Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Venous Flow Profiles on Perfusion CT are Associated with Futile Recanalization After Thrombectomy
Authors Su M, Chen Z, Chen X, Huang J, Li Z, Zhou Y, Xu G
Received 31 January 2022
Accepted for publication 9 April 2022
Published 29 April 2022 Volume 2022:18 Pages 933—942
DOI https://doi.org/10.2147/NDT.S360626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yuping Ning
Mouxiao Su,1,2 Zhonglun Chen,2 Xinyue Chen,3 Jiaxing Huang,4 Zhaokun Li,2 Ying Zhou,4 Gelin Xu1,5
1The First School of Clinical Medicine, Southern Medical University, Guangzhou, 510000, People’s Republic of China; 2Department of Neurology, School of Medicine, Mianyang Central Hospital, University of Electronic Science and Technology of China, Mianyang, 621000, People’s Republic of China; 3CT Collaboration, Siemens Healthineers, Chengdu, 610000, People’s Republic of China; 4Department of Radiology, School of Medicine, Mianyang Central Hospital, University of Electronic Science and Technology of China, Mianyang, 621000, People’s Republic of China; 5Department of Neurology, Jinling Hospital, Medical School of Nanjing University, Nanjing, 210002, People’s Republic of China
Correspondence: Gelin Xu, The First School of Clinical Medicine, Southern Medical University, Guangzhou, 510000, People’s Republic of China, Tel +86 18951919349, Email [email protected]
Background and Purpose: Robust venous outflow (VO) reflects favourable tissue reperfusion in acute ischaemic stroke (AIS) patients with large vessel occlusion (LVO). We aimed to investigate the association of the venous outflow profile on computed tomographic perfusion (CTP) and futile recanalization in anterior circulation AIS patients with LVO after thrombectomy.
Methods: This was a retrospective study of consecutive AIS patients due to anterior circulation LVO who underwent CTP before thrombectomy. Patients who achieved successful recanalization defined as a modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b or 3 after thrombectomy were included. Based on the venous time–intensity curve of CTP, the peak time of venous outflow (PTV), total venous outflow time (TVT), and difference value of arteriovenous peak time (D-value) were recorded. A modified mRS score of 3– 6 at 3 months was regarded as futile recanalization (FR). Logistic regression analysis was applied to assess risk factors for FR. We used receiver operating characteristic curves (ROCs) to evaluate the predictive value of venous outflow time parameters based on VO for FR.
Results: Eighty patients were included; 35 (43.8%) achieved good functional outcomes, and 45 (56.3%) had unfavourable functional outcomes, that is, FR. Adjusting confounding factors, binary stepwise logistic regression analysis showed that delayed PTV was independently associated with FR (odds ratio, 1.374 [95% CI, 1.093– 1.726], P = 0.007). ROCs indicated that PTV effectively predicted unfavourable outcomes at 3 months (area under the curve (AUC) = 0.729, p< 0.001). The combined model was a powerful predictor of FR with an AUC of 0.824 and a cut-off value of 0.631 (p< 0.001).
Conclusion: Delayed PTV is independently related to FR in anterior circulation AIS patients with LVO achieving successful recanalization after thrombectomy. Our results highlight that the time of VO may be a potential marker for FR.
Keywords: time intensity curve, computed tomographic perfusion, futile recanalization, thrombectomy
Introduction
Endovascular thrombectomy (EVT) has been identified as a vital therapy for large vessel occlusion (LVO) acute ischaemic stroke (AIS) patients in anterior circulation.1 The rate of vessel excellent reperfusion status after thrombectomy was 71–95%.2–4 However, many studies have shown that a considerable proportion of AIS patients (41–55%) failed to achieve clinical independence at 3 months despite successful recanalization; this scenario is known as futile recanalization (FR).2,3,5
The pathophysiology of FR remains unclear. The relationship between collateral and FR has been controversial until now. Arterial collaterals cannot reflect the whole tissue perfusion profile in the infarct area.6 Therefore, prior studies found that the status of arterial collateral on single-phase computed tomographic angiography (sCTA) was not associated with FR.5,6 Multiphase CT angiography (mCTA) may be better in predicting clinical outcome than sCTA.7 Venous blood flow accounts for approximately 70% of the blood circulatory volume inside the cranial cavity. Favourable venous outflow (VO) can reflect excellent perfusion and robust collateral, playing a crucial role in the maintenance of cerebral blood flow after ischaemia.8 The association of cortical venous drainage and successful reperfusion in AIS has been described. The cortical vein opacification score (COVES) was shown to predict outcome after thrombectomy in AIS. A COVES value of 0 predicted a poor outcome.9 Robust VO was associated with good functional outcomes in patients with AIS-LVO treated by endovascular therapy.10 These studies assessed VO based on sCTA, which may result in insufficient imaging. Time-resolved CT angiography can compensate for the defect to some extent.9 Computed tomographic perfusion (CTP) provides time-resolved vessel imaging information, including the arterial, arteriovenous and venous phases. The time-density curve based on three-phase maximum intensity picture (MIP) reconstruction reflects profiles of arterial inflow and venous outflow.
To date, the association between the VO profile of CTP and unfavourable outcomes in AIS patients after successful recanalization has not been described. We retrospectively analysed the venous profile on CTP from a prospective cohort to investigate the relationship of VO characteristics derived from CTP and FR at 3 months in anterior circulation AIS-LVO patients with successful vessel recanalization after endovascular thrombectomy.
Methods
Study Protocol and Subjects
We conducted a retrospective analysis of consecutive patients in our prospective data of AIS patients treated with EVT from January 2020 to July 2021. A total of 108 patients with proximal middle cerebral artery (MCA) occlusion (M1 or M2 segments) or occlusion of the internal carotid artery (ICA) were included and achieved successful vessel recanalization (defined as a modified thrombolysis in cerebral infarction score (mTICI) of 2b or 3 on the final digital subtraction angiography images). Other inclusion criteria were (1) age ≥ 18 years; (2) pretreatment modified Rankin Scale (mRS) score ≤ 2; (3) first stroke; (4) baseline head noncontrast CT (NCCT) and CTP; (5) provision of written informed consent by the patient or legal guardian. Patients with poor CTP images caused by excessive motion and incomplete VO flow curves were excluded. And patients with tumors and kidney dysfunction were excluded. We carried out the study in line with the Declaration of Helsinki and was approved by the ethics committee of Mianyang Central Hospital. Figure 1 displays the patient inclusion process of this study.
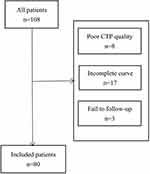 |
Figure 1 Flow chart of the patients included process of the study. |
Demographic characteristics and clinical, laboratory, and radiological data were retrieved from prospective databases, including age, sex, medical history (such as hypertension, diabetes mellitus, and atrial fibrillation), and comorbid conditions (such as renal insufficiency, heart failure, and tumours). Stroke neurologists collected NIHSS (National Institutes of Health Stroke Scale) scores at admission and recorded the characteristics of EVT.
Image Acquisition
All patients were collected prospectively for the study. They all underwent the same radiological examination protocol containing both baseline noncontrast CT (NCCT) and CTP with 24- to 48-hour follow-up NCCT or diffusion-weighted imaging (DWI). Whole-brain perfusion CT was performed with a Dual Source CT scanner (SOMATOM Force CT, Siemens, Germany). The scanner has the spatial resolution of 0.24mm. The scanning protocol was as follows: The acquisition started 4 seconds after an injection of 40 mL of non-ionic iodinated contrast material (Iopromide 370; Bayer Health care, Berlin, Germany) into an antecubital vein at a rate of 5 mL/sec, lasted for 55 seconds, and was followed by a 40 mL saline flush. It consisted of three phases with 29 cycles of scans. The image parameters are listed in Table 1. Perfusion CT data of 1mm slice thickness were postprocessed by using a workstation (syngo.via, Siemens, Germany) operated by a radiologist (JX.H., with more than 4 years of experience in image postprocessing). The deconvolution algorithm was used to generate perfusion maps based on input functions. In our study, we used two input curves: 1) the arterial enhancement time-density curve and 2) the venous enhancement time-density curve.
 |
Table 1 The Image Parameters of Brain CT Perfusion |
Image Processing
The time-intensity curve will be generated automatically for the superior sagittal sinus by the software. Specific time points were then recorded based on this curve. One time point was not related to the contrast in the vein and served as the time for the start of data acquisition. The other time points were the time until the arrival of contrast, the time until peak contrast and the time until the total washout of the contrast in this vein. Subsequently, the time intervals between these specific time points were recorded. See Figure 2 for detailed definitions of the intervals.
Image Analysis
According to the venous outflow duration of the time-intensity curve, the peak time of venous outflow (PTV), total venous outflow time (TVT), and difference value of arteriovenous peak time (D-value) were defined (Figure 2). Arterial collateral vessels were assessed on the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grading system (ASITN/SIR)11 by an experienced neurointerventional neurologist (Z.L.C). Early ischaemic changes were assessed using the Alberta Stroke Program Early CT Score (ASPECTS) applied to perfusion CT source images (CTP-SI)12 by a single neuroradiologist (Y.Z). Symptomatic intracranial haemorrhage (sICH) was determined according to the European Cooperative Acute Stroke Study II (ECAS-S-II) system.13
Outcome Assessment
The functional outcome at 3 months after treatment was obtained by a stroke neurologist or trained staff in accordance with the Modified Rankin Scale (mRS).14
Among patients enrolled in the study who achieved successful recanalization, mRS scores of 3 to 6 at 90 days, indicating a poor outcome, were regarded as FR. Conversely, an mRS score ≤ 2 at 90 days, indicating a good prognosis, was defined as a good outcome.15 In this way, patients were dichotomized into the FR group versus the good outcome group.
Statistical Analysis
Continuous variables are presented as the means (SDs, standard deviations) if normally distributed by kolmogorov–Smirnov test, otherwise, continuous variables are presented by medians (interquartile ranges). Categorical variables are presented as numbers (percentages). Variables were compared between the FR and good outcome groups using the Mann–Whitney U-test, Student’s t-test, the chi-square test or Fisher’s exact test as appropriate. The correlation of continuous variables between the two groups was tested by Pearson test. Bonferroni correction for multiple comparisons was performed for covariate analyses. Binary stepwise logistic regression models were applied to determine the independent factors of FR, and variables with p < 0.05 in univariate analysis were entered into logistic regression as adjusted confounders. The models’ goodness of fit was evaluated by the Hosmer–Lemeshow (HL) test. Receiver operator characteristic (ROC) curves were used to assess the optimal cut-off value of predictors for predicting FR. The ROC of different variables were compared using MedCalc software. All p values were 2 tailed, and a significance level of 0.05 was used. Statistical analyses were computed by SPSS, version 21.0 (IBM, New York, NY) and MedCalc software.
Results
Patient Characteristics
A total of 108 anterior circulation LVO patients undergoing thrombectomy were enrolled. Of these, 80 patients were followed up and analysed in the present study. The other 28 were excluded because of failed CTP and incomplete data. The mean age was 70.74 ± 11.49 years. Thirty-one (38.8%) were female. The median baseline NIHSS score was 16 (interquartile range 11–20), and the median ASPECTS was 8 (interquartile range 7, 9). The mean time from symptom onset to groin puncture (OTP) was 187.4 (61.2) minutes, and the mean time from puncture to recanalization (PTR) was 72.2 (23.1) minutes. Twelve patients (15%) developed sICH. The occlusion sites were as follows: ICA in 23 patients (28.8%), MCA (M1) in 47 patients (58.5%), and MCA (M2) in 10 patients (12.5%). The median PTV was 13.5 (interquartile range 12, 15) seconds. The median TVT was 28.5 (interquartile range 25.5, 34.5) seconds. The median D-value was 6 (interquartile range 4.5, 7.5) seconds. FR occurred in 45 (56.3%) patients, and 35 (43.8%) achieved good functional outcomes. Baseline ASPECTS were significantly correlated with PTV(p=0.047) and TVT (p=0.028). There was no significant difference in the proportions of hypertension, diabetes mellitus or hyperlipidaemia between the FR group and the good outcome group (p > 0.05). The good outcome group tended to be young, but there was no significant difference (p=0.054). More female had FR (p=0.04). The FR group had a higher proportion of atrial fibrillation (AF) (p=0.014) and poor collateral status (p=0.024). No difference was found in baseline blood pressure or subtype of stroke (p>0.05). Lower ASPECTS and higher NIHSS scores on admission were observed in the FR group (p=0.002, p=0.001). FR was not significantly associated with OTP (p= 0.333) or PTR (p=0.716). Compared to the good outcome group, the FR group had longer PTV and TVT (p<0.001, p=0.001) (Table 2). Lower ASPECTS was related to an increased likelihood of FR (p=0.047).
 |
Table 2 Comparison of Baseline Data Between FR Group and Good Outcome Group |
Binary Stepwise Logistic Regression for FR
In the binary stepwise logistic regression model, adjusting potential confounders as follows: sex, baseline NIHSS and ASPECTS score, collateral circulation, occlusion site, TVT, prolonged PTV (OR, 1.374; 95% CI, 1.093–1.726; P=0.007), baseline ASPECTS (OR, 0.677; 95% CI, 0.485–0.947; P=0.023) and baseline NIHSS (OR, 1.113; 95% CI, 1.019—1.215; P=0.018) was independently associated with FR (Hosmer–Lemeshow test χ2=4.676, P=0.792>0.05, Table 3).
 |
Table 3 Binary Stepwise Logistic Regression Analysis for FR in Patients with Successful Recanalization After Thrombectomy |
Predictor Models for FR
ROC analysis revealed that PTV was a significant predictive factor for FR, with an AUC of 0.729 and a cut-off value of 13.5 (sensitivity, 46.47%; specificity, 85.71%). The baseline ASPECTS had an AUC of 0.698 with a cut-off value of 7 (sensitivity, 50%; specificity, 80%). Baseline NIHSS had an AUC of 0.716 with a cut-off 0 value of 12 (sensitivity, 86.67%; specificity, 54.29%). There was no difference between the ASPECTS and NIHSS (p=0.852), between the PTV and ASPECTS (p=0.666) or between the PTV and NIHSS (p=0.846) in the AUC-ROC. The independent factors obtained from the regression analysis were integrated into a comprehensive model, which was superior to any individual model of the ASPECTS, PTV, and NIHSS (p=0.008, p=0.025, p=0.043) in predicting FR (Figure 3). The cut-off value of the combined model was 0.631 with an AUC-ROC of 0.824 (sensitivity, 68.18%; specificity, 85.71%) (Figure 3).
Discussion
Our study demonstrated that prolonged PTV on the venous time-intensity curve of CTP was an independent risk factor for unfavourable functional outcome in anterior circulation LVO patients with successful recanalization after thrombectomy. Patients with favourable VO were prone to achieve good outcomes at 3 months following excellent vessel reperfusion. Furthermore, we developed a combined model composed of identified risk factors after regression. The combined model incorporating PTV was more beneficial to the risk stratification of patients for optimal decision-making and was a more accurate pretreatment tool for predicting FR than the single predictor model. Our findings highlight the important role of VOs in tissue perfusion during cerebral ischaemia. A favourable time from rise to peak for the vein, ie, PTV, suggests robust cerebral blood flow after perfusing ischaemic brain tissue.
A number of clinical trials have confirmed that endovascular therapy within the time window or beyond the time window guided by the ischaemic penumbra is an important strategy for LVO-AIS patients.15–20 However, as shown in our study, approximately 50% of patients could not achieve functional independence at 90 days, which is FR, in accordance with prior studies.2,3,5 The pathophysiological mechanism of FR remains unclear. The identification of patients who can benefit from thrombectomy is crucial and is conducive to patient stratification and individual decision-making regarding endovascular therapy. Many factors are involved in FR, such as clinical data, image variables, and EVT characteristics. Among these, effective tissue reperfusion in the ischaemic area is closely related to prognosis after large vessel recanalization.21 Arterial collateral represents only part of the blood inflow and has difficulty reflecting overall perfusion, which may be the reason for the controversy regarding the relationship between arterial collateral and FR.6,22 In the current study, we also observed that collateral status assessed by DSA in the good outcome group was better than that in the FR group in univariate analysis but was eliminated as an independent predictor in logistic regression, in line with the Imaging Evaluation for Ischaemic Stroke 3 (DEFUSE 3) study.6 The critical perfusion pattern of the cerebral ischaemic region determined by the haemodynamics of distal arterial branches and venous egress most likely reflects the stability of collateral in the case of recanalization of large vessels.23 VO implies the state of blood flow within ischaemic tissue, that is, the microcirculation state. In recent years, an increasing number of studies have focused on the impact of VO on the clinical outcome in AIS patients after thrombectomy. Favourable cortical vein opacification contributed to good clinical outcome after endovascular treatment and vice versa regardless of arterial collaterals.24 Poor VO results in microthrombi formation and occlusion in venules and exacerbates brain oedema.8 These studies selected cortical veins as target veins for qualitative assessment, which may introduce bias due to vein variation. After all, even a certain proportion of the healthy population has congenital absence of cortical veins. VO was evaluated on single-phase CTA, neglecting late-filling veins. We investigated the whole-brain venous flow profile derived from the time-intensity curve on CTP, which provided a dynamic index of venous opacification, including information on venous flow of the ischaemic hemisphere during the whole cerebral perfusion period. Compared with the good outcome group, the PTV and TVT in the FR group were prolonged. After confounders were adjusted, PTV remained an independent predictor for FR. Nevertheless, an independent association between TVT and FR was not established. The underlying mechanism was as follows: a venous time-intensity curve was automatically generated for the superior sagittal sinus, including the time from rise to peak and from peak to baseline. The former showed the duration when the cerebral cortex and subcortical venules converge into the cortical vein and flow into the superior sagittal sinus, reflecting blood flow after penetrating cerebral ischaemic tissue, that is, tissue microperfusion. As mentioned before, the period was closely related to the prognosis of AIS patients treated with EVT. The latter displayed the process of blood flow from the superior sagittal sinus to the confluens sinuum and out of the skull from the internal jugular vein, mainly reflecting cerebral blood egress out of skull after brain tissue permeation. Therefore, the relation between the TVT obtained by adding the two parts and poor clinical outcome did not persist in the binary logistic model, although there was no collinearity between PTV and TVT. Our results supported the hypothesis that in the case of sufficient arterial inflow after excellent vessel recanalization, obstacle of VO due to venous micro-emboli and the destruction of microcirculation involved in exacerbation of brain oedema and haemorrhage transformation delayed the peak of vein opacification.24 Further studies should be conducted to confirm the results. Of note, PTV, baseline NIHSS and ASPECTS had the same predictive effect on FR. The comprehensive model formed by the combination of the three variables was superior to the single model of three variables. Prospective study should be concluded to identify the finding.
The association between collateral circulation and FR is controversial, although good collateral is conducive to reducing infarct volume. Post hoc analysis from MR CLEAN indicated that patients with good collateral were more likely to benefit from EVT than patients with poor collaterals, and collateral was evaluated by single-phase CTA.22 In contrast to this result, collateral status was not significantly correlated with outcome in our study, although the proportion of good collateral circulation assessed by DSA in the good outcome group was higher than that in the FR group, in line with previous studies.5,6 Several reasons could explain the difference. First, we included patients with successful recanalization, excluding patients without unsuccessful recanalization, because patients with good collateral were prone to obtain successful recanalization. Therefore, the majority of patients with poor collateral may be excluded from our study. Second, assessment methods of collateral status were diverse. Vessel recanalization shown by digital subtraction angiography (DSA) did not fully represent the tissue perfusion of cerebral blood flow, much less insufficient collateral status based on single-phase CTA.
The ASPECTS indicated the extent of early ischaemic lesions in anterior circulation and was validated for the selection of patients who would benefit from thrombectomy.25 Along with previous studies,26,27 we observed a higher prevalence of ASPECTS scores in the good outcome group than in the FR group. A lower ASPECTS on admission was a negative independent predictive factor for good 3-month clinical outcomes after adjusting for relevant variables. The ASPECTS was positively correlated with the arterial collateral state, similar to Villringer’s result.28 Notably, we revealed that the baseline ASPECTS was inversely related to PTV and TVT in our study, which indicated an important impact of venous collaterals on the development of ischaemic lesions. Patients with rapid venous reflux had higher ASPECT scores and lower NIHSS scores, which confirms the hypothesis that robust VO through the ischaemic brain reflects superior collateral and brain tissue perfusion. The underlying mechanism is still open to speculation. In our study, a score of <7 in the CTP-SI ASPECTS was identified to be the best cut-off to predict FR, with an AUC of 0.698 (sensitivity 50%; high specificity 80%). In the HERMES meta-analysis, patients with ASPECT ≥ 6 would most likely benefit from EVT. The reason for the different cut-off values of the ASPECTS for FR may be the heterogeneity of the included patients and discrepant evaluation criteria of the ASPECTS.5,29 Nevertheless, there was no definite threshold for EVT. Young patients may have achieved good outcomes even if ASPECT < 5.5
Our study showed FR group tended to be older than good outcome group, consistent with the previous study.30 No significant difference may be due to the small sample size. Our study further demonstrated that NIHSS on admission reflecting pretreatment stroke severity was a powerful risk factor for FR regardless of arterial collateral status, as previous studies have shown. It is worth noting that AIS patients with sufficient salvageable brain tissue and excellent collateral may attain a good outcome even if they have a high NIHSS.31 As a recent study suggested, with an increase in NIHSS, the rate of FR increases as well, but the benefit from EVT still outweighs the risk.32 Although we did not find a difference in OTP and PTR between the two groups, reperfusion of large vessels in the shortest possible time is non-negligible. Other characteristics of EVT are of equal importance apart from OTP and PTR.
Our study is subject to some limitations. First, we conducted retrospective research based on a single-centre database, which inevitably had selection bias. Our results need to be confirmed in multi-centre prospective studies. Second, the sample size was small, which makes it difficult to generalize the overall population of LVO patients and validate the threshold of the peak time of veins for FR. Third, PTV represents the whole cerebral venous reflux, not the ischaemic hemisphere, but that is exactly the point of our research whether the vein is absent or not. Finally, the prediction model based on artificial intelligence (AI) has been proved to be better than classical traditional statistical models in terms of the prediction ability.33 AI-based model should be investigated more in future research.
Conclusion
Our finding underscored the time from rise to peak of venous based on the time-intensity curve originating from CTP as a surrogate biomarker for FR in anterior circulation LVO-AIS. VO should be considered in the stratification strategy of EVT. Further studies are warranted to determine the optimal model to predict FR.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
2. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi:10.1056/NEJMoa1713973
3. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a m ismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi:10.1056/NEJMoa1706442
4. Fiehler J, Thomalla G, Bernhardt M, et al. Eraser. Stroke. 2019;50(5):1275–1278. doi:10.1161/STROKEAHA.119.024858
5. Van Horn N, Kniep H, Leischner H, et al. Predictors of poor clinical outcome despite complete rep erfusion in acute ischemic stroke patients. J Neurointerv Surg. 2021;13(1):14–18. doi:10.1136/neurintsurg-2020-015889
6. De Havenon A, Mlynash M, Kim-Tenser MA, et al. Results from defuse 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke. 2019;50(3):632–638. doi:10.1161/STROKEAHA.118.023407
7. Menon BK, d’Esterre CD, Qazi EM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275(2):510–520. doi:10.1148/radiol.15142256
8. Zhang S, Lai Y, Ding X, et al. Absent filling of ipsilateral superficial middle cerebral vein is a ssociated with poor outcome after reperfusion therapy. Stroke. 2017;48(4):907–914. doi:10.1161/STROKEAHA.116.016174
9. Jansen IGH, van Vuuren AB, van Zwam WH, et al. Absence of cortical vein opacification is associated with lack of intra-arterial therapy benefit in stroke. Radiology. 2018;286(2):731. doi:10.1148/radiol.2017162445
10. Faizy TD, Kabiri R, Christensen S, et al. Favorable venous outflow profiles correlated with favorable tissue-level collaterals and clinical outcome. Stroke. 2021;52(5):1761–1767. doi:10.1161/STROKEAHA.120.032242
11. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–2663. doi:10.1161/STROKEAHA.113.00197
12. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta stroke programme early CT score. Lancet. 2000;355(9216):1670–1674. doi:10.1016/s0140-6736(00)02237-6
13. Zhang X, Yuan K, Wang H, et al. Nomogram to predict mortality of endovascular thrombectomy for ischemic stroke despite successful recanalization. J Am Heart Assoc. 2020;9(3):e014899. doi:10.1161/JAHA.119.014899
14. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin Scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi:10.1161/01.STR.0000258355.23810.c6
15. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi:10.1056/NEJMoa1411587
16. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid end ovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi:10.1056/NEJMoa1414905
17. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi:10.1056/NEJMoa1414792
18. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi:10.1056/NEJMoa1415061
19. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi:10.1056/NEJMoa1503780
20. Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138–1147. doi:10.1016/S1474-4422(16
21. De Silva DA, Fink JN, Christensen S, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke. 2009;40(8):2872–2874. doi:10.1161/STROKEAHA.108.543595
22. Berkhemer OA, Jansen IG, Beumer D, et al. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke. 2016;47(3):768–776. doi:10.1161/STROKEAHA.115.011788
23. Liebeskind DS. Mapping the collaterome for precision Cerebrovascular health: theranostics in the continuum of stroke and dementia. J Cereb Blood Flow Metab. 2018;38(9):1449–1460. doi:10.1177/0271678X17711625
24. Faizy TD, Kabiri R, Christensen S, et al. Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. Radiology. 2021;299(3):682–686. doi:10.1148/radiol.2021203651
25. Broocks G, Hanning U, Flottmann F, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. 2019;142(5):1399–1407. doi:10.1093/brain/awz057
26. Baek JH, Kim BM, Heo JH, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke. 2018;49(9):2088–2095. doi:10.1161/STROKEAHA.118.021320
27. Demeestere J, Scheldeman L, Cornelissen SA, et al. Alberta stroke program early CT score versus computed tomographic perfusion to predict functional outcome after successful reperfusion in acute ischemic. Stroke.Stroke. 2018;49(10):2361. doi:10.1161/STROKEAHA.118.021961
28. Villringer K, Zimny S, Galinovic I, et al. The association between recanalization, collateral flow, and reperfusion in acute stroke patients: a dynamic susceptibility contrast MRI study. Front Neurol. 2019;10:1147. doi:10.3389/fneur.2019.01147
29. Kawiorski MM, Martínez-Sánchez P, García-Pastor A, et al. Alberta stroke program early CT score applied to CT angiography source images is a strong predictor of futile recanalization in acute ischemic stroke. Neuroradiology. 2016;58(5):487–493. doi:10.1007/s00234-016-1652-7
30. Xu H, Jia B, Huo X, et al. Predictors of futile recanalization after endovascular treatment in patients with acute ischemic stroke in a multicenter registry study. J Stroke Cerebrovasc Dis. 2020;29(10):105067. doi:10.1016/j.jstrokecerebrovasdis.2020.105067
31. Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA. 2015;314(17):1832–1843. doi:10.1001/jama.2015.13767
32. Lee S-H, Kim BJ, Han M-K, et al. Futile reperfusion and predicted therapeutic benefits after successful endovascular treatment according to initial stroke severity. BMC Neurol. 2019;19:11. doi:10.1186/s12883-019-1237-2
33. Katsuki M, Kakizawa Y, Nishikawa A, et al. Easily created prediction model using deep learning software (Prediction One, Sony Network Communications Inc.) for subarachnoid hemorrhage outcomes from small dataset at admission. Surg Neurol Int. 2020;11(11):374. doi:10.25259/SNI_636_2020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


