes; physical, mental, emotional, and social domains.22 The tool is known as WHOQOL-HIV BREF and developed by the World Health Organization; Mental Health Department. The questions are measured in 5-scales and we tested the reliability in our context (Cronbach α = 0.727).
Data Processing and Analysis
The data were checked for completeness and consistency during data collection. The data also cleaned during data entry into EPI info version 3.5.1 and has been transported into SPSS version 20. The data exploration technique was used to check the inconsistency and detect outliers in the dataset. The normality test was performed by using Shapiro–Wilk (P-value > 0.05), Kolmogorov–Smirnova (P-value > 0.05), and visual inspection of the Q-Q Normal Plot, P-P Normal Plot, and Histogram. Descriptive statistics were used to express variables in terms of tables, percentages, and frequencies. The bivariable binary logistic regression was used to select predictor variables at a cutoff point (<0.2). Logistic regression assumptions (sample size and multicollinearity test) were used to check whether variables have satisfied the rules in regression or not. Variance Inflation Factor (VIF) > 10 was used to declare the presence and absence of multicollinearity. Forward stepwise conditional logistic regression analysis was used to find the independent predictors of virological failure. To calculate the measures of association, the Adjusted Odds Ratio (AOR) with its 95% Confidence Interval (CI) at a 5% level of significance was used. The model fitness test was performed by Hosmer and Lemeshow test (p=0.573) and the model summary were done by Naglkerke R square (0.461) which expresses the variability of virological treatment failure in terms of CD4 counts, Quality of Life, and ART treatment drug adherence.
Result
Socio-Demographic Characteristics of Patients
A total of 498 HIV infected individuals on first-line ART regimens have voluntarily participated in this study with a response rate of 92.5%. The rest clients were refused to take part in the study due to unwillingness. More than half 290 (58.2%) of the study participants were female. The median age of the participants was 40 with an interquartile range of (IQR = 15). Regarding the educational level, 80 (16.1%) of the study participants had no formal education, 209 (42%) of them completed primary school, 86 (17.3%) completed college or university. Seventy-six (15.3%) were government employees and 230 (46.2%) of them were self-employed. Two-hundred sixty-one (52.4%) of the study participants had a monthly income above 1000 per month (Table 1).
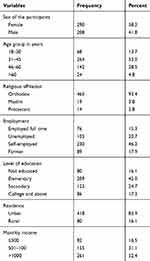 |
Table 1 Socio-Demographic Characteristics of the Respondents in Debre Berhan Referral Hospital and Mehal Meda Hospital, North Showa Zone, Amhara, Ethiopia, 2019 |
Baseline Clinical Characteristics of Patients
The majority, 434 (87.1%) of study participants were working by their functional status and 482 (96.8%) of participants had hemoglobin measurement of greater or equal to 10 mg/dl at the start of HAART. The median CD4 count was 184 cell/μL (IQR=182) and TB infection was confirmed in 138 (27.7%) since the start of HAART. Moreover, 190 (38.2%) and 91 (18.3%) of study participants had started treatment with Tenofovir–Lamivudine–Efavirenz, and Zidovudine-Lamivudine-Efavirenz regimen, respectively. Also, one hundred sixteen (23.3%) of them have a history of malnutrition, and 125 (25.1%) of the participants were non-adhered to their medication (Table 2).
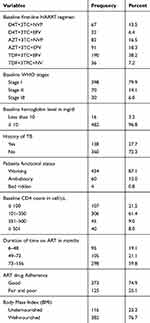 |
Table 2 Baseline and Follow-Up Characteristics of Study Participants in Debre Berhan Referral Hospital and Mehal Meda Hospital, North Shoa Zone, Amhara, Ethiopia, 2019 |
The Magnitude of First-Line ART Virological Failure
The magnitude of virological failure (≥ 1000 RNA copies per mL) was found in 51 (10.24%; 95% CI: 7.57%, 12.91%). Since the start of HAART, 43 (8.63%) of them encountered virological failure within 73–156 months (Table 3).
 |
Table 3 Treatment Failure After Initiation of HAART Among HIV/AIDS Patients in Debre Berhan Referral and Mehal Meda Hospitals, North Shoa Zone, Amhara, Ethiopia, 2019 |
Factors Associated with Virological Treatment Failure
After conducting bivariable analysis on predictor variables with a p-value ≤ 0.2, then multivariable logistic regression analysis has been conducted to control the effects of socio-demographic, behavioral, and clinical factors. Then, poor ART drug adherence (AOR = 4.54; 95% CI: 2.09, 9.87), people who had CD4 counts less than 250 cell/μL (AOR = 24.88; 95% CI: 11.73, 52.81) and PLWHA who had poor quality of life (QoL) (AOR = 2.65; 95% CI: 1.12, 6.25) were found statistically associated with first-line ART virological treatment failure (Table 4).
 |
Table 4 Bivariable and Multivariable Logistic Regression Analysis of Outcome and Predictor Variables in Debre Berhan and Mehal Meda Hospitals, North Shoa Zone, Amhara, Ethiopia, 2019 |
Discussion
The identification and management of virological ART treatment failure is a key challenge for HIV programs in resource-limited settings. ART treatment failure is a serious emerging threat to the global scale-up of HIV treatment access and staying with this failing first-line therapy is associated with an increased risk of morbidity and mortality (22).23 This study particularly designed to determine the magnitude of virological treatment failure (10.24%) and identify the predictors of virological failure (Poor ART drug adherence, CD4 count less than 250cell/µL, and clients’ whose poor quality of life).
The size of the virological treatment failure was 10.24% (51/498). The finding was consistent with the study conducted in guinea 13.7%23 and Bahir Dar 10.7%.24 However, when compared with other studies, for instance, a study conducted in coastal Kenya (6%),25 Debre Markos hospital (21%),26 Colombia (29%),18 Sweden, Honduran (74%),19 and China (28%),20 it was low. This discrepancy may be due to most clients on first-line ART treatment failure shift to second-line ART. This 10.2% virological treatment failure indicated that first-line ART treatment may not be effective, as WHO recommends changing their first-line ART regimen if levels of Non-nucleoside reverse-transcriptase inhibitor (NNRTI) pretreatment HIV drug resistance (PDR) reach 10%,27 so further investigations is needed on pretreatment HIVDR to NNRTIs.
The current study showed that ART treatment adherence was the independent reason for the virological treatment failure and patients with poor ART drug adherence were 4.5 times at higher risk of developing treatment failure compared to patients with good ART drug adherence. This finding is consistent with a systematic review conducted in Ethiopia.28 Medication adherence has an opportunity for best viral suppression, immune recovery, and as a result, the clinical benefit will be gained. Thus, successful ART treatment requires all medications should be taken as prescribed by the physicians (health care service giver). However, this result contradicts studies done in the Tigray region of northern Ethiopia, Gondar, and Felege-Hiwot Referral Hospitals.17,24,31 These variations may be related to the type of data collection method. In our study, the data on adherence were collected from one-month recall self-reported missed doses, and not directly collecting from the patients’ charts.
Another predictor variable that has a significant association with virological failure was CD4 count. Individuals who have a CD4 count of less than 250 cell/μL were 24 times more likely to develop virological failure than their counterparts (AOR = 24.88; 95% CI: 11.449, 52.81). This result was in line with studies conducted in different parts of the world; the University of Gondar Hospital (AOR = 9.03; 95% CI: 4.40, 18.50),29 Zewditu Memorial Hospital (AOR = 2.67; 95% CI: 1.29, 5.51),30 a multi-center study conducted at three selected Hospitals Northwest Ethiopia (AHR = 2.0; 95% CI: 1.20, 3.50),31 and Felege-Hiwot Hospital (AOR = 8.63; 95% CI: 3.32, 22.42).24 Since ART treatment provides opportunities for viral load suppression, recovery of immunity, and a client gets clinical benefits. Thus, all individuals taking ART drugs are expected to take all medications as prescribed. As a consequence, the number of CD4 counts increases and helps in the success of immunological treatment.
Health-Related Quality of Life (HRQoL) was another independent predictor variable for virological treatment failure. Clients with poor Quality of Life were 2.6 times more likely to develop virological failure than clients with good (QoL) (AOR = 2.65; 95% CI: 1.12, 6.25). Maintaining and improving the quality of life among people in ART follow-up clinics is regarded as the most important component of HIV/AIDS care and treatment even though this concept is not emphasized in developing countries where resources are scarce. A similar finding was observed in a study done in Ethiopia where immunologic treatment failure was associated with health-related quality of life among people infected with HIV/AIDS.32 Since measuring the quality of life of people with HIV/AIDS consisted of several dimensions (physical, psychological, independence, social, environmental, and spiritual), stakeholders should work on the betterment of the quality of life among people on HAART. As the result indicated 56.02% (51.65%, 60.40%) of the clients’ quality of life was compromised by the disease (HIV/AIDS) and related complications. Thus, this significant number of clients needs their health-related quality of life to be changed.
Strength and Limitations of the Study
Since the study was conducted in health facilities taking a representative sample, the results can be generalized to health facilities in the North Shoa Zone of Amhara Region, Ethiopia. But, the results are interpreted with limitations. First, due to the cross-sectional nature of the study design, we could not establish the temporal relationship between virological failure and its predictors. Recall and social desirability bias where the participants may not respond correctly for some sensitive questions due to memory loss and social norms. To reduce such systematic errors, during the data collection period data collectors tried to explain the importance of honest response. Moreover, smaller frequency in certain categories of predictor variables may reduce the precision of the measure of association. Thus, the use of this study finding for any concern should be accounted for consideration of having these inherent limitations of the study.
Conclusion
The magnitude of virological failure in this study was relatively high. Poor ART drug adherence, CD4 counts less than 250 µL/mL and poor health-related quality of life were found to be significant predictors of virological treatment failure. Thus, Early ART failure detection is one of the most important key improvement areas of the health care providers with close follow up of the patients. Moreover, interventions that enhance the quality of life of clients on ART should be established. Behavior change communication on the benefits of good ART drug adherence should be strengthened which in turn is expected to reduce the risks of the decrement of CD4 counts.
Abbreviations
ADR, acquired HIV drug resistance; ART, antiretroviral therapy; ARV,anti-retroviral HAART, highly active antiretroviral therapy; HIV/AIDS, human immune deficiency virus/acquired immune deficiency syndrome; HIVDR, HIV drug resistance; PDR, pretreatment HIV drug resistance; PLHIV, people living with HIV; TDR, transmitted HIV drug resistance.
Data Sharing Statement
The data used to support the findings of this study are included in this manuscript.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from Debre Berhan University (DBU), College of Health Sciences (HSC), and the Ethical Review Committee (ERC) (Protocol number 08/19/SPH). An official letter was submitted to Debre Berhan Comprehensive Specialized Hospital and Mehal Meda Hospital to inform and support the data collection process. Verbal informed consent was gained from the study participants by explaining the potential benefits of the study. Moreover, participants were told that they can stop the interview at any point during data collection if they are uncomfortable and if they don’t want to answer a particular question. In addition, all the process of this study followed the principles of the Declaration of Helsinki and was approved by the Ethical Review Committee of DBUHSC.
Acknowledgment
We would like to express our heartfelt gratitude to Debre Berhan University, College of Health Sciences, and the Department of Public Health for monitoring and evaluating this project. This work is impossible without the support of staff working at Debre Berhan Comprehensive Specialized Hospital and Mehal Meda Hospital - thanks for your support during data collection time. Moreover, study participants and data collectors deserve appreciations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. El Programa Conjunto de las Naciones Unidas sobre el VIH/SIDA (ONUSIDA). Fact sheet - WORLD AIDS DAY 2018. 2017 Global HIV Statistics. El Programa Conjunto las Nac Unidas sobre el VIH/SIDA (ONUSIDA); 2018:6. Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf.
2. Averting Hiv and Aids. Global HIV and AIDS Statistics | AVERT; 2018.
3. UNAIDS. FACT SHEET – World AIDS Day 2018 Global HIV and AIDS Statistics. Unaids; 2018:1–6.
4. Ethiopian Pubilc Health Institute. HIV Related Estimates and Projections for Ethiopia; 2017.
5. U.S. Department of Health & Human Services. Global Statistics | HIV.gov; 2017. Available from: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics.
6. Pollakis G, Abebe A, Kliphuis A, et al. Recombination of HIV type 1C (C’/C”) in Ethiopia: possible link of EthHIV-1C’ to subtype C sequences from the high-prevalence epidemics in India and Southern Africa. AIDS Res Hum Retroviruses. 2003;19(11):999–1008. doi:10.1089/088922203322588350
7. Abebe A, Kuiken CL, Goudsmit J, et al. HIV type 1 subtype C in Addis Ababa, Ethiopia. AIDS Res Hum Retroviruses. 1997;13(12):1071–1075. doi:10.1089/aid.1997.13.1071
8. Tully DC, Wood C. Chronology and evolution of the HIV-1 subtype C epidemic in Ethiopia. AIDS. 2010;24(10):1577–1582. doi:10.1097/QAD.0b013e32833999e1
9. World Health Organization. Global action plan on HIV drug resistance 2017–2021; 2017. Available from::http://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf?sequence=1.
10. Joint United Nations Programme on HIV/AIDS, UNAIDS 2017. Ending aids progress towards the 90- 90-90Targets. Glob Aids Update. 2017;198.
11. World Health Organization. Consolidated Guidelines on the Use of ART Drugs for Treating and Preventing HIV Infection. Vol. 16. Geneva: World Health Organization; June 2016:1–272
12. WHO. Global report on early warning indicators of HIV drug resistance. World Health Organ. 2016:9. doi:10.1016/j.quaint.2018.06.014
13. Federal Ministry of Health. National Guidelines for HIV Prevention, Treatment, and Care (2016); 2016. doi:10.1016/j.anbehav.2014.09.030
14. WHO. Tackling HIV drug resistance: trends, guidelines, and global action. Policy Br. 2017;1–4.
15. Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346–355. doi:10.1016/S1473-3099(17)30702-8
16. Mpondo BC, Kilonzo SB, Meda JR, Gunda DW. Prevalence and predictors of Immunological failure among HIV-infected adults on HAART in Northwestern Tanzania: a cross-sectional study. Tanzania Med J. 2016;27(1):55–69. doi:10.4314/tmj.v27i1.188
17. Genet Gebrehiwet H, Dawit Gebregziabher H, Amlsha Kahsay H, Araya Gebreyesus W, Tsehaye Asmelash D. Virological and immunological failure of HAART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS One. 2018;13(5).
18. de La Hoz JM, Bolaño L, Cárdenas O, et al. Characterization of treatment failure in HIV positive patients in the Colombian Caribbean region. Colomb Med. 2014;45(4):162–167. doi:10.25100/cm.v45i4.1566
19. Murillo W, de Rivera IL, Parham L, et al. Prevalence of drug resistance and the importance of viral load measurements in Honduran HIV-infected patients failing antiretroviral treatment. HIV Med. 2010;11(2):95–103. doi:10.1111/j.1468-1293.2009.00747.x
20. Ma Y, Zhao D, Yu L, et al. Predictors of virologic failure in HIV-1-infected adults receiving first-line antiretroviral therapy in 8 provinces in China. Clin Infect Dis. 2010;50(2):264–271. doi:10.1086/649215
21. WHO (World Health Organisation). Guidance on operations and service delivery. In: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing Hiv Infection; 2013.
22. World Health Organization. WHOQOL-HIV Instrument User’s Manual: Scoring and Coding for the WHOQOL-HIV Instruments; 2002.
23. Gare J, Kelly-Hanku A, Ryan CE, et al. Factors influencing antiretroviral adherence and virological outcomes in people living with HIV in the Highlands of Papua New Guinea. PLoS One. 2015;10(8):e0134918. doi:10.1371/journal.pone.0134918
24. Gidey Brhane B, Nibret E, Kahsu Abay G. HIV/AIDS treatment failure and its determinant factors among first line HAART patients at Felege-Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. J AIDS Clin Res. 2017;8(11). doi:10.4172/2155-6113.1000744
25. Hassan AS, Nabwera HM, Mwaringa SM, et al. HIV-1 virologic failure and acquired drug resistance among first-line antiretroviral experienced adults at a rural HIV clinic in coastal Kenya: a cross-sectional study. AIDS Res Ther. 2014;11(1). doi:10.1186/1742-6405-11-9
26. Melsew YA, Terefe MW, Tessema GA, Ayele TA. Rate of immunological failure and its predictors among patients on highly active antiretroviral therapy at Debremarkos Hospital, Northwest Ethiopia: a retrospective follows up study. J AIDS Clin Res. 2013;4(5). doi:10.4172/2155-6113.1000211
27. Endalamaw A, Mekonen M, Geremew D, Ambaw F, Hiwot Tesera TDH. Evidence that poor HAART adherence has a great impact on HIV/AIDS treatment failure more than the severity of illness and opportunity of infection in Ethiopia: systematic review and meta-analysis. bioRxiv. 2018. doi:10.1101/440743
28. Ayalew MB, Kumilachew D, Belay A, et al. First-line antiretroviral treatment failure and associated factors in HIV patients at the University of Gondar teaching hospital, Gondar, Northwest Ethiopia. HIV/AIDS Res Palliat Care. 2016;8:141–146. doi:10.2147/HIV.S112048
29. Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. Determinants of virological failure among patients on highly active antiretroviral therapy at the University of Gondar Referral Hospital, Northwest Ethiopia: a case-control study. HIV/AIDS Res Palliat Care. 2017;9:153–159. doi:10.2147/HIV.S139516
30. Sisay C, Bekele A, Sisay A, et al. Incidence and predictors of anti-retroviral treatment (ART) failure among adults receiving HIV care at Zewditu Memorial Hospital, Addis Ababa, Ethiopia. J AIDS Clin Res. 2017;8(12). doi:10.4172/2155-6113.1000749
31. Tsegaye AT, Wubshet M, Awoke T, Addis Alene K. Predictors of treatment failure on second-line antiretroviral therapy among adults in northwest Ethiopia: a multicentre retrospective follow-up study. BMJ Open. 2016;6(12):e012537. doi:10.1136/bmjopen-2016-012537
32. Abera K, Gedif T, Engidawork E, Gebre-Mariam T. Quality of life of people living with HIV/AIDS and on highly active antiretroviral therapy in Ethiopia. Afr J AIDS Res. 2010;9(1):31–40. doi:10.2989/16085906.2010.484560
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
