Back to Journals » Risk Management and Healthcare Policy » Volume 13
Venous Thromboembolism Risk and Thromboprophylaxis Assessment in Surgical Patients Based on Caprini Risk Assessment Model
Authors Tadesse TA , Kedir HM , Fentie AM, Abiye AA
Received 22 July 2020
Accepted for publication 6 October 2020
Published 10 November 2020 Volume 2020:13 Pages 2545—2552
DOI https://doi.org/10.2147/RMHP.S272852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Marco Carotenuto
Tamrat Assefa Tadesse, Hanan Muzeyin Kedir, Atalay Mulu Fentie, Alfoalem Araba Abiye
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Tamrat Assefa Tadesse
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, P.O. Box: 9086, Addis Ababa, Ethiopia
Email [email protected]
Purpose: Venous thromboembolism (VTE) is the most common preventable cause of hospitalization-associated mortality. In the absence of optimal prophylaxis and depending on the type of surgery and patient-related factors, the risk of developing VTE increases by 10% to 50%. We aimed to assess VTE risk and thromboprophylaxis among surgical patients hospitalized at surgical wards of Tikur Anbessa Specialized Hospital (TASH). Addis Ababa, Ethiopia.
Materials and Methods: A retrospective cross-sectional study was conducted from September 1, 2018 to February 28, 2019. Data were collected using a pretested observational checklist which is prepared based on the VTE Caprini risk assessment model. Then, the collected data were checked for completeness and finally entered and analyzed using Statistical Package for Social Sciences (SPSS) version 25.
Results: Out of 155 admitted patients, almost equal numbers of males (49.68%) and females (50.32%) participated in the study with a mean age of 41.87± 16.84 and an age range of 13 to 89 years. Undergoing major surgery, resting in bed for more than 3 days and having acute infections (including pneumonia) were the most frequently seen VTE risk factors. Most of the study participants (135, 87.10%) were at risk of developing VTE (> 1 Caprini risk score), and 47.11% were in the highest risk category (≥ 5 Caprini score). The maximum and minimum total risk scores were 19 and 1, respectively with a mean score of 4.53± 2.31. Among patients who were at risk of developing VTE and eligible for thromboprophylaxis, only 17.78% received thromboprophylaxis and two ineligible patients received prophylaxis. Parental unfractionated heparin twice or three times per day was the most widely used thromboprophylaxis regimen. A total of 29 (18.71%) patients had one or more contraindication(s) for thromboprophylaxis and three of them took prophylaxis despite the contraindications. Only 3 (1.93%) patients admitted to surgical wards developed VTE during hospitalization.
Conclusion: As per the Caprini risk assessment model, the majority of surgical patients treated at TASH were at risk of developing VTE. However, thromboprophylaxis was underutilized. The incidence of VTE was 1.93% in our study.
Keywords: VTE risk, Caprini risk score, thromboprophylaxis, surgical patients, Tikur Anbessa Specialized Hospital, Ethiopia
Introduction
Venous thromboembolism (VTE), which consists of deep venous thrombosis (DVT) and its sequela pulmonary embolism (PE), are the prominent causes of mortality following surgical procedures.1,2 In the United States, an estimated 350,000–900,000 people develop VTE, of whom approximately 100,000 die. In addition, 30–50% of people with lower-extremity DVT develop postthrombotic syndrome. Re-occurrence of VTE is seen in 10–30% of individuals who survive the first occurrence of VTE within 5 years.3 In developing countries of Africa, VTE is a serious issue which was indicated by a recent systematic review study. The prevalence of DVT varied between 2.4% and 9.6% in postoperative patients and the prevalence of VTE and associated mortality were high following surgery, and in pregnant and postpartum women in Africa.4
In a multinational cross-sectional study called the ENDORSE Study (2008), out of 30,827 (45%) surgical patients enrolled in the study, 64·4% were judged to be at risk for VTE.5 In patients undergoing major surgery, the risk of developing VTE increases by 30% when there is an absence of optimal prophylaxis, and the risk associated with VTE in general surgery patients varies between 10 and 50% depending on the type of surgery and patient risk factors.6 Although most surgical procedures carry some risk for VTE, this risk varies considerably across surgical procedures and among individual patients undergoing surgery. Surgical procedures carrying the highest risk of developing postoperative VTE include hip and knee arthroplasty, invasive neurosurgical procedures, and major vascular procedures. However, surgical procedures like laparoscopic cholecystectomy, appendectomy, transurethral prostatectomy and mastectomy have the lowest risk of developing DVT.7
Both pharmacological and non-pharmacological methods are utilized in preventing the consequences associated with VTE.8 Thromboprophylaxis with unfractionated heparin (UFH 5000 or 7500 units subcutaneously every 8 hours)9 low molecular weight heparin (eg, 30 mg subcutaneously of enoxaparin)10 or Vitamin K antagonist (VKAs) (Warfarin should be monitored so that the dose can be titrated to achieve an International Normalized Ratio of 2–3)11,12 are commonly used and have well-established effectiveness. The American College of Chest Physician (ACCP) 2016 guidelines recommends UFH for general, vascular, gynecologic, and urologic surgeries.13
Based on current adult literature, direct oral anticoagulants (DOACs) are also used for treatment and prevention of DVT and PE.14 DOACs are categorized into two main classes: oral direct factor Xa inhibitors (ie, rivaroxaban, apixaban, edoxaban, and betrixaban) and direct thrombin inhibitors (ie, dabigatran).15 Mechanical methods serve to prevent venous stagnation in the lower limbs by promoting venous outflow and include elastic compression stockings and various intermittent compression devices. All surgical and trauma patients should be assessed as soon as possible after admission to the hospital to identify the risk of VTE and bleeding.16
Caprini risk assessment model (RAM) is the widely used tool to stratify patients at a different level of VTE risks based on the risk factors that exist (risk assessment points) in hospitalized patients. Zero to 1 point indicates the patient has very low VTE risk and early and frequent ambulation is recommended. When the point is 2, it means that the patient has moderate VTE risk and mechanical prophylaxis is enough. High VTE risk and with points between 3 and 4 suggest initiation of pharmacological prophylaxis is necessary. A combination of mechanical and pharmacological prophylaxis is required for patients with the highest VTE risk and low bleeding risk with points 5 and more score.17
All patients are not candidates for VTE prophylaxis (both mechanical and pharmacological) and all prophylactic agents and techniques are not applicable in all patients.18 Patients with active bleeding (gastrointestinal bleeding, cerebral hemorrhage, retroperitoneal bleeding), bleeding risk, and thrombocytopenia are some of the reasons that exclude patients from being a candidate to pharmacological prophylaxis.19
Apart from the contraindicated cases, proper risk stratification followed by thromboprophylaxis is needed for successful prevention and treatment of VTE in the candidate patients. In developing countries like Ethiopia, the service of pharmacological prophylaxis is greatly compromised and patients’ quality of life is pledged to morbidity and mortality.20 Therefore, the current study assessed VTE risks and the appropriateness of thromboprophylaxis in surgical patients admitted to TASH. The rational of this study was to characterize patients’ clinical data related to VTE and to identify associated practice gaps. When it is possible to anticipate the occurrences of VTE associated in surgery patients, it would be rational to develop strategies to tackle possible events by improving VTE risk assessment practice and optimize thromboprophylaxis prescribing pattern in this patient population.
Materials and Methods
Study Area
The study was carried out at Tikur Anbessa Specialized Hospital (TASH, Addis Ababa, Ethiopia). It is one of the oldest and largest teaching specialized hospitals owned by Addis Ababa University. The hospital has about 700 beds and serves approximately 500,000 patients per year in its 20 outpatient specialty clinics, inpatient and emergency departments. A total of 150 beds are allocated for adult surgical patients.
Study Design and Period
A retrospective cross sectional study involving patients’ chart review was conducted among surgical patients admitted to TASH from September 1, 2018 to February 28, 2019.
Inclusion and Exclusion Criteria
All patients (≥ 13 years old) who were admitted to adult surgical wards of the hospital from September 1, 2018 to February 28, 2019 were included in the study. However, patients admitted with established VTE and on treatment, those who stayed in the hospital only for 1 day or who were admitted to surgical pediatric wards were excluded from our study.
Sample Size and Sampling Method
We have included all surgically operated patients for a 6-month study period that fulfilled inclusion criteria. Accordingly, a total of 155 patients who fulfilled inclusion criteria admitted to surgical wards of TASH during the 6-month admission period were included for analysis.
Data Collection, Management, Quality Assurance and Analysis
A structured instrument for data collection which was developed from different literature and guidelines16,17,21 was used to collect all necessary data from patients’ charts. The instrument was specifically designed to capture sociodemographic (age, sex) and surgery-related information (preoperative hospital stay, site of surgery, duration of surgery, surgery type, wound class, hospital stay after surgery), VTE risk assessment, contraindications to VTE prophylaxis, prophylaxis provided and VTE-related outcomes. The VTE risk assessment tool was taken from Caprini RAM.13 Patients’ data was collected from their admission to discharge dates. VTE events were identified as recorded by attending physician on medical charts of patients. In the study setting, VTE was diagnosed clinically and sometimes by Doppler studies. The data was collected by two pharmacists after training was given for 1 day on how to collect the required information from patients’ charts. Pre-test was done on 5% of the study population before going to the actual data collection for checking its clarity, simplicity, understandability and necessary modification were made to the data collection tool. Data was checked for its completeness. Then it was entered and analyzed using SPSS version 25. Descriptive statistics were used to summarize the data.
Ethical Approval
Ethical clearance was obtained from the ethical review board of the School of Pharmacy, College of Health Sciences, Addis Ababa University with Ref No: ERB/SOP/27/10/2018 and permission to access patient charts was obtained from the hospital clinical service director and surgical department. For the purpose of confidentiality, patients’ names were not used at the time of data collection; instead a specific identification number was given for each patient. All other personal and health information were de-identified and kept separately, so every effort was made to maintain confidentiality throughout the study period and afterwards. Besides, information obtained in the course of study was only handled by the research team, and data are analyzed in aggregates.
Results
Sociodemographic and Clinical Characteristics
Out of 155 patients, almost equal numbers of males (50.32%) and females (49.68%) participated in this study with a mean age of 41.87±16.84 and an age range of 13 to 89 years. The maximum preoperative hospital stay was 37 days with a median of 11 days and they stayed in surgical wards from 2 to 96 days with a mean of 14.39±12.36 days. Urologic procedure (37, 23.87%) was the leading procedure followed by orthopedic surgery (35, 22.58%) and surgery for gynecological cancer (27, 17.42%). The majority of wound categories were clean (85.12%) and 83.9% of the surgical procedures were elective. The mean duration of operation was 2.34 ± 1.12 hours with a median of 2 hours (Table 1).
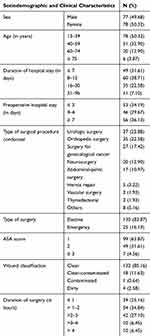 |
Table 1 Sociodemographic and Clinical Characteristics of Patients Admitted to Surgical Wards of TASH |
VTE Risk Factors
Acute infection including pneumonia (10.3%), undergoing major surgical procedures (65.81%), and resting on the bed for more than 3 days (42.58%) were the most frequently seen VTE risk factors in this study (Table 2).
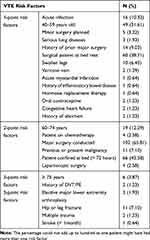 |
Table 2 VTE Risk Characteristics of Patients Admitted to Surgical Wards of TASH Stratified by Caprini RAM Risk Factors |
VTE Risk Stratification and Thromboprophylaxis Status
Based on Caprini RAM, 87.10% of study participants were at risk of developing VTE (>1 Caprini risk score). Nearly half of the patients (47.09%) were in the highest risk category for VTE development and a few were in the lower risk category. The maximum and minimum total risk scores were 18 and 0, respectively with a mean score ± SD of 4.53 ± 2.37 (Table 3).
 |
Table 3 VTE Risk Stratification, Thromboprophylaxis and VTE Outcomes in Patients Admitted to Surgical Wards of TASH |
Out of 135 (87.10%) subjects who were at risk of developing VTE, parenteral thromboprophylaxes were given only for 24 (17.78%) patients (Table 3). The remaining 111 (82.22%) patients who were at risk of developing VTE, did not receive prophylaxis which may be due to ineligibility and/or contraindication, fear of risk of bleeding and failure of prescribing them even for legible patients by prescribers. Unfractionated heparin two or three times per day was the most widely used thromboprophylaxis regimen in the studied population. All thromboprophylaxis were provided 6 hours before surgery and resumed at least after 12 hours if they were continued (Table 4). Pharmacological prophylaxis was continued until mobility returned to an anticipated or clinically acceptable level or when the patient was discharged from hospital whichever was sooner in the study setting.
 |
Table 4 Regimen Used for VTE Prophylaxis of Patients Admitted to Surgical Wards of TASH (N=135) |
Contraindications to Pharmacological Prophylaxis
In this study, 29 (18.71%) patients had one or more contraindication(s) to thromboprophylaxis with 9.68% and 9.03% with absolute and relative contraindications, respectively, as shown in detail in Table 5. The listed contraindications to prophylaxis were related to risk of bleeding.
 |
Table 5 Contraindication to Thromboprophylaxis of Patients Admitted to Surgical Wards of TASH (N=155) |
Thromboprophylaxis Appropriateness and VTE Outcomes
Thromboprophylaxis was given inappropriately for two patients despite the fact that they did not fulfill the criteria for prophylaxis, ie, they were at low risk of developing VTE (Table 3). Moreover, two and one patients with absolute and relative contraindications received prophylaxis without considering the harm, respectively. In this study, 3 (1.93%) of the patients admitted to surgical wards developed VTE during their stay in hospital and all of them were from high and highest risk categories (Table 3) while one patient had a history of VTE. Among three patients who developed VTE, two of them did not receive thromboprophylaxis and only one received it. All of them received treatment regimen for VTE.
Discussion
In this study, the most common VTE risk factors were being in the age range of 40 to 59 years (31.61%), having acute infections (10.32%), being bed ridden or immobility (38.71%), undergoing major surgery (65.81%), and having a longer hospital stay (>3 days) (42.58%) (Table 2). Pannucci et al22 documented a different prevalence of individual VTE risk factors based on Caprini RAM in patients undergoing surgery.22 In Panucci et al, at one point Caprini risk factor patients between 41 and 49 years accounted for 54.2% but in our study it was only 31.61%, patients undergoing major surgery comprised 13.6% in Panucci et al study while in ours it was 65.81%. With respect to 3-point risk factors, >75 years accounted for 4.6% in the Panucci et al study but 3.87% in our study, a history of DVT/PE was 3.4% in the Panucci et al study and 1.23% in our study. Also, the Panucci et al study included many other 3-point risk factors which are not mentioned in our study because of lack of data for such variables.
The observed inconsistency could be due to differences in sample size, population of studies and clinical practice among the two settings. Immobility of more than 3 days and acute infection were also reported as the most common risk factors for VTE in surgical patients from an Asian hospital study.23 In addition, many patients who underwent major surgery and were confined to bed for more than 3 days in our study put them at moderate risk of developing VTE which is supported by a systematic review done by Ahmad and Clayburgh.24 Moreover, patient factors that carry greater risks for thrombosis (3- or 5-point risk factors) including age ≥ 75 years, history of thrombosis, elective major lower extremity surgery arthroplasty, hip or leg fracture, multiple trauma were common in our study and all needed pharmacological prophylaxis according to the 2019 American Society of Hematology (ASH) guidelines recommendation.7
The median VTE Caprini risk score (4) in our study was lower than that reported by the VTEPS study in 2005 and 2010 (5 and 6) using the same model.22 The difference might be explained by the small sample size in our study, data collection method and study population difference. Just over a quarter (26.45%) and close to half (47.09%) of patients were classified as high risk (Caprini scores of 3–4) and highest risk (Caprini scores ≥5), respectively. Tan and Tan23 also reported an almost similar percentage of patients with VTE highest risk score (44.71%). However, a higher percentage (36.58%) was documented for high risk category in their study.23
In our study, thromboprophylaxis was provided only for 17.78% patients among the eligible participants. This result is more than three times lower than that reported by Fang et al25 in the study of VTE prophylaxis in spinal fusion surgery.25 Furthermore, it was lower when compared with ASH and ACCP guidelines recommendations.7,13 In our study, 100%, 85.36%, 78.08% of patients did not receive prophylaxis in the moderate, high and highest risk groups, respectively (Table 3). This indicates provision of VTE prophylaxis was higher than in a report from surgical patients in Asian hospital except for the highest risk groups.22 In general, underutilization of thromboprophylaxis in our setting may be reasoned out by perception of the low incidence of DVT and PE in these subjects, failure to recognize high risk patients, unfamiliarity with published recommendations, concerns regarding complications from anticoagulation (ie, bleeding) especially in surgical patients postoperatively.
In the present study, the incidence of VTE events was 1.93%. It was higher than findings of other studies done elsewhere in surgical patients which reported 0.89%,23 0.45%,25 0.2%,26 and 0.8%.27 Different reasons for high incidence in our study hospital could be failure to assess risk factors, underutilization of thromboprophylaxis for patients in need, fear of bleeding risk upon provision of prophylaxis. However, higher VTE incidence rates (8.4%), were reported in major thoracic surgery in Chinese patients.28
Since the data was collected through patient chart review, other undocumented VTE risk factors may exist and which we could not assess in our study. Furthermore, we have not assessed the timing of prophylaxis as we could not find the exact information on this from patients’ charts.
While reviewing patients’ charts, we faced continuous challenges to collect necessary information due to poor organization in auditing patients’ history chronologically, unreadable physicians’ handwriting and absence of a large number of charts from the medical room which might have important information. Alternative VTE prevention nonpharmacological options (advising patients on the importance of leg elevation, early ambulation) were not assessed in this study due to lack of documentation on patient charts. This might affect our results like risk assessment and stratification; and prophylaxis given to the patients.
Conclusions
In this study, the majority of patients were at risk of developing VTE, but there was underutilization of thromboprophylaxis among surgical patients in TASH. Despite fewer patients receiving thromboprophylaxis among the eligible patients, there were also inappropriate uses of thromboprophylaxis. Appropriate VTE risk stratification and utilization of prophylaxis for surgical patients lead to a better VTE prevention. Hence, a concerted effort must be made to improve utilization of thromboprophylaxis to prevent VTE and there is a need for implementation of existing evidence-based guidelines proposed by ACCP and TASH.
Abbreviations
ACCP, American College of Chest Physicians; ASH, American Society of Hematology; DVT, deep vein thrombosis; DOACs, direct oral anticoagulants; PE, pulmonary embolism; RAM, risk assessment model; SPSS, Statistical Package for Social Sciences; TASH, Tikur Anbessa Specialized Hospital; UFH, unfractionated heparin; VTE, venous thromboembolism; VKAs, vitamin K antagonists.
Data Sharing Statement
All data used and/or analyzed during this study are included in this article and are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
Ethical clearance was obtained from the Ethical Review Board of the School of Pharmacy, College of Health Sciences Addis Ababa University, Addis Ababa, Ethiopia and then permission was obtained from the hospital to conduct the study. Information obtained from the data collected during the study was only handled by the research team. Informed consent was waived as the study was conducted retrospectively and also we did not encounter the study participants directly. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors acknowledge Tikur Anbessa Specialized Hospital for allowing us to conduct this research. Furthermore, we would like to thank the study participants and data collectors and hospital record room staff for their time and facilitation of the data collection process.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We have not received funding for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maynard G. Preventing Hospital-Associated Venous Thromboembolism: A Guide for Effective Quality Improvement.
2. Ali N, Nawaz A, Junaid M, Kazi M, Akhtar S. Venous thromboembolism—incidence of deep venous thrombosis and pulmonary embolism in patients with head and neck cancer: a tertiary care experience in Pakistan. Int Arch Otorhinolaryngol. 2015;19:200–204. doi:10.1055/s-0035-1549153
3. Streiff MB, Brady PJ, Grant AM, Grosse SD, Wong B, Popovic T. CDC grand rounds: preventing hospital-associated venous thromboembolism. Morb Mortal Wkly Rep. 2014;63:190–193.
4. Danwang C, Temgoua MN, Agbor VN, Tankeu AT, Noubiap JJ. Epidemiology of venous thromboembolism in Africa: a systematic review. J Thromb Haemost. 2017;15:1770–1781. doi:10.1111/jth.13769
5. Cohen AT, Tapson VF, Bergmann J-F, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–394. doi:10.1016/S0140-6736(08)60202-0
6. White RH, Henderson MC. Risk factors for venous thromboembolism after total hip and knee replacement surgery. Curr Opin Pulm Med. 2002;8:365–371. doi:10.1097/00063198-200209000-00004
7. Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3:3898–3944.
8. Spyropoulos AC, Raskob GE. New paradigms in venous thromboprophylaxis of medically ill patients. Thromb Haemost. 2017;117:1662–1670. doi:10.1160/TH17-03-0168
9. Beall J, Woodruff A, Hempel C, Wovkulich M, Zammit K. Efficacy and safety of high-dose subcutaneous unfractionated heparin prophylaxis for the prevention of venous thromboembolism in obese hospitalized patients. Hosp Pharm. 2016;51:376–381. doi:10.1310/hpj5105-376
10. Minze MG, Kwee -Y-Y, Hall RGH. Low-molecular-weight heparin prophylaxis dosing: is weight an issue? J Pharm Technol. 2016;32:75–80. doi:10.1177/8755122515617200
11. Al Saleh AS, Berrigan P, Anderson D, Shivakumar S. Direct oral anticoagulants and vitamin K antagonists for treatment of deep venous thrombosis and pulmonary embolism in the outpatient setting: comparative economic evaluation. CJHP. 2017;70:188–199. doi:10.4212/cjhp.v70i3.1658
12. O’Donnell M, Weitz JI. Thromboprophylaxis in surgical patients. Can J Surg. 2003;46:129–135.
13. Kearon C, Akl EA, Omelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. CHEST. 2016;149:315–352. doi:10.1016/j.chest.2015.11.026
14. McMillan KN, Ozment CP Coagulation disorders in congenital heart disease: 14.
15. Chen A, Stecker E, A Warden B. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc. 2020;9:e017559. doi:10.1161/JAHA.120.017559
16. National Insititute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism | guidance | NICE; 2019. Available from: https://www.nice.org.uk/guidance/ng89.
17. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3–S10. doi:10.1016/j.amjsurg.2009.10.006
18. Yhim H-Y, Lee J, Lee JY, Lee J-O, Bang S-M. Pharmacological thromboprophylaxis and its impact on venous thromboembolism following total knee and hip arthroplasty in Korea: a nationwide population-based study. PLoS One. 2017;12:e0178214. doi:10.1371/journal.pone.0178214
19. Yamamura H, Morioka T, Yamamoto T, Kaneda K, Mizobata Y. Spontaneous retroperitoneal bleeding: a case series. BMC Res Notes. 2014;7:659. doi:10.1186/1756-0500-7-659
20. Chu -C-C, Haga H. Venous thromboembolism associated with lower limb fractures after trauma: dilemma and management. J Orthop Sci. 2015;20:364–372. doi:10.1007/s00776-014-0690-4
21. Gebremedhin A. FINAL version of Venous thromboembolism Prophylaxis and Treatment Guidelines.pdf. 1st ed. 2014 Addis Ababa University College of Health Sciences School of Medicine Tiku Anbessa Specialized Hospital.
22. Pannucci CJ, Barta RJ, Portschy PR, et al. Assessment of postoperative venous thromboembolism risk in plastic surgery patients using the 2005 and 2010 Caprini risk score. Plast Reconstr Surg. 2012;130:343–353. doi:10.1097/PRS.0b013e3182589e49
23. Tan LH, Tan SC. Venous thromboembolism prophylaxis for surgical patients in an Asian hospital. ANZ J Surg. 2004;74:455–459. doi:10.1111/j.1445-1433.2004.03025.x
24. Ahmad FI, Clayburgh DR. Venous thromboembolism in head and neck cancer surgery. Cancers Head Neck. 2016;1:13. doi:10.1186/s41199-016-0014-9
25. Fang MC, Maselli J, Lurie JD, Lindenauer PK, Pekow PS, Auerbach AD. Use and outcomes of venous thromboembolism prophylaxis after spinal fusion surgery: venous thromboembolism after spinal surgery. J Thromb Haemost. 2011;9:1318–1325. doi:10.1111/j.1538-7836.2011.04326.x
26. Assareh H, Chen J, Ou L, Hollis SJ, Hillman K, Flabouris A. Rate of venous thromboembolism among surgical patients in Australian hospitals: a multicentre retrospective cohort study. BMJ Open. 2014;4:e005502. doi:10.1136/bmjopen-2014-005502
27. Nelson RE, Grosse SD, Waitzman NJ, et al. Using multiple sources of data for surveillance of postoperative venous thromboembolism among surgical patients treated in Department of Veterans Affairs hospitals, 2005–2010. Thromb Res. 2015;135:636–642. doi:10.1016/j.thromres.2015.01.026
28. Tian B, Li H, Cui S, Song C, Li T, Hu B. A novel risk assessment model for venous thromboembolism after major thoracic surgery: a Chinese single-center study. J Thorac Dis. 2019;11:1903–1910. doi:10.21037/jtd.2019.05.11
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
