Back to Archived Journals » Core Evidence » Volume 15
Vedolizumab in the Treatment of Ulcerative Colitis: An Evidence-Based Review of Safety, Efficacy, and Place of Therapy
Authors Takatsu N , Hisabe T, Higashi D , Ueki T , Matsui T
Received 1 September 2019
Accepted for publication 8 March 2020
Published 1 April 2020 Volume 2020:15 Pages 7—20
DOI https://doi.org/10.2147/CE.S179053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Garry Walsh
Noritaka Takatsu,1 Takashi Hisabe,1 Daijiro Higashi,2 Toshiharu Ueki,1 Toshiyuki Matsui1
1Department Of Gastroenterology, Fukuoka University Chikushi Hospital, Fukuoka, Japan; 2Department Of Surgery, Fukuoka University Chikushi Hospital, Fukuoka, Japan
Correspondence: Toshiyuki Matsui Tel +81-92-921-1011
Fax +81-92-929-2630
Email [email protected]
Introduction: Selective blockade of the integrins and mucosal adhesion molecules is a promising therapeutic strategy for ulcerative colitis (UC). Vedolizumab (VDZ), a humanized IgG1 monoclonal antibody against α 4β 7 integrin, selectively blocks the trafficking of the leukocytes into the gastrointestinal tract through its binding with the α 4β 7 integrin.
Aim: In this review, we provide an overview of the unique mechanism of VDZ, along with its efficacy, safety, and pharmacokinetic and pharmacodynamic data obtained from clinical trials, observational studies, and meta-analyses.
Evidence Review: A positive exposure–efficacy relationship with regard to clinical remission and clinical response was apparent in VDZ induction therapy. No drug-specific safety signals are currently available.
Place in Therapy: VDZ has been shown to be effective as first- or second-line induction and maintenance therapy in UC.
Conclusion: VDZ is a safe and effective treatment option for patients with UC. Prolonged VDZ induction therapy may contribute to improved outcomes in patients with UC, particularly those previously treated with tumor necrosis factor-α. Prospective head-to-head study of VDZ and other biologics would alter the positioning of VDZ much more clearly.
Keywords: integrin antagonist, vedolizumab, ulcerative colitis, inflammatory bowel disease, safety, efficacy
Core Evidence Place in Therapy Summary for Vedolizumab in Moderate-to-Severe Ulcerative Colitis
 |
|
Scope, Aims, and Objective
The integrin antagonists can selectively inhibit the interaction between the integrins and mucosal adhesion molecules. Thus, such a selective blockade of the integrins can prevent the translocation of the lymphocytes into the inflamed gastrointestinal mucosa, thereby, reducing local inflammation. Vedolizumab (VDZ), a humanized IgG1 monoclonal antibody against α4β7 integrin, inhibits the activity of the α4β7 integrin in the blood vessels; thus, it can exert a therapeutic effect against ulcerative colitis (UC).
This review focuses on the unique mechanism of VDZ and describes its efficacy, safety, and pharmacokinetic and pharmacodynamic data obtained from clinical trials, observational studies, and meta-analyses.
We primarily aimed to evaluate the efficacy and safety of VDZ for the induction and maintenance of remission in patients with UC.
Methodology for Systematic Review
We performed a systematic search of Pub Med and the Cochrane Library in March 2019 to evaluate and compare the efficacy and safety outcomes of ulcerative colitis treated using VDZ. The search terms that were used included the following: “vedolizumab”, “ulcerative colitis”, “inflammatory bowel disease”, “efficacy”, “safety”, “adverse events”, “infusion reaction”, “infection”, “surgery or perioperative periods”, “pregnancy”, “pharmacokinetics”, “pharmacodynamics”, “immunogenicity”, “therapeutic drug monitoring”, and “cost effectiveness”. Given the large number of studies comparing the efficacy and safety profile, we included not only randomized trials but also real-world evidence. In addition, we searched review articles, conference proceedings, and abstracts to identify additional studies.
We found a total of six randomized trials comparing efficacy and safety profiles; additionally, we identified real-world evidence and systematic reviews on efficacy and safety.
Data extraction: Data from each study were extracted using a standardized data collection form (Table 1). Year of publication, study design, number of cases, total sample size, population type, and relevant clinical outcomes are summarized in Tables 2 and 3 (details shown later).
 |
Table 1 Evidence Base Included in the Review |
 |
Table 2 Clinical and Endoscopic Outcomes and Safety Among Patients with UC Treated with Vedolizumab in Randomized Controlled Trials |
 |
Table 3 Clinical and Endoscopic Outcomes Among Patients with Vedolizumab in Real World Evidence of Ulcerative Colitis Results at Week 14 |
 |
Table 4 Association of Serum Vedolizumab Concentration Thresholds with Therapeutic Outcomes in Ulcerative Colitis |
Disease Overview of Ulcerative Colitis
Crohn’s disease (CD) and ulcerative colitis (UC), the two major types of inflammatory bowel disease (IBD), are chronic remitting/relapsing conditions, which result from uncontrolled inflammation of the intestinal mucosa.4–6 UC is a diffuse, non-specific inflammatory disease of unknown cause. It is characterized by persistent irritation of the colonic and rectal mucosa, resulting in the formation of erosions and/or ulcers, which can lead to various clinical symptoms. As UC commonly occurs at a younger age, quality of life (QOL) is often impaired in patients with UC who present with symptoms such as abdominal pain, diarrhea, and bloody stool. UC chronically progresses with repeated cycles of relapse and remission. Extraintestinal manifestations (EIM) may develop in various organs, such as the joints, skin, and eyes of patients with UC. Although the cause of IBD remains unknown, there is an international consensus that inflammation is associated with a genetic predisposition as a result of impaired regulatory mechanisms by which various environmental factors are involved in the intestinal mucosal immune system. Considering that a slightly higher prevalence of IBD has been reported in blood-relatives, there is a possibility that genetic factors may be involved in the pathogenesis of IBD. However, consistent results have not been obtained, to date, in populations from around the world. Moreover, extensive research is ongoing to identify disease susceptibility genes.
Because the pathophysiology of IBD is complicated, accurately determining the disease condition is essential to plan the appropriate treatment, which for UC varies depending on the stage, extent, and severity of the disease. The pathologic stage of UC is commonly classified as either active (in which the patients complain of bloody stools, and the endoscopy reveals the loss of vascular pattern with the occurrence of friable mucosa, erosions, and/or ulcers) or remission (in which the incidence of bloody stools has been resolved and the endoscopy reveals the reappearance of the vascular pattern and the loss of the friable mucosa, erosions, and/or ulcers). In addition, UC can be divided into the following types depending on the extent of the lesions: proctitis, distal colitis, left-sided colitis, and pancolitis. Using the Trulove–Witts score,7 the severity of UC is graded as “mild,” “moderate,” or “severe” according to the frequency of defecation and bloody stools and systemic symptoms such as fever, palpitation, and anemia.
Endoscopic evaluation of the severity of intestinal damage is essential to determining the outcome of UC treatment. Among endoscopic indices for UC, the Mayo endoscopic subscore8 has been widely used in clinical trials. Mucosal healing is reportedly scored as 0 (normal or inactive disease) or 1 (mild disease: erythema, decreased vascular pattern, and mild friability). Endoscopic evaluation of mucosal healing is a useful procedure that allows the determination of appropriate treatment for the maintenance of remission and prediction of UC relapse, although controversies remain regarding the precise definition of mucosal healing, including histological findings.
Overview of Current Therapy (Figure 1)
Preparations of 5-aminosalicylic acid are effective in inducing and maintaining remission of UC. Corticosteroids with potent anti-inflammatory properties also effectively induce remission in patients with UC. Azathioprine and 6-mercaptopurine contribute to preventing relapse in patients with UC in remission, especially in those dependent on steroids or those in whom remission cannot be maintained with 5-aminosalicylic acid preparations.4–6 According to meta-analyses and randomized controlled trials (RCTs), the use of infliximab (IFX)9,10 and adalimumab (ADA)10,11 plays an important role in inducing remission in patients with steroid-refractory or steroid-dependent moderate-to-severe UC. A report has shown that secondary loss of response may occur in approximately 60% of patients who have responded to anti-tumor necrosis factor (TNF) agents during an approximately 5-year follow-up period.12 A meta-analysis in 2011 assessing the side effects of IFX, which was used as remission induction therapy for UC, found no statistically significant differences in the incidence of infusion reactions, injection site reactions, headaches, skin lesions, or arthralgia between the IFX treated and placebo groups.12,13
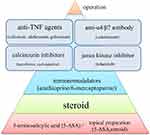 |
Figure 1 Overview of current therapy. |
In both UC and CD, surgery is required to avoid life-threatening complications when patients develop severe disease refractory to drug therapy.9,10 Further, patients whose QOL is severely impaired owing to IBD symptoms, EIMs, or drug side effects may be candidates for surgery.13 Postoperative resolution of these symptoms can improve patients’ QOL.14
Unfortunately, patients with IBD who have been previously treated with anti-TNF-α agents are predisposed to either primary non-response or secondary loss of response.10,12 Moreover, considering that these drugs have been associated with an increased risk of developing serious adverse events, including infections, a substantial proportion of patients require alternative therapeutic options. A novel IBD therapy with better safety profiles is thus warranted.
Clinical Evidence of Vedolizumab Use in UC
Mechanism of Action of Vedolizumab
VDZ, which is a fully humanized monoclonal IgG1 antibody, selectively inhibits the interaction between α4β7 integrin and mucosal address in cell adhesion molecule-1 (MAdCAM-1). It exerts a preventative effect on lymphocyte translocation from the blood to the inflamed gut tissue, thereby reducing local inflammation.15,16 (Figure 2)
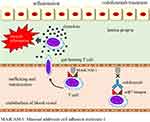 |
Figure 2 A mechanism of action of vedolizumab. |
Natalizumab, the first integrin antagonist, is a humanized IgG4 monoclonal antibody, eventually inhibiting α4 integrin. It was approved in 2008 for the treatment of CD. However, the widespread use of natalizumab was limited owing to the associated increased incidence of progressive multifocal leukoencephalopathy (PML), a rare but fatal demyelinating disease of the central nervous system caused by the opportunistic human polyoma John Cunningham (JC) virus.17,18 Natalizumab inhibits not only α4β7, which is expressed on T lymphocytes in the inflamed gut, but also α4β1, which mediates lymphocyte homing exclusively in the central nervous system. This process disrupts immune surveillance in the kidney and blocks T-cell trafficking to the brain, ultimately leading to reactivation of the JC virus.17,19,20 Considering its unique mechanism of action, it is unlikely that the therapeutic use of VDZ would be associated with the risk of developing PML.21–26
Randomized Controlled Trials
Phase I Trial
In 2000, Feagan et al conducted a phase Ib/IIa proof-of-concept RCT involving 29 patients with moderate-to-severe UC; the patients were assigned to receive either LPD-02 a humanized monoclonal antibody to α4β7 derived from an NS0 mouse myeloma cell line or a placebo in single doses.27 They found that 40% of patients in the LPD-02 group (0.5 mg/kg) achieved deep remission whereas no patients in the placebo group achieved remission.
Phase II Trials
In 2005, Feagan et al conducted a multicenter, double-blind, placebo-controlled trial of α4β7 antibody (MLN02) in 181 patients with active UC.28 In this trial, patients with moderate-to-severe disease were administered 0.5 mg/kg MLN02, 2.0 mg/kg MLN02, or a placebo intravenously on days 1 and 29. At 6 weeks after starting therapy, the remission rates were higher in the treatment groups than in the placebo group for both those with clinical remission (0.5 mg/kg, 33%; 2 mg/kg, 32%; placebo, 14%; p = 0.03) and those with endoscopic remission (0.5 mg/kg, 28%; 2 mg/kg, 12%; placebo, 8%; p = 0.007).
Parikh et al conducted a further phase II dose ranging, randomized controlled trial involving 47 patients with UC in 2012.29 The patients were randomly assigned to receive either VDZ (n = 38) (2 mg/kg [n =13], 6 mg/kg [n = 14], or 10 mg/kg [n = 11]) or a placebo (n = 9) on days 1, 15, 29, and 85, with a follow-up period of 253 days. The doses of VDZ used in this study were higher and the frequency between doses was shorter than that in previous trials. The clinical response rate in the VDZ groups exceeded 50% between day 29 and day 253, as compared with 22–33% in the placebo group. For patients with active disease at baseline, the clinical remission rate ranged from 53% to 79% in the VDZ groups, as compared with 25% to 50% in the placebo group. Fecal calprotectin levels were also shown to be reduced in the VDZ groups compared to those in the placebo group.29 During the follow-up, a greater proportion of patients treated with VDZ showed clinical response than those who were assigned to placebo.30
Phase III Trial
Feagan et al reported the results of the GEMINI 1 trial in 2013.31 This was a Phase III, randomized, double-blind, placebo-controlled study investigating the efficacy, safety, and tolerability of VDZ (MLN002) as an induction and maintenance therapy in patients with moderate-to-severe UC. The patients were randomly assigned to receive a single dose of VDZ (300 mg IV) or a placebo on days 1 and 15. At week 6, there were statistically significant differences between the VDZ and placebo groups regarding clinical response (47.1% vs 25.5%, p < 0.001), clinical remission (16.9% vs 5.4%, p = 0.001), and mucosal healing (40.9% vs 24.8%, p = 0.001) rates. Clinical responders at week 6, in addition to patients who responded to open-label VDZ induction therapy, were enrolled in the maintenance trial and received VDZ or a placebo every 4 or 8 weeks until week 52. The VDZ groups were superior to the placebo group in terms of clinical remission (VDZ [every 8 weeks], 41.8%, p < 0.001; VDZ [every 4 weeks], 44.8%, p < 0.001; placebo, 15.9%) and mucosal healing rates. An open-label long-term extension study showed a higher rate of clinical response (98%) and remission (90%) at week 248 among the patients who had responded to induction therapy and finished GEMINI 1 maintenance trial.32
In 2019, Motoya et al conducted a phase III, randomized, double-blind, placebo-controlled trial of VDZ in Japanese patients with moderate to severe UC.33 Exploratory analyses of GEMINI 1 suggested that greater efficacy may have been obtained with a longer induction treatment.31 However, thus far, the efficacy of VDZ at >6 weeks induction was not investigated as a primary endpoint. Therefore, in this Japanese population trial, the primary endpoint was clinical response at week 10, for the induction phase, and clinical remission at week 60, for the maintenance phase. A total of 292 patients were enrolled in the induction phase; of these, 83 patients achieved a response to VDZ and were subsequently enrolled in the maintenance phase. Clinical response rates at week 10 were 39.6% and 32.9% in the VDZ and placebo groups, respectively (adjusted odds ratio = 1.37, 95% confidence interval (CI) 0.779–2.399; p = 0.2722). In the maintenance phase, clinical remission rate at week 60 was significantly higher in the VDZ group, at 56.1%, versus the placebo group, at 31.0% (adjusted odds ratio = 2.88, 95% CI 1.168–7.108; p = 0.0210). This RCT had a primary endpoint at week 10; hence, comparison with other RCTs is difficult.
Systematic Review and Meta-Analysis
Systematic review and meta-analysis of the aforementioned RCTs27–29,31 were conducted by Mosli et al and Bickston et al.1,34 In a pooled analysis involving patients with active UC, VDZ was superior to a placebo for the induction of clinical remission (relative risk [RR] 0.86, 95% CI 0.80–0.91), clinical response (RR 0.82, 95% CI 0.75–0.91), and endoscopic remission (RR 0.82, 95% CI 0.75–0.91). Furthermore, maintenance therapy with VDZ achieved better clinical remission (RR 2.73, 95% CI 1.78–4.18) and endoscopic remission (RR 2.71, 95% CI 1.88–3.93) than a placebo.
Real-World Evidence
Since regulatory approval of VDZ in 2014, the drug has been widely used in clinical settings for the treatment of both UC and CD. Now, data from multiple real-world cohorts are available. In 2018, Schreiber et al reported a systematic review with meta-analysis to assess the real-world effectiveness and safety of VDZ in patients with UC or CD.35 Clinical remission rates for the treatment of UC were 24% at week 6 (95% CI 13–41%) and 32% at week 14 (95% CI 27–39%), increasing to 39% at 6 months (95% CI 30–48%) and 46% at 12 months (95% CI 37–56%). Clinical response rates for the treatment of UC were 43% at week 6 (95% CI 38–49%), 56% at week 14 (95% CI 50–62%), and 52% at 12 months (95% CI 37–65%). In patients with UC, corticosteroid-free clinical remission rates were 14% at week 6 (95% CI 6–32%), 26% at week 14 (95% CI 20–34%), and 32% at 6 months (95% CI 21–45%), with the rate increasing to 42% at 12 months (95% CI 31–53%). At month 12, the rate of mucosal healing ranged from 33% to 77% in patients with UC. In biologic-naïve patients with UC, clinical remission was achieved in 51% of patients at week 14 (95% CI 40–62%) and 61% of patients at 12 months (95% CI 48–72%). Subgroup analyses involving biologic-naïve patients with UC treated with VDZ have shown that their remission rates were substantially improved as compared to that of the overall patient population.35–41
Another study of open-label VDZ therapy, conducted by Narula et al, reported results from the multicenter hospital-based US VICTORY (Vedolizumab for Health Outcomes in Inflammatory Bowel Disease) consortium to evaluate outcomes in 321 VDZ-treated patients with UC, the majority of whom (71%) had failed treatment with a TNF antagonist. The 12-month cumulative rate was 51% for clinical remission and 41% for endoscopic remission. Corresponding rates for corticosteroid-free remission and deep remission were 37% and 30%, respectively. On multivariable analyses, prior exposure to a TNF-α antagonist was associated with a reduced probability of achieving clinical remission (HR 0.53, 95% CI 0.38–0.75) and endoscopic remission (HR 0.51, 95% CI 0.29–0.88). Overall cumulative rates of colectomy over 12 months were 13%, with lower rates observed in patients naive to anti-TNF-α therapy (2%) than in those who had been exposed to TNF-α antagonists (19%).42 Taken together, these open-label studies have shown that VDZ is likely more effective in patients with UC who are naive to TNF antagonists, while a significant proportion of patients previously treated with TNF antagonists can also achieve important clinical and endoscopic outcomes over time when treated with VDZ.
Pharmacokinetics, Pharmacodynamics, and Immunogenicity
One study evaluated the pharmacokinetic profile of VDZ in patients with IBD and healthy volunteers using a two-compartment model (parallel components of linear and nonlinear elimination).43 The linear elimination half-life of VDZ was estimated to be 25.5 days, with linear clearance values of 0.159 L/day for UC and 0.155 L/day for CD.26,43 Based on pooled population data from the GEMINI program, both a low albumin concentration and very high body mass were identified as predictors of accelerated VDZ clearance.44 Indeed, real-world studies have suggested these factors to be clinically important, linking them with lower drug levels and worse therapeutic outcomes.45–47
In GEMINI 1, the immunogenicity of VDZ was low; 3.7% of patients had at least one positive sample testing for anti-VDZ antibodies at any time and 1% of patients persistently tested positive for anti-VDZ antibodies.31 This has been confirmed even in a real-world cohort using a drug-resistance assay.48 This might explain why the addition of an immunomodulator to VDZ therapy may neither enhance drug levels nor restore therapeutic response.49
Emerging evidence supports that serum VDZ concentrations are associated with efficacy. The GEMINI 1 trial showed that clinical response and remission rates were higher with increased serum concentrations of VDZ.31 An exposure–response analysis of VDZ from these clinical studies revealed that the probability rates of clinical remission, clinical response, and mucosal healing increased by 31%, 34%, and 43%, respectively, in patients with UC at week 6, from concentration quartiles 1 to 4.31,50,51
Several cohort studies have shown a positive relationship between VDZ serum concentrations and efficacy outcomes (Table 4).46,49-54 Dreesen et al conducted a study involving 179 patients (66 with UC, 113 with CD) reporting that thresholds of >30.0 μg/mL at week 2, >24.0 μg/mL at week 6, and >14.0 μg/mL during maintenance therapy were associated with a higher probability of attaining effectiveness endpoints.26,46 Similarly, Yacoub et al reported the outcomes of 82 patients (43 with UC and 39 with CD); a VDZ serum concentration of >18 μg/mL at week 6 led to mucosal healing in the first year of therapy.49 Finally, according to multicentric data from a study conducted by Williet et al in France, when VDZ trough levels were below 18.5 μg/mL at week 6, additional doses were required during the first 6 months of therapy.52 Pouillon et al summarized in his review using accumulating evidence from clinical trials, in addition to real-world data, suggests an exposure–efficacy relationship in the treatment of UC with VDZ, although these results are not as straightforward as those for anti-TNF-α therapy.47 Although the exposure-outcome relationship of VDZ has been demonstrated, the time to assess the drug concentrations and the related therapeutic drug window for VDZ remains undefined. As stated by Pouillon et al, prior to the recommendation of therapeutic monitoring of VDZ, prospective studies are required to explore the effect of dose optimization on objective disease markers and changes in drug levels of VDZ.47 In a consensus meeting that was held to gather expert opinion regarding the clinical utility of TDM for biologic therapies in IBD, Papamichael and colleagues proposed55 the following recommendations regarding the use of TDM in VDZ: (1) It is appropriate to order drug/antibody concentration testing for vedolizumab in non-responders at the end of induction. (2) It is appropriate to order drug/antibody concentration testing for vedolizumab in patients with confirmed secondary loss of response. (3) Although there are emerging data that may show an association between drug concentrations and outcomes, they are not sufficient to guide specific induction and maintenance drug concentrations for vedolizumab and ustekinumab other than confirming that there is detectable drug.55
General Safety
In Feagan’s pivotal study (GEMINI 1, 2013), 374 patients with UC were randomized to receive either a 300 mg intravenous dose of VDZ (n = 225) or a placebo (n = 149). The follow-up period was 52 weeks.31 No important differences were observed among the study groups in the most commonly reported adverse events. Serious infections were not more common with VDZ than with placebo. No cases of PML occurred. No significant differences in hematologic or serum chemical profiles or liver function test results were identified among the study groups. Unlike other anti-integrin therapeutic regimens, VDZ treatment did not increase peripheral-blood total lymphocyte counts. Clinically important infusion reactions were few.31
Bickston et al conducted a systematic review of moderate-to-high quality data from four studies.1 Based on these studies, the incidence of adverse events in the patients treated with VDZ was found to be comparable to that in the patients treated with the placebo. Two studies examined the proportion of patients who experienced at least one adverse event.29,31 According to the results of a pooled analysis of these studies (n = 941 patients), there was no statistically significant difference in the incidence of adverse events between patients administered VDZ and a placebo. At least one adverse event was experienced by 79% of patients administered VDZ compared to 80% of patients administered placebo (RR 0.99, 95% CI 0.93 to 1.07). Two studies reported withdrawals owing to adverse events as an outcome.29,31 A pooled analysis from these studies involving 941 patients showed that drug withdrawal owing to adverse events was less in patients administered VDZ than that in patients administered a placebo. A further pooled analysis of 1122 patients from three studies28,29,31 showed that VDZ was not significantly associated with an increased likelihood of serious adverse events. The incidence of serious adverse events was 12% in both groups of patients treated with VDZ and a placebo (RR 1.01, 95% CI 0.73 to 1.42).28,29,31 Commonly reported adverse events in the Parikh et al (2012) study included: headache, worsening UC, upper respiratory tract infection, and nasopharyngitis; none of the patients showed systemic opportunistic infections or neoplasms.29 Common adverse events reported in the GEMINI 1 study31 were worsening UC, headache, nasopharyngitis, arthralgia, upper respiratory tract infection, nausea, cough, anemia, abdominal pain, fatigue, and influenza. Serious adverse events included the exacerbation of colitis, infusion reaction with angioedema, infection, nausea and vomiting, and degenerative disk disease, as reported by Feagan et al (2005),28 and compression fractures of the thoracic vertebrae and gastroduodenitis, as reported by Parikh et al (2012).29 There were no reports of PML in any of the four studies.27–29,31
Safety data (May 2009–June 2013) from six trials of VDZ were integrated by Colombel et al.2 In total, 2830 patients had 4811 PYs of VDZ exposure. No increased risk of any infection or serious infection was associated with VDZ exposure. Serious clostridial infections, sepsis, and tuberculosis were reported infrequently (≤0.6% of patients). No cases of PML were observed. Independent risk factors for serious infection in UC included prior failure of TNF-α antagonist therapy (HR, 1.99; 95% CI 1.16 to 3.42; p = 0.0122) and narcotic analgesic use (HR, 2.68; 95% CI 1.57 to 4.58; p = 0.0003). Investigator-defined infusion-related reactions were reported for ≤5% of patients in each study. Eighteen VDZ-exposed patients (<1%) were diagnosed with a malignancy. Thus, it was concluded that the integrated clinical trial data set of 2932 patients with moderately to severely active UC or CD provides evidence that there are no significant safety concerns associated with VDZ treatment. VDZ offers a gut-selective mechanism of action without any clear increase in the risk of serious systemic opportunistic infections or other common complications.
The VICTORY consortium reported the safety data from 321 patients with UC.42 Serious infections (defined as those requiring treatment with antibiotics or resulting in discontinuation of VDZ therapy, hospitalization, or death) and serious adverse events (defined as contracting a serious infection or having a non-infectious complication, resulting in the discontinuation of VDZ therapy, hospitalization, or death) were reported in 4% and 6% of the cases, respectively.42
Schreiber et al reviewed real-world safety outcomes from 46 studies over a VDZ exposure/follow-up period of 0.5–12 months.35 Real-world safety data were consistent with those from the GEMINI trials, and no new or unexpected safety signals emerged.35 Real-world data are helpful for determining the effectiveness of VDZ in clinical settings representative of heterogenous and more complex patient populations. The safety data presented may support the positive long-term risk–benefit profile of VDZ in the treatment of IBD.
EIM
Feagan et al have performed post hoc analyses of data from the GEMINI studies to evaluate the effect of VDZ on arthritis/arthralgia.56 In this study, sustained resolution of baseline arthritis/arthralgia, worsening, and the occurrence of new arthritis/arthralgia were evaluated. In patients with UC, VDZ and placebo showed a similar incidence of new/worsening of arthritis/arthralgia. Thus, they concluded that VDZ therapy was associated with no increased incidence of these EIM events in UC. Dubinsky et al researched the incidence rates of EIMs in two cohorts.57 Descriptive analyses were performed and generalized linear models estimated the impact of treatment on the likelihood of developing EIMs. Patients with UC receiving VDZ did not exhibit a statistically significant increase in any EIMs versus patients receiving anti-TNFs; however, they were more likely to develop specific EIMs (aphthous stomatitis, pyoderma gangrenosum, and PSC). They concluded that patients with IBD receiving VDZ may be more likely to develop EIMs versus patients receiving anti-TNF therapies. The gut-selective inflammatory control of VDZ may potentially limit its clinical effect on the prevention of EIMs.
Safety in the Perioperative Period
VDZ is typically considered to be a safe drug, but its effects when administered perioperatively are unknown.58 VDZ targets leukocytes; therefore, there are concerns that it would increase postoperative infectious complications. A recent meta-analysis focused on the impact of preoperative VDZ treatment on the rate of postoperative complications in real-world patients with IBD. They reviewed five studies and analyzed 307 patients receiving VDZ, 490 patients receiving anti-TNF therapy, and 535 patients not receiving preoperative biologic therapy.59 They found that VDZ did not significantly increase the risk of postoperative infection or complications in patients with IBD undergoing abdominal surgery.59 Yung et al analyzed four studies including 1080 patients and found no significant differences between patients with IBD receiving VDZ or anti-TNF therapy preoperatively.60 Additionally, they found that patients with UC who received VDZ had significantly fewer complications than similar patients who received anti-TNF therapy. VDZ is considered to be related to an increase in postoperative infectious complications in patients with CD; however, according to Lightner et al such patients warrant further study because they were receiving multiple drug therapies, including corticosteroids.61 In another study, Lightner et al examined 146 patients who received VDZ within 121 weeks of abdominal surgery and 289 patients who received anti-TNF therapy.62 They found that patients receiving VDZ exhibited higher incidence of surgical site infections; however, patients receiving VDZ often received corticosteroid therapy up to 12 weeks preoperatively. At present, VDZ does not appear to increase the risk of postoperative complications compared to other biologic therapies.
Safety During Pregnancy
There are important issues that need to be addressed regarding the treatment of IBD during pregnancy or when pregnancy is planned. Mainly, there could be effects on IBD activity due to pregnancy and effects on the fetus due to treatment. VDZ is a humanized monoclonal IgG1 antibody to the α4β7 integrin that does not cross the blood-brain barrier and is classified as pregnancy risk B. It has not been shown to be associated with PML.63 Mahadevan et al reported that among 24 VDZ-treated females (23 with CD/UC, 1 healthy volunteer), there were 11 live births, 5 elective terminations, 4 spontaneous abortions, and 4 undocumented outcomes. A congenital corpus callosum agenesis anomaly was reported in one live birth from a healthy volunteer with extensive obstetric history of exposure to single-dose VDZ 79 days before estimated conception.64 Moens et al reported a retrospective study that evaluated pregnancy outcomes in 24 VDZ-treated female patients with IBD. Complications were observed in 25% of pregnancies (premature rupture of membranes, pre-eclampsia, miscarriage, elective termination, and stillbirth) and 35% of infants (prematurity, intra-uterine growth retardation, small for gestational age, and congenital malformations, including hip dysplasia, pulmonary valve stenosis, and Hirschsprung’s disease). For live born children, the median (interquartile range) gestational age, weight, and Apgar score 5 min after birth were 39 weeks, 3270 g, and 10, respectively.65 VDZ has a longer half-life than ADA or IFX and would theoretically result in significant concentrations in the neonate, even if VDZ was discontinued in the third trimester.66 Lahat et al reported a prospective observational study of VDZ-treated breastfeeding patients with IBD. VDZ can be detected in the breast milk of nursing mothers. VDZ was measurable in all lactating women who received VDZ (n = 5). However, on serial measurements in breast milk after an infusion, drug levels did not surpass 480 ng/mL, which was roughly 1/100 of the comparable serum levels. Thus, they concluded that the concentrations of VDZ in breast milk are minute and, therefore, unlikely to result in systemic or gastrointestinal immunosuppression of the infant.67 Clearly, more information is needed about the safety of VDZ in pregnancy; however, given the mechanism of action and limited immunosuppression associated with use, the risk is likely to be low. VDZ should only be used in pregnancy if the benefits for the mother outweigh the potential risks for the mother and child.
Place in Therapy
A network meta-analysis of RCTs involving patients with UC determined that VDZ ranked high for the induction of clinical remission and mucosal healing in biologic-naïve patients.68 For biologic-experienced patients, low-quality evidence supported the use of VDZ. Unlike other evaluated agents, VDZ did not carry an increased risk of infection. Allamneni et al conducted an ambidirectional cohort study, in patients with moderate to severe UC, comparing the rates of clinical response to induction by VDZ versus IFX.69 This study revealed an overall numerically higher proportion of patients who responded to VDZ than IFX induction among patients who had moderately to severely active UC. However, when adjusting for time between induction and assessment of clinical response, the rates of clinical response were similar. A key difference between the two groups was the higher response rate in the VDZ group among anti-TNF agent-experienced patients.
Recently, a prospective head-to-head study of VDZ and ADA, called VARSITY, was conducted by Schreiber and colleagues.70 This was a phase IIIb, double-blind, double-dummy, multicenter, active-controlled trial; patients with moderate-to-severe active UC who had failed other conventional therapies (NCT02497469) were enrolled. Prior TNF antagonist exposure was capped at 25% of the patient population. Patients were randomized 1:1 to receive either active VDZ intravenous (IV) infusions (300 mg)/placebo subcutaneous (SC) injections or placebo IV infusions/active ADA SC injections (160/80/40 mg). The primary endpoint was clinical remission (a complete Mayo score ≤ 2) with no sub-score > 1 at week 52. In total, 769 patients were randomly assigned to VDZ (n = 383) or ADA (n = 386) at 330 sites in 37 countries and received at least one dose of the study drug. At week 52, patients in the VDZ group showed significantly higher rates of clinical remission (primary endpoint) and mucosal healing, and both VDZ and ADA were generally safe and well tolerated in patients with moderate-to-severe active UC. The study results could alter the positioning of VDZ as the first or second line of treatment in the near future.
Conflicting data have been reported regarding the cost-effectiveness of VDZ compared with anti-TNF agents. Wilson et al examined the clinical and economic impact of VDZ compared with IFX, ADA, and golimumab in the treatment of moderately to severely active UC in the United Kingdom.71 Compared with the other biologics, VDZ was the more effective treatment. When considering costs and a lifetime time horizon, VDZ was dominant (more effective and less costly) compared with all other biologics. Trigo-Vicente et al conducted cost-effectiveness analysis of IFX, ADA, golimumab, and VDZ for moderate to severe UC in Spain.72 Among the drugs studied, ADA was the most cost-effective drug for the treatment of moderate to severe UC. In the UK, NICE guidelines allow the use of VDZ as the first line of treatment in patients with moderate to severe UC73 considering cost-effectiveness. There is a need for further research comparing VDZ with other biologic therapies, which may alter the perceptions of cost-effectiveness. Finally, VDZ with a high therapeutic index can be used as a gut-specific agent for either first- or second-line biologic treatment of UC.
Conclusions
The efficacy of VDZ for UC has been proven in both the randomized controlled and real-world trials. VDZ is effective both as a first-line therapy and when given after failure of anti-TNFα agents. Considering that VDZ has a safety profile comparable to the placebo, it may have advantages over the other treatments if safety profiles must be taken into consideration. A prospective, direct comparison study of VDZ with the other therapeutic agents can further clarify the positioning of VDZ. Although the exposure-outcome relationship of VDZ has been demonstrated, the time to assess the drug concentrations and the related therapeutic drug window for VDZ remains undefined. To use VDZ more effectively, prospective interventional trials are needed to establish TDM in VDZ.
Acknowledgments
We are grateful to Yoshinori Takeyama at Medical Translation Division Tokyo Office. This manuscript has been checked by an experienced proofreader who is a native speaker of English and who is under the direct supervision of Honyaku Center Inc.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Toshiyuki Matsui has received honoraria from EA Pharma Co. Ltd., Abbvie GK, Eisai Co. Ltd., Kyorin Pharmaceutical Co. Ltd., Zeria Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation and Janssen Pharmaceutical K.K.; has received research grants from Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation and Otsuka Pharmaceutical Co. Ltd.; has received scholarship grants from EA Pharma Co. Ltd., Otsuka Pharmaceutical Co. Ltd., JIMRO Co. Ltd. and Nippon Kayaku Co. Ltd.; and was a recipient of endowed chairs funded by AbbVie GK, Asahi Kasei Medical Co. Ltd., Ajinomoto Seiyaku, Inflammatory Bowel Disease Advanced Clinical Treatment endowed chair, Regional/Emergency Medical Management endowed chair (Fukuoka). The authors report no other conflicts of interest in this work.
References
1. Bickston SJ, BW B, Tsoulis DJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;(8):
2. Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66(5):839–851. doi:10.1136/gutjnl-2015-311079
3. Batta R, Ma C, Jairath V, et al. Benefit-risk assessment of vedolizumab in the treatment of crohn’s disease and ulcerative colitis. Drug Saf. 2019;42(5):617–632. doi:10.1007/s40264-018-00783-1
4. Ko CW, Singh S, Feuerstein JD, et al.; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748–764. doi:10.1053/j.gastro.2018.12.009
5. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. doi:10.14309/ajg.0000000000000152
6. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353.
7. Trulove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2(4947):1041–1048. doi:10.1136/bmj.2.4947.1041
8. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi:10.1056/NEJM198712243172603
9. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi:10.1056/NEJMoa050516
10. Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–659. doi:10.1038/ajg.2011.73
11. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265. doi:10.1053/j.gastro.2011.10.032
12. Ma C, Huang V, Fedorak DK, et al. Outpatient ulcerative colitis primary anti-TNF responders receiving adalimumab or infliximab maintenance therapy have similar rates of secondary loss of response. J Clin Gastroenterol. 2015;49:675–682. doi:10.1097/MCG.0000000000000265
13. Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030.
14. Heikens JT, de Vries J, van Laarhoven CJ. Quality of life, health-related quality of life and health status in patients having restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis: a systematic review. Colorectal Dis. 2012;14:536–544. doi:10.1111/j.1463-1318.2010.02538.x
15. Fiorino G, Correale C, Fries W, et al. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:567–572. doi:10.1586/eci.10.40
16. Cominelli F. Inhibition of leukocyte trafficking in inflammatory bowel disease. N Engl J Med. 2013;369(8):775–776. doi:10.1056/NEJMe1307415
17. Chen Y, Bord E, Tompkins T, et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med. 2009;361(11):1067–1074. doi:10.1056/NEJMoa0904267
18. Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–1880. doi:10.1056/NEJMoa1107829
19. Kuehn BM. Rare neurological condition linked to newer monoclonal antibody biologics. JAMA. 2009;301(14):1423–1424. doi:10.1001/jama.2009.451
20. Hunt D, Giovannoni G. Natalizumab-associated progressive multifocal leucoencephalopathy: A practical approach to risk profiling and monitoring. Pract Neurol. 2012;12:25–35. doi:10.1136/practneurol-2011-000092
21. Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. doi:10.1124/jpet.109.153973
22. Erle DJ, Briskin MJ, Butcher EC, et al. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–528.
23. Fedyk ER, Wyant T, Yang -L-L, et al. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107–2119. doi:10.1002/ibd.22940
24. Haanstra KG, Hofman SO, Estevao DML, et al. Antagonizing the α4β1 integrin, but not α4β7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol. 2013;190:1961–1973. doi:10.4049/jimmunol.1202490
25. Milch C, Wyant T, Xu J, et al. Vedolizumab does not reduce the CD4 +: CD8 + ratio in the CSF of healthy volunteers: P-136. Inflamm Bowel Dis. 2011;17:S54. doi:10.1097/00054725-201112002-00173
26. Battat R, Dulai PS, Jairath V, et al. A product review of vedolizumab in inflammatory bowel disease. Hum Vaccin Immunother. 2019;15(10):2482–2490. doi:10.1080/21645515.2019.1591139
27. Feagan BG, McDonald J, Greenberg G, et al. An ascending dose trial of a humanized A4 B7 antibody in ulcerative colitis (UC). Gastroenterology. 2000;118(4):A874. doi:10.1016/S0016-5085(00)85637-1
28. Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the α 4β7 integrin. N Engl J Med. 2005;352(24):2499–2507. doi:10.1056/NEJMoa042982
29. Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: a randomized controlled Phase 2 dose-ranging study. Inflamm Bowel Dis. 2012;18(8):1470–1479. doi:10.1002/ibd.21896
30. Parikh A, Fox I, Leach T, et al. Long-term clinical experience with vedolizumab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(8):1691–1699. doi:10.1097/MIB.0b013e318281f538
31. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi:10.1056/NEJMoa1215734
32. Loftus EV, Colombel JF, Feagan BG, et al. Long-term effectiveness and safety of vedolizumab in patients with ulcerative colitis: 5-year cumulative exposure of GEMINI 1 completers rolling into the GEMINI open-label extension study. Gastroenterology. 2017;152(5):S602. doi:10.1016/S0016-5085(17)32150-9
33. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: A Phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;11:14(4):e0215491. doi:10.1371/journal.pone.0215491
34. Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21(5):1151–1159. doi:10.1097/MIB.0000000000000396
35. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol. 2018;53(9):1048–1064. doi:10.1007/s00535-018-1480-0
36. Amiot A, Grimaud J-C, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14(11):1593–1601. doi:10.1016/j.cgh.2016.02.016
37. Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice–a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090–1102. doi:10.1111/apt.13594
38. Kopylov U, Ron Y, Avni-Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real world experience. Inflamm Bowel Dis. 2017;23(3):404–408. doi:10.1097/MIB.0000000000001039
39. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21(12):2879–2885. doi:10.1097/MIB.0000000000000561
40. Vivio EE, Kanuri N, Gilbertsen JJ, et al. Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. J Crohns Colitis. 2016;10(4):402–409. doi:10.1093/ecco-jcc/jjv226
41. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol. 2017;8(3):196–202. doi:10.1136/flgastro-2016-100720
42. Narula N, Peerani F, Meserve J, et al. Vedolizumab for ulcerative colitis: treatment outcomes from the VICTORY consortium. Am J Gastroenterol. 2018;113(9):1345–1354. doi:10.1038/s41395-018-0162-0
43. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 2015;42(2):188–202. doi:10.1111/apt.13243
44. Osterman MT, Rosario M, Lasch K, et al. Vedolizumab exposure levels and clinical outcomes in ulcerative colitis: determining the potential for dose optimisation. Aliment Pharmacol Ther. 2019;49:408–418. doi:10.1111/apt.15113
45. Al-Bawardy B, Ramos GP, Willrich MAV, et al. Vedolizumab drug level correlation with clinical remission, biomarker normalization, and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(3):580–586. doi:10.1093/ibd/izy272
46. Dreesen E, Verstockt B, Bian S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(12):1937–1946. doi:10.1016/j.cgh.2018.04.040
47. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019;17(1):89. doi:10.1186/s12916-019-1323-8
48. Bian S, Dreesen E, Tang HT, et al. Antibodies toward vedolizumab appear from the first infusion onward and disappear over time. Inflamm Bowel Dis. 2017;23(12):2202–2208. doi:10.1097/MIB.0000000000001255
49. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47(7):906–912. doi:10.1111/apt.14548
50. Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis. 2017;11(8):921–929. doi:10.1093/ecco-jcc/jjx021
51. Maria R, Brihad A, Serap S, et al. O-003 relationship between vedolizumab pharmacokinetics and endoscopic outcomes of patients with ulcerative colitis. Inflamm Bowel Dis. 2014;20:S1–S3. doi:10.1097/01.MIB.0000456699.04243.9e
52. Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol. 2017;15(11):1750–1757. doi:10.1016/j.cgh.2016.11.023
53. Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti-drug antibodies, and alpha4beta7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(5):697–705. doi:10.1016/j.cgh.2017.11.050
54. Yarur A, Bruss A, Jain A, et al. Higher vedolizumab levels are associated with deep remission in patients with Crohn’s disease and ulcerative colitis on maintenance therapy with vedolizumab. J Crohns Colitis. 2017;11(suppl_1):S38–S38. doi:10.1093/ecco-jcc/jjx002.057
55. Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655–1668. doi:10.1016/j.cgh.2019.03.037
56. Feagan BG, Sandborn WJ, Colombel JF, et al. Incidence of arthritis/arthralgia in inflammatory bowel disease with long-term vedolizumab treatment: post hoc analyses of the GEMINI trials. J Crohns Colitis. 2019;13(1):50–57. doi:10.1093/ecco-jcc/jjy125
57. Dubinsky MC, Cross RK, Sandborn WJ, et al. Extraintestinal manifestations in vedolizumab and anti-TNF-treated patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(9):1876–1882. doi:10.1093/ibd/izy065
58. Wang MC, Zhang LY, Han W, et al. PRISMA–efficacy and safety of vedolizumab for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2014;93(28):e326. doi:10.1097/MD.0000000000000326
59. Law CCY, Narula A, Lightner AL, et al. Systematic review and meta-analysis: preoperative vedolizumab treatment and postoperative complications in patients with inflammatory bowel disease. J Crohn Colitis. 2018;12(5):538–545. doi:10.1093/ecco-jcc/jjy022
60. Yung DE, Horesh N, Lightner AL, et al. Systematic review and meta-analysis: vedolizumab and postoperative complications in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(11):2327–2338. doi:10.1093/ibd/izy156
61. Lightner AL, Loftus EV
62. Lightner AL, Mathis KL, Tse CS, et al. Postoperative outcomes in vedolizumab-treated patients undergoing major abdominal operations for inflammatory bowel disease: retrospective multicenter cohort study. Inflamm Bowel Dis. 2018;24(4):871–876. doi:10.1093/ibd/izx076
63. Gaidos JK, Kane SV. Overcoming challenges of treating inflammatory bowel disease in pregnancy. Expert Rev Clin Immunol. 2016;12(8):871–878. doi:10.1586/1744666X.2016.1166958
64. Mahadevan U, Vermeire S, Lasch K, et al. Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(7):941–950. doi:10.1111/apt.13960
65. Moens A, van Hoeve K, Humblet E, et al. Outcome of pregnancies in female patients with inflammatory bowel diseases treated with vedolizumab. Belgian IBD Research and Development group (BIRD). J Crohns Colitis. 2019;13(1):12–18. doi:10.1093/ecco-jcc/jjy142
66. Bryant RV, Sandborn WJ, Travis SP. Introducing vedolizumab to clinical practice: who, when, and how? J Crohns Colitis. 2015;9(4):356–366. doi:10.1093/ecco-jcc/jjv033
67. Lahat A, Shitrit AB, Naftali T, et al. Vedolizumab levels in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2018;12(1):120–123. doi:10.1093/ecco-jcc/jjx120
68. Singh S, Fumery M, Sandborn WJ, et al. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(2):162–175. doi:10.1111/apt.14422
69. Allamneni C, Venkata K, Yun H, et al. Comparative effectiveness of vedolizumab vs. infliximab induction therapy in ulcerative colitis: experience of a real-world cohort at a tertiary inflammatory bowel disease center. Gastroenterology Res. 2018;11(1):41–45. doi:10.14740/gr934w
70. Available from: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2019/item/op34-varsity-a-double-blind-double-dummy-randomised-controlled-trial-of-vedolizumab-versus-adalimumab-in-patients-with-active-ulcerative-colitis.html.
71. Wilson MR, Bergman A, Chevrou-Severac H, et al. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ. 2018;19(2):229–240. doi:10.1007/s10198-017-0879-5
72. Trigo-Vicente C, Gimeno-Ballester V, Montoiro-Allué R, et al. Cost-effectiveness analysis of infliximab, adalimumab, golimumab and vedolizumab for moderate to severe ulcerative colitis in Spain. Expert Rev Pharmacoecon Outcomes Res. 2018;18(3):321–329. doi:10.1080/14737167.2018.1411193
73. National Institute for Health and Care Excellence. Vedolizumab for treating moderately to severely active ulcerative colitis. Technol Appraisal Guidance (TA342). 2015.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
