Back to Journals » International Journal of General Medicine » Volume 14
Venous Thromboembolism in ICU Patients with Intracerebral Hemorrhage: Risk Factors and the Prognosis After Anticoagulation Therapy
Authors Chu Q, Liao L, Wei W, Ye Z, Zeng L, Qin C, Tang Y
Received 3 July 2021
Accepted for publication 26 August 2021
Published 8 September 2021 Volume 2021:14 Pages 5397—5404
DOI https://doi.org/10.2147/IJGM.S327676
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Quanhong Chu,1 Lin Liao,2 Wenxin Wei,1 Ziming Ye,1 Li Zeng,1 Chao Qin,1 Yanyan Tang1
1Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 2Department of Clinical Laboratory, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
Correspondence: Yanyan Tang
Department of Neurology, The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road, Nanning, 530021, Guangxi, People’s Republic of China
Email [email protected]
Purpose: Venous thromboembolism (VTE) is a common complication of intracerebral hemorrhage (ICH) patients in intensive care unit (ICU), but anticoagulation therapy of ICH patients with VTE remains controversial. We aim to explore the risk factors and prognosis of anticoagulation therapy in ICH patients with VTE.
Patients and Methods: Medical records of ICH patients were collected from the Medical Information Mart for Intensive Care III (MIMIC-III version 1.4) database. The risk factors and prognosis of anticoagulation therapy in ICH patients with VTE were assessed by multivariable logistic regression analysis and Kaplan–Meier survival analysis, respectively.
Results: A total of 848 ICH patients were included in our study, of whom 69 ICH patients with VTE were screened, including 58 patients with deep vein thrombosis (DVT), 12 patients with pulmonary embolism (PE), and 1 patient with DVT and PE. In the multivariable logistic regression analysis, malignancy (odds ratio (OR): 4.262, 95% confidence interval (CI): 2.263– 8.027, P=0.000), pulmonary circulation disease (OR: 28.717, 95% CI: 9.566– 86.208, P=0.000), coagulopathy (OR: 2.453, 95% CI: 1.098– 5.483, P=0.029), age > 60 years old (OR: 2.138, 95% CI: 1.087– 4.207, P=0.028) and hospitalization time > 16 days (OR: 2.548, 95% CI: 1.381– 4.701, P=0.003) were independent risk factors for VTE in ICH patients. Kaplan–Meier survival analysis and log-rank test found that, compared to non-anticoagulation group, anticoagulation group had higher cumulative survival rates during hospitalization, 28-day, 3-month, 1-year, and 4-year after admission, respectively.
Conclusion: Malignancy, pulmonary circulation disease, coagulopathy, age > 60 years old and hospitalization time > 16 days were independent risk factors for VTE in ICH patients, and anticoagulation therapy for VTE in ICH patients may be safe and effective. These findings need to be verified by more high-quality and well-designed randomized controlled trials.
Keywords: intracerebral hemorrhage, venous thromboembolism, risk factors, anticoagulation, prognosis
Introduction
Stroke is the primary cause of mortality and disability worldwide and the overall burden of stroke remains very high.1 Intracerebral hemorrhage (ICH), as a severe subtype, accounts for 23.8% of patients with stroke in China.2 Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common complication in patients with ICH.3 Furthermore, the risk of VTE after hemorrhagic stroke was reported to be dramatically higher than that after ischemic stroke.4,5 Additionally, in-hospital VTE was associated with poor outcome in patients with ICH.6 Therefore, VTE in ICH patients causes large socio-economic burden.
Due to the severity of VTE, it is important to explore and prevent the risk factors of VTE in patients with ICH. According to the previous studies, risk factors identified between VTE and ICH include female gender, black/African American race, infection, National Institute of Health Stroke Scale (NIHSS) score ≥12, elevated admission D-dimer levels, external ventricular drainage, a prior history of VTE, intubation and presence of intraventricular hemorrhage (IVH).7–13 Although our knowledge about risk factors for VTE after ICH has increased in the last few years, part of patients with VTE after ICH do not have identifiable risk factor.14 Therefore, it is necessary to further explore the risk factors for VTE in ICH patients.
On the other hand, due to the occurrence of VTE after ICH, most patients still have a poor prognosis.6 Therefore, many studies focus on the prevention of VTE in ICH patients. Prophylactic approaches, such as intermittent pneumatic compression (IPC) and heparin, are recommended for the VTE prevention after ICH.15,16 Meanwhile, according to American Heart Association/American Stroke Association (AHA/ASA) and Chinese Stroke Association guidelines for ICH, systemic coagulation or inferior vena cava (IVC) filter placement is recommended in ICH patients with symptomatic DVT or PE (Class IIa; Level of Evidence C).17,18 However, European Stroke Organization (ESO) guidelines do not have explicit recommendations for VTE after ICH.19 Furthermore, there are little data on the VTE treatment in ICH patients.20 Anticoagulation is the primary treatment for VTE. IVC filter is only an option when patients have absolute contraindications of anticoagulation, such as active bleeding.21 However, for VTE in ICH patients, anticoagulation therapy presents a therapeutic dilemma. We need to consider not only the risk of recurrent fatal VTE in the absence of anticoagulation treatment but also the risk of hematoma expansion if treating patients with anticoagulation therapy. In addition, because the evidence recommendation for anticoagulation therapy is low, current guidelines are not widely accepted in clinical practice, and most of treatment decisions regarding anticoagulation are empirical.
Therefore, the objectives of this study were (1) to explore the risk factors of VTE in ICH patients, and (2) to study the prognosis of anticoagulation therapy for VTE in ICH patients.
Patients and Methods
Database
We conducted a retrospective cohort study on a large US-based database called the Medical Information Mart for Intensive Care III (MIMIC-III version 1.4). Access to the MIMIC-III database was approved by the institutional review boards of both the Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology Affiliates after finishing the required training course (certification number 23307636). One author obtained access to the database and was responsible for data extraction. No informed consent was required because the private information of patients was deidentified in the database.
Study Design and Participants
Using the MIMIC-III database, we identified all adult patients (>18 years old) with ICH according to the International Classification of Diseases, Ninth edition (ICD-9) code 431 between June 2001 and October 2012. We included only patients who survived day 2 of hospitalization. We excluded patients who were younger than 18 years old or who survived to less than day 2 of hospitalization.
Included patients were classified as the VTE group, while the rest of the patients comprised the non-VTE group. VTE included DVT detected with compressible ultrasound or PE diagnosed with CT pulmonary angiography or ventilation-perfusion lung scanning. DVT and PE were identified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).22
Variable Extraction
Baseline characteristics were extracted with the Structured Query language (SOL), including gender, age, comorbidities (hypertension, hyperlipidemia, diabetes, congestive heart failure, atrial fibrillation, COPD, respiratory failure, chronic kidney injury (48 h), malignancy, ARDS, pneumonia, renal failure, liver failure, hypothyroidism, rheumatoid arthritis, coagulopathy, deficiency anemia, electrolyte disturbances), ICU scale (SOFA, LODS, GCS, MELD, MELD-initial), mechanical ventilation and coagulation function test.
Study Outcomes
The outcomes included in-hospital outcomes, 28-day, 3-month, 1-year and 4-year time-to-event outcomes after admission. The primary outcome was in-hospital mortality. The secondary outcomes were 28-day, 3-month, 1-year and 4-year mortality after admission. Out-of-hospital mortality data were acquired from the Social Security Death Registry.
Statistical Analysis
Values were expressed as median (interquartile range) for continuous variables with abnormal distribution, and categorical variables were presented as number (percent). Comparisons between ICH patients with VTE and without VTE were evaluated using the Mann–Whitney U-test for continuous variables with abnormal distribution, and the χ2 test or Fisher’s exact test for categorical variables, as appropriate. Baseline variables that were considered clinically relevant or that showed a univariate relationship with VTE (P<0.05), including congestive heart failure, malignancy, renal failure, coagulopathy, electrolyte disturbances, pulmonary circulation disease, respiratory failure, age >60 years old, hospitalization time >16 days, LODS, MELD, MELD initial, PT-max, INR-max and APTT-max were entered into a multivariate logistic regression model as covariates. The risk factors of VTE in ICH patients were assessed by multivariable logistic regression analysis. Kaplan–Meier (K-M) survival analysis and log-rank test were used to compare the cumulative survival rate during hospitalization, 28-day, 3-month, 1-year and 4-year after admission between the heparin anticoagulation group and the non-heparin anticoagulation group.
All statistical analyses were performed using SPSS (version 22.0), and P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 848 ICH patients were included in our study. Table 1 presents the baseline characteristics of the study subjects. Among the study cohort, 779 patients (91.8%) were ICH patients without VTE. Sixty-nine patients (8.2%) were ICH patients with VTE, including 58 DVT patients, 12 PE patients, and 1 patient with DVT and PE. Compared with ICH patients without VTE, ICH patients with VTE had higher incidence of congestive heart failure (24/779, 3.10% vs 6/69, 8.70%), renal failure (56/779, 7.20% vs 10/69, 14.50%), malignancy (101/779, 13.00% vs 22/69, 31.90%), respiratory failure (243/779, 31.20% vs 35/69, 50.70%), coagulopathy (60/779, 7.70% vs 14/69, 20.30%), electrolyte disturbances (209/779, 26.80% vs 28/69, 40.60%), age >60 years old (517/779, 66.40% vs 54/69, 78.30%), pulmonary circulation disease (8/779, 1.00% vs 13/69, 18.80%), hospitalization time >16 days (211/779, 27.10% vs 33/69, 47.80%), LODS (3.0, 2.0–5.0 vs 4.0, 2.0–6.0), MELD (9.0, 7.0–14.0 vs 11.0, 7.0–18.0), MELD initial (9.0, 7.0–13.0 vs 11.0, 7.0–17.0), PT-max (13.40, 12.70–15.10 vs 14.70, 13.00–17.95), INR-max (1.20, 1.10–1.40 vs 1.30, 1.10–1.80), APTT-max (27.30, 24.60–31.10 vs 30.50, 26.55–35.75) (all P < 0.05).
 |
Table 1 Patient Characteristics According to the Presence of Venous Thromboembolism (VTE) |
Multivariable Logistic Regression Analysis
The results of multivariable logistic regression analysis are presented in Table 2. Multivariable logistic regression analysis showed that malignancy (OR: 4.262, 95% CI: 2.263–8.027, P=0.000), pulmonary circulation disease (OR: 28.717, 95% CI: 9.566–86.208, P=0.000), coagulopathy (OR: 2.453, 95% CI: 1.098–5.483, P=0.029), age >60 years old (OR: 2.138, 95% CI: 1.087–4.207, P=0.028) and hospitalization time >16 days (OR: 2.548, 95% CI: 1.381–4.701, P=0.003) were independent risk factors for VTE in ICH patients.
 |
Table 2 Logistic Regression Analysis of the Risk Factors for VTE in ICH Patients |
ROC Curve Analysis
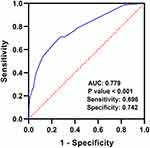
As shown in Figure 1, ROC curve revealed that combined coagulopathy, pulmonary circulation disease, hospitalization time >16 days, age >60 years old and malignancy had predictive power for VTE in ICH patients, with an AUC of 0.779 (95% CI: 0.718–0.839, P < 0.0001, sensitivity 0.696, specificity 0.742).
Outcome
The results of comparison of mortality outcomes between the anticoagulation and non-anticoagulation groups are presented in Table 3. In-hospital mortality, 28-day, 3-month, 1-year and 4-year cumulative mortality rates were 17.86%, 21.43%, 32.14%, 42.86%, 57.14%, respectively, in the anticoagulation group. In-hospital mortality, 28-day, 3-month, 1-year and 4-year cumulative mortality rates were 53.85%, 61.54%, 69.23%, 76.92%, 84.62%, respectively, in the non-anticoagulation group.
 |
Table 3 Comparison of Mortality Outcome Between the Anticoagulation and Non-Anticoagulation Group |
K-M Survival Analysis
As shown in Figure 2A–E, K-M survival analysis and log-rank test showed that the cumulative survival rates during hospitalization (log-rank: χ2=10.69, P=0.0011), 28-day (log-rank: χ2=12.09, P=0.0005), 3-month (log-rank: χ2 =10.66, P=0.0011), 1-year (log-rank: χ2=10.53, P=0.0012), and 4-year after admission (log-rank: χ2=10.23, P=0.0014) in the heparin anticoagulation group were significantly higher than those in the non-heparin anticoagulation group.
Discussion
Our study revealed that malignancy, coagulopathy, age >60 years, hospitalization time >16 days and pulmonary circulation disease were independent risk factors for VTE in ICH patients, and using anticoagulation therapy for VTE in ICH patients may be safe and effective. VTE, including DVT and PE, was a common complication in patients with ICH,3 which was associated with poor prognosis in patients with ICH.6 We should explore the risk factors for VTE in ICH patients because of the high fatality of VTE. Our knowledge of risk factors for VTE in ICH patients has increased in the past few years, such as female gender, black/African American race, infection, National Institute of Health Stroke Scale (NIHSS) score ≥12, elevated admission D-dimer levels, external ventricular drainage, a prior history of VTE, intubation and presence of intraventricular hemorrhage (IVH) were risk factors found at present.7–13 However, some of ICH patients with VTE have no identified risk factors. Our study found that malignancy and coagulopathy were independent risk factors for VTE in ICH patients, which was consistent with the results of previous studies.23,24 Furthermore, our study revealed that age >60 years old was an independent risk factor for VTE in ICH patients. According to a previous study, from the age of 45 years old, the lifetime risk of developing VTE was 8%, which represented that age was a risk factor for VTE.22 In addition, we found that hospitalization time >16 days was an independent risk factor for VTE in ICH patients. Patients with hospitalization time >16 days maybe more likely to have some complications, such as limb paralysis and infection, therefore heightening their susceptibility to VTE. Additionally, we also found pulmonary circulation disease was an independent risk factor for VTE in ICH patients. This was a new risk factor for VTE in ICH patients. Patients with pulmonary circulation disease may be physiologically predisposed to VTE complications.
Due to the high mortality and morbidity of VTE, many studies have focused on the prevention of VTE in ICH patients. At present, prophylactic approaches include anticoagulation and IPC, which are recommended for VTE prevention in ICH patients.15,16,25 However, there are little data on the treatment of VTE in ICH patients.20 Anticoagulation therapy for VTE complications in ICH patients varies widely across different ICH management guidelines.17–19 Moreover, anticoagulation therapy for VTE in ICH patients is not well understood. Anticoagulation is the primary treatment of VTE in ICH patients. Meanwhile, IVC filter placement is indicated in patients who have contraindications of anticoagulation.26,27 However, for VTE in ICH patients, anticoagulation presents a very difficult therapeutic dilemma because it has enormous risk of recurrent fatal VTE in the absence of treatment; additionally, it also has substantial risk of hematoma enlargement if treated with anticoagulants. Furthermore, there is no data from randomized controlled trials to guide the anticoagulation therapy in ICH patients with VTE. Therefore, the decision-making is mainly empirical. Our study found that the cumulative survival rates during hospitalization in the heparin anticoagulation group were significantly higher than those in the non-heparin anticoagulation group through K-M survival analysis and log-rank test. The result suggested that anticoagulation therapy may be significantly associated with in-hospital mortality, implying that we may perform active anticoagulation in ICH patients with VTE. Anticoagulation is a first-line treatment for most VTE patients. Unfortunately, there are currently no clear guidelines for the anticoagulation therapy of VTE in ICH patients. Furthermore, there are few meaningful data to guide decision-making in anticoagulation therapy of VTE in ICH patients, especially in randomized controlled trial. However, our study can provide clinicians with potential evidence of anticoagulation therapy for VTE in ICH patients.
In addition, our study also found that the cumulative survival rates during 28-day, 3-month, 1-year, and 4-year after admission in the heparin anticoagulation group were significantly higher than those in the non-heparin anticoagulation group through K-M survival analysis and log-rank test. The result suggested that anticoagulation therapy may be incomplete, significantly associated with 28-day, 3-month, 1-year, and 4-year mortality after admission, implying that anticoagulation may be beneficial to both short-term and long-term outcomes of VTE in ICH patients. Meanwhile, anticoagulation decision should consider several factors, such as hematoma stability, time from ICH onset, cause of ICH and general patient condition.17,18 However, more high-quality and well-designed randomized controlled trials are needed to verify the association between anticoagulation therapy and in-hospital mortality, 28-day, 3-month, 1-year, and 4-year mortality after admission in ICH patients with VTE. Hopefully, it will provide clinicians with evidence for anticoagulation therapy in ICH patients with VTE.
Our study has several limitations. First, this is a retrospective study, which has selective bias. Therefore, we cannot draw robust conclusions. Second, the MIMIC III database in the present study only includes data on critically ill patients admitted between 2001 and 2012. The definition of VTE has evolved in 2012. Therefore, it is possible that our participants are not exactly meeting the latest definition of VTE. Third, our sample size of ICH patients with VTE is small, and we cannot remove other confounding factors’ disturbance by the Cox regression analysis. In future, we will intend to perform Cox regression analysis to verify the association between anticoagulation therapy and short-term and long-term prognosis through more high-quality and well-designed randomized controlled trials. Fourth, the imaging features of ICH, such as hematoma volume, hematoma location and intraventricular hemorrhage, are not available in MIMIC-III database. In future, if these imaging features of ICH are available, we will add some imaging information to this study and explore the value of these imaging features in ICH patients with VTE.
Conclusion
In conclusion, malignancy, coagulopathy, age >60 years, hospitalization time >16 days and pulmonary circulation disease were independent risk factors for VTE in ICH patients, and anticoagulation therapy for VTE in ICH patients may be safe and effective, but the results need further to be verified in more high-quality and well-designed randomized controlled trials.
Acknowledgment
This study was supported by grants from the National Natural Science Foundation of China (No. 82001252, No. 82060226 and No. 81960220), the Natural Science Foundation of Guangxi (No. 2020JJB140052) and “Medical Excellence Award” funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):439–458. doi:10.1016/S1474-4422(19)30034-1
2. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
3. Otite FO, Khandelwal P, Malik AM, Chaturvedi S, Sacco RL, Romano JG. Ten-year temporal trends in medical complications after acute intracerebral hemorrhage in the United States. Stroke. 2017;48(3):596–603. doi:10.1161/STROKEAHA.116.015746
4. Ji R, Li G, Zhang R, Hou H, Zhao X, Wang Y. Higher risk of deep vein thrombosis after hemorrhagic stroke than after acute ischemic stroke. J Vasc Nurs. 2019;37(1):18–27. doi:10.1016/j.jvn.2018.10.006
5. Skaf E, Stein PD, Beemath A, Sanchez J, Bustamante MA, Olson RE. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. 2005;96(12):1731–1733. doi:10.1016/j.amjcard.2005.07.097
6. Li J, Wang D, Wang W, et al. In-hospital venous thromboembolism is associated with poor outcome in patients with spontaneous intracerebral hemorrhage: a multicenter, prospective study. J Stroke Cerebrovasc Dis. 2020;29(8):104958. doi:10.1016/j.jstrokecerebrovasdis.2020.104958
7. Cheng X, Zhang L, Xie NC, Ma YQ, Lian YJ. High plasma levels of D-dimer are independently associated with a heightened risk of deep vein thrombosis in patients with intracerebral hemorrhage. Mol Neurobiol. 2016;53(8):5671–5678. doi:10.1007/s12035-015-9487-5
8. Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther. 2008;25(9):831–841. doi:10.1007/s12325-008-0092-0
9. Ding D, Sekar P, Moomaw CJ, et al. Venous thromboembolism in patients with spontaneous intracerebral hemorrhage: a multicenter study. Neurosurgery. 2019;84(6):E304–E310. doi:10.1093/neuros/nyy333
10. Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care. 2009;10(1):28–34. doi:10.1007/s12028-008-9134-3
11. Shah JN, Murthy SB, Dlugash R. Venous thromboembolism after intraventricular hemorrhage: results from the CLEAR III trial. Neurosurgery. 2019;84(3):709–716. doi:10.1093/neuros/nyy189
12. Kawase K, Okazaki S, Toyoda K, et al. Sex difference in the prevalence of deep-vein thrombosis in Japanese patients with acute intracerebral hemorrhage. Cerebrovasc Dis. 2009;27(4):313–319. doi:10.1159/000202006
13. Ogata T, Yasaka M, Wakugawa Y, Inoue T, Ibayashi S, Okada Y. Deep venous thrombosis after acute intracerebral hemorrhage. J Neurol Sci. 2008;272(1–2):83–86. doi:10.1016/j.jns.2008.04.032
14. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480–1483. doi:10.1111/jth.13336
15. Pan X, Li J, Xu L, Deng S, Wang Z. Safety of prophylactic heparin in the prevention of venous thromboembolism after spontaneous intracerebral hemorrhage: a meta-analysis. J Neurol Surg a Cent Eur Neurosurg. 2020;81(3):253–260. doi:10.1055/s-0039-3400497
16. Yogendrakumar V, Lun R, Hutton B, Fergusson DA, Dowlatshahi D. Comparing pharmacological venous thromboembolism prophylaxis to intermittent pneumatic compression in acute intracerebral haemorrhage: protocol for a systematic review and network meta-analysis. BMJ Open. 2018;8(11):e024405. doi:10.1136/bmjopen-2018-024405
17. Cao Y, Yu S, Zhang Q, et al. Chinese stroke association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of intracerebral haemorrhage. Stroke Vasc Neurol. 2020;5(4):396–402. doi:10.1136/svn-2020-000433
18. Hemphill JC
19. Steiner T, Salman RA-S, Beer R, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9(7):840–855. doi:10.1111/ijs.12309
20. Kelly J, Hunt BJ, Lewis RR, Rudd A. Anticoagulation or inferior vena cava filter placement for patients with primary intracerebral hemorrhage developing venous thromboembolism? Stroke. 2003;34(12):2999–3005. doi:10.1161/01.STR.0000102561.86835.17
21. Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398(10294):64–77.
22. Bell EJ, Lutsey PL, Basu S, et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129(3):339 e319–326. doi:10.1016/j.amjmed.2015.10.014
23. MacCallum P, Bowles L, Keeling D. Diagnosis and management of heritable thrombophilias. BMJ. 2014;349(7):g4387. doi:10.1136/bmj.g4387
24. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi:10.1182/blood-2013-04-460121
25. Cherian LJ, Smith EE, Schwamm LH, et al. Current practice trends for use of early venous thromboembolism prophylaxis after intracerebral hemorrhage. Neurosurgery. 2018;82(1):85–92. doi:10.1093/neuros/nyx146
26. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388(10063):3060–3073. doi:10.1016/S0140-6736(16)30514-1
27. Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320(15):1583–1594. doi:10.1001/jama.2018.14346
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.