Back to Journals » Drug Design, Development and Therapy » Volume 14
Video Education Reduces Pain and Anxiety Levels in Cancer Patients Who First Use Fentanyl Transdermal Patch: A Randomized Controlled Trial
Authors Ye Z , Chen J , Zhang Y, Hu X, Xuan Z, Yang S, Mao X, Rao Y
Received 25 May 2020
Accepted for publication 6 August 2020
Published 25 August 2020 Volume 2020:14 Pages 3477—3483
DOI https://doi.org/10.2147/DDDT.S264112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Ziqi Ye,1,* Jie Chen,2,* Yanfang Zhang,1 Xi Hu,1 Zixue Xuan,3 Si Yang,1 Xiaohong Mao,3 Yuefeng Rao1
1Department of Clinical Pharmacy, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Pharmacy, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Department of Pharmacy, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuefeng Rao Department of Clinical Pharmacy
The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou 310003, People’s Republic of China
Tel +86-571-8723-6531
Email [email protected]
Xiaohong Mao Department of Pharmacy
Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, 158 Shangtang Road, Hangzhou 310014, People’s Republic of China
Tel +86-15990151723
Email [email protected]
Objective: We sought to evaluate the efficacy of using a quick response (QR) code within video education to guide proper use of fentanyl transdermal patches and control pain, depression, and anxiety levels in cancer patients.
Methods: Patients using a fentanyl transdermal patch for the first time were enrolled in the study and then given an information leaflet as well as an informed consent form. We asked them to complete the first questionnaire (Q1) prior to first use of the fentanyl transdermal patch, and then used a random number table to randomize those who completed it into two groups. Participants in group A received a QR code (to make it easier for them to obtain additional video information) and a traditional information leaflet, whereas those in group B (control group) only received a traditional information leaflet. Thereafter, we requested all participants to complete standard questionnaires, which comprised a Numeric Rating Scale (NRS), a Spielberger State-Trait Anxiety Inventory (STAI), as well as a Hospital Anxiety and Depression Scale (HADS). The resulting continuous (with a normal distribution) and categorical data were analyzed using Student’s t- and chi-square tests, respectively. We also recorded parameters such as NRS, STAI, and HADS, as well as the frequency of rescue medication in both groups.
Results: A total of 154 cancer patients who first used a fentanyl transdermal patch were recruited during the study period, from April to May 2020. Among these, 138 completed follow-up, with 70 and 68 in group A and B, respectively. Participants in both groups had similar baseline and clinical characteristics, whereas significant differences were observed between the groups with regard to the other parameters. Specifically, participants in group A recorded a lower STAI state (38.2 vs 38.9, P=0.027) and HADS (3.9 vs 4.2, P=0.001) anxiety scores, as well as NRS (2.1 vs 2.4, P=0.025) and frequency of rescue medication (0.4 vs 1.4, P< 0.001) than those in group B, following 14 days of using a fentanyl transdermal patch.
Conclusion: Our results indicated that incorporating a QR code within additional video education leads to proper use of a fentanyl transdermal patch and relieves pain and anxiety levels in patients with cancer. Based on this, we recommend a new style of education during care of cancer patients who first use a fentanyl transdermal patch.
Keywords: QR code, video education, fentanyl transdermal patch, anxiety, depression
Introduction
Pain is one of the most common symptoms associated with cancer, with previous studies showing that 59% of patients undergoing cancer treatment, 64% of those with advanced disease, and 33% of those who have had curative treatment experience pain.1 Fentanyl is a “strong opioid” that is commonly used by cancer patients who experience pain, including difficulty in swallowing, poor tolerance to morphine or oxycodone, or poor compliance. Previous studies have also shown that fentanyl is a highly lipophilic opioid receptor agonist, and can be administered via spinal, transdermal, parenteral, buccal, transmucosal, and intranasal routes.2,3 When administered through these routes, a fentanyl transdermal patch, rather than rapid opioid titration, is recommended for opioid-tolerant patients whose pain cannot be effectively controlled by other opioids.4
However, several cautions during the use of fentanyl transdermal patches, as well as misuse, have been associated with lower efficacy and exacerbation of adverse events. Consequently, unrelieved pain severely affects a patient’s mood, motivation, activities, and the overall quality-of-life.5 Accurate information on drug use plays a key role in treatment of cancer pain. For example, previous studies have reported significantly higher anxiety levels in patients with cancer pain, who get inadequate information from physicians, indicating that accurate understanding of how to use drugs, such as fentanyl transdermal patches, may alleviate patients’ pain and anxiety levels.5
Patient education has proven to be an effective way for ensuring proper use of drugs. For example, use of video education was found to be superior to traditional information leaflets in patients undergoing colposcopy.6 A quick response (QR) code, which can easily be created to link a video online, enables people to watch the video by scanning the QR code with their mobile device.7 Recently, the QR code has generated interest from healthcare practitioners, including its application in proper use of medications by elderly patients.8 In China, low educational levels among many cancer patients limit their understanding of how to properly use therapies such as fentanyl transdermal patches. However, widespread use of smartphones presents an excellent opportunity for access to education for proper use of drugs. Therefore, the present study sought to evaluate whether incorporating a QR code within additional video education is more effective than traditional leaflets in ensuring proper use of fentanyl transdermal patches in cancer patients.
Methods
Patient Recruitment and Randomization
A total of 154 cancer patients, who were experiencing pain and were hospitalized at the First Affiliated Hospital, Zhejiang University School of Medicine, were enrolled in the study between April and May 2020.
All enrolled patients were over 18 years, used a fentanyl transdermal patch for the first time, and had adequate knowledge of the Chinese language. Patients with liver and kidney dysfunction, ECOG scores >2, as well as those with refractory cancer pain were excluded from the study.
Patients received study information, via a leaflet, and then willingly consented to the study, prior to inclusion. The patients were given a questionnaire (Q1), prior to first use of fentanyl transdermal patch, then those who completed it were randomized into two groups using a random number table. Blinding was not possible in this study. Patients in group A received a QR code, which was linked to an education video, as well as a traditional information leaflet, whereas those in group B (control group) only received a traditional information leaflet.
The study was registered in the Chinese Clinical Trial Registry (ChiCTR2000031054), and approved by the Institutional Review Board. All procedures were conducted in accordance with the guidelines of the Declaration of Helsinki.
Questionnaires
Enrolled participants were asked to complete two sets of different questionnaires: Q1 at baseline before commencing use of fentanyl transdermal patch for the first time (to compare two groups); Q2 14 days after use of the drug (to assess the effect of the video intervention). Specifically, information contained in Q1 included State and Trait Anxiety Inventory (STAI), Hospital Anxiety and Depression Scale (HADS), and pain with the Numeric Rating Scale (NRS). Q2 was completed via follow-up telephone calls.
We adopted HADS, a 14-item self-report screening scale created by Zigmond and Snaith,9 to measure depression and anxiety levels, before and after first use of a fentanyl transdermal patch. This tool contains two 7-items scales: one for depression and another for anxiety. All items are scored on a 4-point scale, ranging from 0–3. Moreover, we used the short version of the STAI to evaluate the trait and state anxieties of patients.10 Trait and state denote anxiety “in general” and “at the present moment”, respectively, and can reflect the differences in anxiety levels. In addition, STAI contains 20 items (10 items each for state and trait anxiety) measured on a 4-point scale, ranging from “not at all” to “very much”. The overall scores range from 20–80, with higher scores indicating greater anxiety levels. On the other hand, NRS scores and frequency of rescue medication were used to measure pain levels, before and 14 days following use of a fentanyl transdermal patch. The NRS scales range from 0 (no pain) to 10 (worst imaginable pain).
Video Information
An information video that helps patients understand how to correctly use fentanyl transdermal patches, by providing vivid and detailed information and aid in reduction of anxiety, depression, as well as pain levels, was designed. Specifically, the video included cartoon images of the hospital, the inpatient area, and a physician. In the video, a physician vividly explained the matters needing attention before, during, and after the use of fentanyl transdermal patches, including appropriate patch sites, proper skin preparation procedures, keeping away from sources of heat, among others. The video was 2 minutes long, which is an appropriate time for cancer patients with pain and fatigue. A QR code was also generated to let the patients easily obtain the video, even after getting discharged.
Statistical Analyses
The G*Power 3.1.9.4 program was used to perform a power calculation and effect size determination. Thereafter, Student’s t- and chi-square tests were applied for analysis of continuous (with a normal distribution) and categorical variables, respectively. All data analyses were performed in SPSS version 20 software (IBM, Armonk, NY).
Results
A total of 154 patients were enrolled in this study between April and May 2020. Ten (6.5%) of the patients did not complete Q1 prior to first use of fentanyl transdermal patch, and were subsequently excluded from the study. The remaining 144 patients were randomly assigned into two groups of 72 participants each, although two and four in groups A and B, respectively, were excluded because they did not complete Q2. Finally, a total of 138 participants, 70 in group A and 68 in group B, were used for analysis (Figure 1).
 |
Figure 1 A flowchart indicating study design. |
Baseline and Clinical Characteristics
Based on feedback from Q1, which was completed prior to first use of a fentanyl transdermal patch, we found no significant differences in clinical characteristics between the two groups (Table 1). All patients were opioid tolerant, and the fentanyl transdermal patches were not tapered during the observation period. In addition, slightly higher depression and anxiety levels were found in participants in group A relative to those in group B, although these differences were not significantly different. Specifically, according to HADS-based anxiety levels were 6.4±2.0 vs 6.2±2.3 (P=0.362), whereas depression levels were 2.7±1.0 vs 2.5±0.7 (P=0.142), in groups A and B, respectively. On the other hand, STAI state anxiety levels were 39.7±5.6 vs 39.5±7.6 (P=0.722), whereas trait anxiety levels were 37.7±5.7 vs 37.6±6.9 (P=0.703) in groups A and B, respectively. Participants in both groups also exhibited NRS pain scores of 4.0 (P=0.752).
 |
Table 1 Patients’ Baseline and Clinical Characteristics |
Outcome Measures
Results of outcome measures, 14 days after the video intervention, revealed significant differences between groups A and B (Table 2). Specifically, HADS-based anxiety levels were 3.9±1.1 vs 4.2±1.3, (mean difference (MD)=−0.26, 95% CI=−0.42 to −0.11, P=0.001), STAI-based state anxiety levels were 38.2±5.8 vs 38.9±4.3 (MD=−0.61, 95% CI=−1.15 to −0.07, P=0.027), and pain scores were 2.1±1.2 vs 2.4±1.1 (MD=−0.21, 95% CI=−0.39 to −0.03, P=0.025), whereas frequency of rescue medication was 0.4±0.6 vs 1.4±0.7 (MD=−1.03, 95% CI=−1.25 to −0.81, P<0.001) in group A and B, respectively. Conversely, no significant differences were found between the groups, with regards to HADS-based depression levels (2.2±1.0 vs 2.0±0.9, P=0.224).
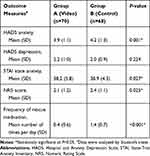 |
Table 2 Patients’ Outcome Measures, 14 Days After the First Use of the Fentanyl Transdermal Patch |
Discussion
Use of a fentanyl transdermal patch for management of pain and anxiety in cancer patients has increased worldwide, owing to it's marked superiority over conventional methods in patients who cannot receive prescribed oral medication. Previous studies have associated this therapy with serious or life-threatening hypoventilation, especially in opioid-naïve patients.11 In fact, patients wearing a fentanyl transdermal patch should not be exposed to external heat sources, such as hot tubs, saunas, and heat lamps, because heat accelerates release of fentanyl. In addition, fever has also been shown to enhance fentanyl absorption.12 Misuse of a fentanyl transdermal patch may reduce drug efficacy and increase chances of adverse events. There is a need, therefore, to increase patients’ knowledge on proper use of this therapy. In the present trial, analysis of cancer patients, using STAI, HADS, NRS scores, and frequency of rescue medication, showed that a QR code, within additional video education, reduced pain and anxiety levels in participants using fentanyl transdermal patches.
In recent years, numerous studies have reported the use of video education in cancer patients experiencing pain. For example, video education was found to significantly improve the ratings of pain-related interference with function, as well as general health, vitality, and mental health.13 Similarly, video education reportedly reduced barriers to pain relief, thereby lowering usual pain.14 In another RCT, significantly lower average and worst pain scores were recorded who received video education (by 1.17 and 1.12).15 However, to date, the use of video education in reducing pain levels in cancer patients who first use a fentanyl transdermal patch remains unknown. Results of the present study revealed that video education corresponded with pain relief in cancer patients, relative to use of a traditional information leaflet. Our NRS scores indicated a reduction of only 8.8%, suggesting a small clinical relevance between the video education and reduction of pain intensity. This was contrary to previous studies that have suggested that at least a 30% reduction in pain intensity or an absolute reduction in the value of at least 2 to be meaningful pain relief in patients.16 Conversely, frequency of rescue medication indicated that video education might significantly reduce the frequency of breakthrough pain.
Poor pain control may lead to an increase in anxiety and depression levels in cancer patients. Besides pain levels, most previous studies have not reported on the impact of video education on anxiety and depression levels of patients who experience cancer pain. Use of video education in patients who underwent colonoscopy has been reported. Despite the use of sedation, colonoscopy is not well tolerated by many patients since it is accompanied by pain and anxiety. One RCT found significantly lower STAI-S scores in participants in the educational video group relative to those in the control group (P=0.001).17 Conversely, another RCT reported that educational video did not significantly affect anxiety levels of patients undergoing colonoscopy (P>0.05).18 In the present study, our results revealed that video education significantly reduced state-anxiety scores but not depression levels compared to conventional education. This might be attributed to a greater impact of short-term study on anxiety levels. Future studies are needed to elucidate the impact of video education on depression levels in patients. Generally, we expect small clinical relevance if differences in actual anxiety levels are less than 5%.19 In the present study, difference in HADS scores was 6.2%, whereas that of STAI scores was only 1.6%. The heterogeneity between HADS and STAI scores suggested that incorporating a QR code within additional video education might reduce anxiety levels in patients relative to traditional information, although the actual clinical significance needs to be further studied.
In this trial, the average patient age was over 60 years. Furthermore, most of the participants only had primary or preparatory secondary vocational education, and they rarely obtained information from the internet. Based on these education levels, it is difficult for traditional information leaflets to achieve satisfactory results since the patients may not correctly use transdermal fentanyl at home. Availability of an educational video may be a convenient and time-saving strategy for such patients, owing to their literacy levels.20 A key concern during use of this tool was how patients could easily access the educational video upon discharge. Fortunately, widespread use of smartphones made it easy for QR code links to be sent to the participants. Encouragingly, patients’ access to the QR code within video education effectively enhanced the safe use of fentanyl transdermal patches, and relieved their anxiety and pain levels.
This study is the first report on the use of a QR code within additional video education to guide safe use of fentanyl transdermal patches and relieve pain and anxiety levels in cancer patients. The study had some limitations. Firstly, we assessed outcomes 14 days after the first use of fentanyl transdermal patches. This period was not sufficient to allow assessment of long-term effects of video education on relieving pain in cancer patients. Secondly, we did not analyze the effect of video education on overall survival or progression-free survival of the patients.
Conclusion
Taken together, our results indicate that incorporating a QR code within video education can effectively guide proper use of fentanyl transdermal patches, thereby relieving pain and anxiety levels in patients with cancer pain. These findings affirm the need for a new style of education during care of cancer patients who first use fentanyl transdermal patches.
Data Sharing Statement
Readers can contact the corresponding authors to obtain the individual de-identified participant data by email, after 6 months of publication. The study protocol, statistical analysis plan, and clinical report will also be available.
Ethics and Consent Statement
This study received approval from the Clinical Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Approval number: IIT20200013A), and was conducted in accordance with the tenets of the Declaration of Helsinki. All patients signed an informed consent form, agreeing to the use of their anonymized clinical data for research purposes, prior to enrolment in the study.
Funding
This work was supported by Joint Fund of Zhejiang Provincial Natural Science Foundation, China (Grant number LYY18H310002) and Funding of Zhejiang Pharmaceutical Association (Grant number ZYYZL01).
Disclosure
The authors report no conflicts of interest in this work.
References
1. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi:10.1093/annonc/mdm056
2. Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Phys. 2008;11(2 Suppl):S133–153.
3. Mercadante S, Vellucci R, Cuomo A, et al. Long-term efficacy and tolerability of intranasal fentanyl in the treatment of breakthrough cancer pain. Support Care Cancer. 2015;23(5):1349–1354. doi:10.1007/s00520-014-2491-x
4. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–68. doi:10.1016/S1470-2045(12)70040-2
5. Te Boveldt N, Vernooij-Dassen M, Burger N, Ijsseldijk M, Vissers K, Engels Y. Pain and its interference with daily activities in medical oncology outpatients. Pain Phys. 2013;16(4):379–389.
6. Freeman-Wang T, Walker P, Linehan J, Coffey C, Glasser B, Sherr L. Anxiety levels in women attending colposcopy clinics for treatment for cervical intraepithelial neoplasia: a randomised trial of written and video information. BJOG. 2001;108(5):482–484. doi:10.1111/j.1471-0528.2001.00121.x
7. Karia CT, Hughes A, Carr S. Uses of quick response codes in healthcare education: a scoping review. BMC Med Educ. 2019;19(1):456. doi:10.1186/s12909-019-1876-4
8. Mira JJ, Guilabert M, Carrillo I, et al. Use of QR and EAN-13 codes by older patients taking multiple medications for a safer use of medication. Int J Med Inform. 2015;84(6):406–412. doi:10.1016/j.ijmedinf.2015.02.001
9. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
10. Julian LJ. Measures of anxiety: state-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S467–472. doi:10.1002/acr.20561
11. Wong JO, Chiu GL, Tsao CJ, Chang CL. Comparison of oral controlled-release morphine with transdermal fentanyl in terminal cancer pain. Acta Anaesthesiol Sin. 1997;35(1):25–32.
12. Southam MA. Transdermal fentanyl therapy: system design, pharmacokinetics and efficacy. Anticancer Drugs. 1995;6(Suppl 3):29–34. doi:10.1097/00001813-199504003-00005
13. Thomas ML, Elliott JE, Rao SM, Fahey KF, Paul SM, Miaskowski C. A randomized, clinical trial of education or motivational-interviewing-based coaching compared to usual care to improve cancer pain management. Oncol Nurs Forum. 2012;39(1):39–49. doi:10.1188/12.ONF.39-49
14. Syrjala KL, Abrams JR, Polissar NL, et al. Patient training in cancer pain management using integrated print and video materials: a multisite randomized controlled trial. Pain. 2008;135(1–2):175–186. doi:10.1016/j.pain.2007.10.026
15. Lovell MR, Forder PM, Stockler MR, et al. A randomized controlled trial of a standardized educational intervention for patients with cancer pain. J Pain Symptom Manage. 2010;40(1):49–59. doi:10.1016/j.jpainsymman.2009.12.013
16. Farrar JT, Portenoy RK. Neuropathic cancer pain: the role of adjuvant analgesics. Oncology (Williston Park). 2001;15(11):
17. Arabul M, Kandemir A, Celik M, et al. Impact of an information video before colonoscopy on patient satisfaction and anxiety. Turkish J Gastroenterol. 2012;23(5):523–529. doi:10.4318/tjg.2012.0416
18. Bytzer P, Lindeberg B. Impact of an information video before colonoscopy on patient satisfaction and anxiety - a randomized trial. Endoscopy. 2007;39(8):710–714. doi:10.1055/s-2007-966718
19. Ye Z, Chen J, Rao Y, Yang W. Should S-1 be better than capecitabine for patients with advanced gastric cancer in Asia? A systematic review and meta-analysis. Onco Targets Ther. 2019;12:269–277. doi:10.2147/OTT.S187815
20. Zapka JG, Lemon SC, Puleo E, Estabrook B, Luckmann R, Erban S. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med. 2004;141(9):683–692. doi:10.7326/0003-4819-141-9-200411020-00009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
