Back to Journals » Breast Cancer: Targets and Therapy » Volume 11
Variation in the management of elderly patients in two neighboring breast units is due to preferences and attitudes of health professionals
Authors Morrow ES , Dolan RD , Doughty J, Stallard S, Lannigan A, Romics L
Received 10 November 2018
Accepted for publication 19 February 2019
Published 8 May 2019 Volume 2019:11 Pages 179—188
DOI https://doi.org/10.2147/BCTT.S194124
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Elizabeth S Morrow,1 Ross D Dolan,1 Julie Doughty,2 Sheila Stallard,2 Alison Lannigan,3 Laszlo Romics1,4
1Academic Unit of Surgery, University of Glasgow, Glasgow, UK; 2Department of Surgery, Gartnavel General Hospital, Glasgow, UK; 3Department of Surgery, Wishaw General Hospital, Lanarkshire, UK; 4Department of Surgery, New Victoria Hospital, Glasgow, UK
Introduction: Elderly breast cancer patients have been shown to be managed less aggressively than younger patients. There is evidence that their management varies between institutions. We audited the management of elderly patients in two neighboring units in Glasgow and aimed to identify reasons for any differences in practice found.
Methods: Patients aged ≥70 years, who were managed for a new diagnosis of breast cancer in the two units between 2009 and 2013, were identified from a prospectively maintained database. Tumor pathology, treatment details, postcode and consultant in charge of care were obtained from the same database. Comorbidities were obtained from each patient’s electronic clinical record. Questionnaires were distributed to members of each multidisciplinary teams.
Results: 487 elderly patients in Unit 1 and 467 in Unit 2 were identified. 76.2% patients in Unit 1 were managed surgically compared to 63.7% in Unit 2 (p<0.0001). There was no difference between the two units in patient age, tumor pathology, deprivation or comorbidity. 16.2% patients managed surgically in Unit 1 had a comorbidity score of 6 and above compared to 11% of surgically managed patients in Unit 2 (p=0.036). Responses to questionnaires suggested that staff at Unit 1 were more confident of the safety of general anesthetic in elderly patients and were more willing to consider local anesthetic procedures.
Conclusion: A higher proportion of patients aged >70 years with breast cancer were managed surgically in Unit 1 compared to Unit 2. Reasons for variation in practice seem to be related to attitudes of medical professionals toward surgery in the elderly, rather than patient or pathological factors.
Keywords: breast cancer, old age, variation in treatment
Introduction
Approximately one-third (33.7% in 2012–2014) of female breast cancer cases in the UK are diagnosed in people aged ≥70 years, and breast cancer incidence rates are highest in people aged ≥85 years.1 Older breast cancer patients are more likely to suffer comorbidities and frailty. For these patients, deemed not fit for surgery, there is the option of nonsurgical treatment, usually in the form of primary endocrine therapy (PET) for those with ER-positive disease, which is present in the majority of patients in this age group.2–4
Guidelines are clear that age should not be a factor in treatment decisions.5,6 The reported 30-day mortality following standard breast surgery is negligible.7 Despite this, there is evidence that elderly patients are sometimes treated less aggressively than younger patients8–10 and that management varies between institutions.11 Patients aged >70 years are less likely to have surgical management of their breast cancer and are less likely to receive adjuvant radiotherapy.12,13
In Glasgow, breast cancer patients are managed in three separate units, depending on geographical location. We aimed to audit the management of breast cancer patients aged ≥70 years in two of these units, which have 3 consultant surgeons each, with separate multidisciplinary teams (MDTs). Both units serve similar populations. Our secondary aim was to identify reasons for any differences in practice observed.
Methods
Patients who were managed for breast cancer between 2009 and 2013 at either the Western Infirmary (Unit 1) or Victoria Infirmary (Unit 2) in Glasgow were identified from a prospectively maintained database within the West of Scotland Managed Clinical Network. Data collection within this network is systematic and its standards are checked regularly. Those patients aged <70 years at the date of diagnosis were excluded. Data regarding clinicopathological characteristics, hospital of treatment, consultant in charge of care and treatment received were collected from the same database. The postcode obtained from this database for each patient was used to determine the Scottish Index of Multiple Deprivation 2012 (SIMD) score. The SIMD ranks small areas from most deprived to least deprived by combining 38 indicators across seven weighted domains (income, employment, health, education, skills and training, housing, geographic access and crime).14 For the sake of our analysis, we then divided these rankings into quintiles.
Comorbidity data were collected from each patient’s electronic clinical record. Clinic letters, pre-assessment forms and diagnosis codes within GP referral letters, which preceded the date of diagnosis of breast cancer, were examined by hand by EM to identify comorbid diagnoses for each patient. These were then used to calculate the Charlson comorbidity index (CCI). The CCI is a combined age-comorbidity score, devised as a predictor of mortality.15 In addition, surrogate marker of comorbidity, number of inpatient bed days in the two years preceding diagnosis, and number of emergency admissions in the year preceding diagnosis, were obtained for each patient from the National Services Scotland Information Services Division, within the SMR01-General/Acute Inpatient and Day Case database. Approval for this was obtained from the Caldicott Guardian for NHS Greater Glasgow & Clyde.
To ascertain the attitudes of medical staff on each site, questionnaires were designed and distributed via e-mail to all staff who were members of the MDT on each site for part or all of the time between 2009 and 2013. The design of the questionnaires was based on those used in previous work by Morgan et al.16 They were returned and responses analyzed anonymously. A sample of the questionnaire is attached as Supplementary material.
All data analysis was carried out in SPSS version 22.0 (IBM Corp, Armonk, NY, USA). Graphs were created in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). Chi-square test was used for comparisons.
Results
Management in the two units
In total, 3,850 patients were treated for breast cancer at the two units over the 5-year period. 954 patients were aged ≥70 years at the time of diagnosis so were included in the study. 487 patients were treated at Unit 1 and 467 patients were treated at Unit 2. Treatment regimens involved different combinations of surgery, endocrine therapy, radiotherapy and chemotherapy with 16 different combinations used altogether. These combinations were simplified to those whose initial management plan included surgery at any point, involved endocrine treatment only and other. Over the 5-year period, 371 (76.2%) patients were managed surgically in Unit 1, compared to 300 (63.7%) patients in Unit 2 (p<0.0001). When broken down by year, this difference was maintained throughout, though was not statistically significant in 2011 or 2012 (Figure 1).
Clinicopathological characteristics
The median age of patients in both units was 77 years (range 70–97 years Unit 1, 70–101 years Unit 2). There was no difference between the two units in tumor pathological characteristics, namely histological type, clinical T stage, tumor grade, number of involved lymph nodes, estrogen status or human epidermal growth factor 2 status (Table 1).
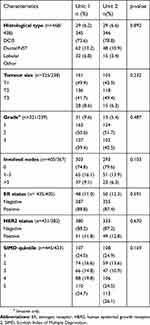 | Table 1 Pathological characteristics of breast cancers and SIMD quintile of patients treated within each unit |
Deprivation
SIMD scores were available for 445 patients from Unit 1 and 433 patients from Unit 2. Within each unit, they were divided into quintiles, with quintile 1 representing the most deprived patients and quintile 5, the least deprived. 107 (24.0%) Unit 1 patients were in quintile 1 compared to 108 (24.9%) of Unit 2 patients. 110 (24.7%) of Unit 1 patients were in quintile 5 compared to 113 (26.1%) Unit 2 patients. Overall, there was no difference in levels of deprivation demonstrated between the two units (Table 1).
Comorbidity
No difference in CCI was seen overall between the two units. Both units had a median CCI of 4 (range 3–13 Unit 1, 3–11 Unit 2). However, a difference in CCI was seen in patients who were managed surgically in each unit. When grouped into low (3–5), medium (6–9) and high (10+) CCI groups, a higher proportion of Unit 1 surgical patients (16.2%) were in the two higher comorbidity groups compared to Unit 2 (11%) (p=0.036) (Table 2).
 | Table 2 Distribution of Charlson Comorbidity Index scores for patients who underwent surgery in each unit |
There was no difference between the two units in median number of emergency admissions for patients in the year preceding diagnosis (Unit 1: 0 [0–6], Unit 2: 0 [0–7]). Similarly, there was no difference demonstrated between the two units in terms of inpatient days per patient in the two years preceding diagnosis (Unit 1: median 0 [0–232], Unit 2: 0 [0–327]). 66.1% patients in Unit 1 had 0 inpatient days compared to 64.5% for Unit 2. For 1–7 inpatient days, it was 19.5% and 19.1%, respectively, for 8–21 days 8.4% and 8.8%, for 22–90 days 4.7% and 5.8% and 1.2% and 1.9% had more than 90 inpatient days.
Attitudes of health care staff
The questionnaire was distributed by e-mail to 29 people on two separate occasions. There were 18 respondents, 6 from Unit 1 (3 surgeons, 1 breast care nurse and 2 oncologists), 11 from Unit 2 (3 surgeons, 2 breast care nurses, 2 oncologists, 2 radiologists, 1 pathologist and 1 anesthetist) and 1 (radiologist) who covered both sites equally so was excluded from comparative analysis. There was little difference in the responses to most of the questions in the questionnaire between the two sites. However, there was a suggestion of a stronger feeling amongst staff in Unit 1 that surgery is superior to PET, compared to Unit 2 (Figure 2A). Staff in Unit 1 felt more strongly that general anesthetic (GA) is safe in elderly breast cancer patients (Figure 2B) and seem more willing to perform a wide local excision under local anesthetic (Figure 2C).
There was general agreement that comorbidities (17/17) (with respondents rating it as very or moderately important), life expectancy (17/17), patient choice (16/16), frailty (16/16) and dementia (16/17) were the most important factors in deciding whether a patient should undergo surgery with age and family preference felt to be the least important factors (Figure 3). Assessment tools used to help make these decisions were most commonly “end of the bed test” or simple tests such as walking up a flight of stairs, while formal comprehensive geriatric assessments were rarely used (Figure 4).
Discussion
In this study, we report a significant difference in the rates of surgical treatment of breast cancer, in patients aged ≥70 years, at two neighboring city hospitals. There was no difference in patient or tumor factors between the two cohorts to explain this difference. Unit 1 operated on a higher proportion of patients with high levels of comorbidity than Unit 2, which may be explained by differences in attitudes of health professionals towards surgery in the elderly.
Our findings are in keeping with a number of other studies which have reported variation in the rates of surgical management of breast cancer in the elderly. It is known that elderly patients are often treated less aggressively for breast cancer than their younger counterparts. Several studies have reported lower rates of surgical treatment with increasing age.8,10,17–21 Most recently, the National Audit of Breast Cancer in Older Patients (NABCOP) 2017 Annual Report reported rates of breast cancer surgery of 90% in patients aged 50–74 years which fell to 15% in those aged >90, between 2011 and 2015.21 It has been reported that axillary surgery is less aggressive in older age groups,17,19,20 and rates of adjuvant treatment fall with increasing age.3,17,19,20,22 These differences have been reported despite adjustment for tumor characteristics,20 levels of comorbidity and functional status23,24 and patient choice.25 A 2007 review by Bouchardy et al described rates of substandard treatments from 13% to 57% in older women.24
Our key finding in this study is the difference in rates of surgical treatment of patients aged ≥70 years between the two units studied. Regional variation in surgical management of breast cancer in older patients has been reported previously. Data from other countries in Europe describe higher surgical rates in elderly patients than those in the UK.17–19 A Canadian study reported that all of their patients with stage I–III primary breast cancer aged ≥70 years underwent surgery in some form9 and only 1.7% of patients in an American study of 49,616 women did not undergo surgery.3 Beyond these international differences, variation in surgical management of older patients has also been reported between different regions of the UK, even after adjustment for case mix.11,21,26 The Breast Cancer Clinical Outcome Measures (BCCOM) project reported rates of nonsurgical treatment which ranged from 12% to 40% across different regions of England and Wales.26 A national interview and questionnaire-based study reported variable rates of PET use, ranging from 37.9% respondents using PET in <10% women ≥70 years to 7.1% saying they used it in >30% women.16 The results of our study add to this body of evidence by reporting that this variability in treatment is not only international or regional, but even extends to adjacent hospitals within the same health board.
Our results showed that Unit 1 operated on patients with higher levels of comorbidity. The questionnaire results suggest that it may be differences in attitudes of health professionals which are responsible for this variation. Members of the Unit 1 MDT were more confident in the safety of general anesthetic in elderly patients, supported in this view by a perioperative mortality rate of 0% over the study period, and were more willing to carry out breast-conserving surgery under local anesthetic. These differences may have a number of explanations, including differences in training and experience of the surgeons and anesthetist. It should also be noted, however, that while an equal number of respondents from each unit were surgeons, they made up a higher proportion of total respondents from Unit 1 which may affect these results. While guidelines clearly state that the decision regarding treatment should not be based on age but on fitness for surgery,6 there is a paucity of guidelines regarding who should be regarded as fit. Our results also show that objective measures of fitness for surgery are rarely used in this setting. A previous study involving questionnaires and interviews with health professionals nationwide reported similar results. As in our study, most respondents felt that level of comorbidity, frailty and life expectancy were more important than age in decision-making, while opinion was divided regarding dementia. As in our study, there was variable experience with local and regional anaesthetic.16
Another factor which could influence patient management is patient choice, which was impossible to reliably assess retrospectively in this study. However, some studies have suggested that patient preference is not a strong factor in determining management in this patient group. Lavelle et al reported that patients’ role in decision-making made no difference to whether they had surgery or not.25 A review by Bouchardy et al reported that only a small proportion of patients refused all or part of their proposed treatment, a minority of patients were prepared to consider lighter treatment and refusal rates for clinical trials were similar for all ages.24 In contrast to this, Vetter et al reported that 13% patients aged >80 years refused endocrine therapy when it was recommended and 49% refused radiotherapy.19 Similarly, Hamaker et al reported that patient choice was the reason for omitting surgery in 32% patients aged ≥75 years.18
Other limitations of this study include its retrospective nature. Though details of tumor characteristics and treatment are recorded prospectively, there is a reliance on accurate coding of data. Comorbidity data were collected retrospectively, but a number of sources within the electronic clinical record were used to optimize retrieval of diagnoses. The questionnaires required practitioners to respond to questions in the present relating to their attitudes and practice several years ago. These may have changed over time and findings may be subject to recall bias. There was a 62% response rate but 100% surgeons responded. As a measure of comorbidity, the CCI is limited in that it measures a diagnosis and not the extent to which that diagnosis limits the patient functionally, neither does it assess frailty. For this reason, we employed a second, surrogate marker of comorbidity, inpatient bed days and emergency admissions, as this may provide a clearer representation of a patient’s function and frailty in the years immediately preceding to diagnosis. Statistically, our study is limited by sample size, particularly as regards analysis of questionnaire results, but this was unavoidable since the aim of our study was to investigate whether the known regional variation in the management of breast cancer in the elderly was born out in two neighboring hospitals where it might be expected that practice would be similar.
Conclusion
A higher proportion of patients aged >70 years with breast cancer were managed surgically in Unit 1 compared to Unit 2. Reasons for variation in practice seem to be related to attitudes of medical professionals toward surgery in the elderly, rather than patient or pathological factors. Further research and guidelines are required to aid practitioners in deciding who is fit for surgery, to improve uniformity of treatment.
Ethics
This study is an audit, and the patients included in it are all patients of the authors of this article, and therefore, in Scotland, formal ethical approval is not required. Permission to obtain the comorbidity data from the National Services Scotland Information Services Division was granted by the Caldicott Guardian for NHS Greater Glasgow and Clyde. The study was conducted in compliance with the Declaration of Helsinki.
Acknowledgment
This study was presented at theAssociation of Breast Surgery Conference 2017 (Published in: EuropeanJournal of Surgical Oncology 2017; 43(5), S32, P075).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cancer Research UK Statistics. Available from:
2. Chatzidaki P, Mellos C, Briese V, Mylonas I. Does primary breast cancer in older women (>/=80 years) have unfavorable histological characteristics? Arch Gynecol Obstet. 2011;284(3):705–712. doi:10.1007/s00404-010-1697-5
3. Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–2045. doi:10.1200/JCO.2009.25.9796
4. Glaser R, Marinopoulos S, Dimitrakakis C. Breast cancer treatment in women over the age of 80: a tailored approach. Maturitas. 2018;110:29–32. doi:10.1016/j.maturitas.2018.01.014
5. Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–e160. doi:10.1016/S1470-2045(11)70383-7
6. Nice CG80: early and locally advanced breast cancer: diagnosis and treatment. February 2009. Available from:
7. El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W. Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg. 2007;245(5):665–671. doi:10.1097/01.sla.0000245833.48399.9a
8. Bates T, Kearins O, Monypenny I, Lagord C, Lawrence G. Clinical outcome data for symptomatic breast cancer: the Breast Cancer Clinical Outcome Measures (BCCOM) project. Br J Cancer. 2009;101(3):395–402. doi:10.1038/sj.bjc.6605155
9. Angarita FA, Chesney T, Elser C, Mulligan AM, McCready DR, Escallon J. Treatment patterns of elderly breast cancer patients at two Canadian cancer centres. Eur J Surg Oncol. 2015;41(5):625–634. doi:10.1016/j.ejso.2015.01.028
10. Richards P, Ward S, Morgan J, et al. The use of surgery in the treatment of ER+ early stage breast cancer in England: variation by time, age and patient characteristics. Eur J Surg Oncol. 2016;42(4):489–496. doi:10.1016/j.ejso.2015.12.012
11. Morgan J, Richards P, Ward S, et al. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg. 2015;102(9):1056–1063. doi:10.1002/bjs.9842
12. Cheung SGN, Lagord C, Williams L, Kearins O, Lawrence G. All Breast Cancer Report: A UK Analysis of All Symptomatic and Screen-Detected Breast Cancers Diagnosed in 2006. West Midlands Cancer Intelligence Unit. National Cancer Intelligence Network
13. G KO L, Lagord C, Cheung S, Sidhu J, Sagar C. The Second All Breast Cancer Report. Focussing on Inequalities: Variation in Breast Cancer Outcomes with Age and Deprivation. National Cancer Intelligence Network. West Midlands Cancer Intelligence Unit; 2007.
14. Scottish government Scottish Index of Multiple Deprivation. Available from:
15. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251.
16. Morgan JL, Collins K, Robinson TG, et al. Healthcare professionals’ preferences for surgery or primary endocrine therapy to treat older women with operable breast cancer. Eur J Surg Oncol. 2015;41(9):1234–1242. doi:10.1016/j.ejso.2015.05.022
17. Bastiaannet E, Liefers GJ, de Craen AJ, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124(3):801–807. doi:10.1007/s10549-010-0898-8
18. Hamaker ME, Bastiaannet E, Evers D, et al. Omission of surgery in elderly patients with early stage breast cancer. Eur J Cancer. 2013;49(3):545–552. doi:10.1016/j.ejca.2012.08.010
19. Vetter M, Huang DJ, Bosshard G, Guth U. Breast cancer in women 80 years of age and older: a comprehensive analysis of an underreported entity. Acta Oncol. 2013;52(1):57–65. doi:10.3109/0284186X.2012.731523
20. Lavelle K, Todd C, Moran A, Howell A, Bundred N, Campbell M. Non-standard management of breast cancer increases with age in the UK: a population based cohort of women > or =65 years. Br J Cancer. 2007;96(8):1197–1203. doi:10.1038/sj.bjc.6603709
21. National Audit of Breast Cancer in Older Patients 2017 Annual Report. 2017. Available from: https://www.nabcop.org.uk › Reports. Accessed July 17, 2018.
22. van de Water W, Markopoulos C, van de Velde CJ, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. Jama. 2012;307(6):590–597. doi:10.1001/jama.2012.84
23. Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C. Older women with operable breast cancer are less likely to have surgery. Br J Surg. 2007;94(10):1209–1215. doi:10.1002/bjs.5834
24. Bouchardy C, Rapiti E, Blagojevic S, Vlastos AT, Vlastos G. Older female cancer patients: importance, causes, and consequences of undertreatment. J Clin Oncol. 2007;25(14):1858–1869. doi:10.1200/JCO.2006.10.4208
25. Lavelle K, Sowerbutts AM, Bundred N, et al. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer. 2014;110(3):573–583. doi:10.1038/bjc.2013.734
26.
In each case, please replace the relevant □ with “X”.
NB. For the purposes of this questionnaire, “elderly” is defined as age ≥70 years.
Of which MDT were you a member?
West □ South □
Over what time period between 2009 and 2013 were you a member of that MDT?
–––––––––––––––––––––––––
What was your role on the MDT during the above time period?
Surgeon□ BC nurse□ Oncologist□ Radiologist□ Pathologist□ Anaesthetist□ Other □
Attitudes to surgery
Please indicate the degree to which you agree with the following statements.
1. Surgery is superior to PET in elderly patients.
Strongly agree □ Agree □ Unsure □ Disagree□ Strongly disagree□
2. All women aged >70 years should be offered an operation regardless of age.
Strongly agree □ Agree □ Unsure □ Disagree □ Strongly disagree □
3. Surgery under general anesthesia (GA) is generally safe in elderly breast cancer patients.
Strongly agree □ Agree □ Unsure □ Disagree□ Strongly disagree □
4. I would be happy to perform wide local excision under local anesthetic for an elderly patient deemed high risk for GA.
Strongly agree □ Agree □ Unsure □ Disagree □ Strongly disagree □ Not applicable to me □
5. I would be happy to carry out mastectomy under LA for an elderly patient deemed high risk for GA.
Strongly agree □ Agree □ Unsure □ Disagree □ Strongly disagree □ Not applicable to me □
6. In my institution, there would be no difficulty in arranging for an anesthetist to carry out a regional block for breast cancer surgery.
Strongly agree □ Agree □ Unsure □ Disagree □ Strongly disagree □ Not applicable to me □
Attitudes to PET
7. In your experience, how effective is PET at managing breast cancer in the elderly?
Very effective □ Reasonably effective □ Equivocal □ Not particularly □ Not at all □
Please indicate the degree to which you agree with the following statements.
8. PET should be offered to all ER-positive patients over 70 years.
Strongly agree □ Agree □ Unsure □ Disagree □ Strongly disagree □
Perception of patient preference
9. Given the choice, most elderly patients would opt for surgery rather than PET.
Strongly agree □ Agree □ Unsure □ Disagree □ Strongly disagree □
Assessment tools and important factors
10. In your practice, how frequently do you use the following tests to assess fitness for surgery in elderly patients? (If not applicable, please skip to question 11.)
a) End of the bed test
Always □ Frequently □ Sometimes □ Rarely/Seldom □ Never □
b) Simple tests, eg, walk up flight of stairs
Always □ Frequently □ Sometimes □ Rarely/Seldom □ Never □
c) Formal anesthetic assessment
Always □ Frequently □ Sometimes □ Rarely/Seldom □ Never □
d) Comprehensive geriatric assessments
Always □ Frequently □ Sometimes □ Rarely/Seldom □ Never □
11. In your opinion, how important are the following factors in determining whether an elderly patient undergoes surgery or not?
a) ER status
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
b) HER-2 status
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
c) Tumor size
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
d) Axillary disease
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
e) Age
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
f) Comorbidities
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
g) Frailty
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
h) Dementia
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
i) Patient choice
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
j) Life expectancy
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
k) Functional status
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
l) Anxiety about surgery
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
m) Anxiety about breast cancer
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
n) Family preference
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
12. In your experience, how important are the following factors in explaining the wide variation in treatment for elderly patients with breast cancer?
a) Medical staff attitudes
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
b) Patient attitudes
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
c) Lack of guidelines
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
d) More variability in patients/heterogeneous group
Very Important □ Moderately important □ Equivocal □ Not very important □ Not at all important
13. Please use the box below to provide any other comments you have on this subject.
 |
Many thanks for taking the time to complete this questionnaire.
Figure S1 Questionnaire for MDT members Western Infirmary & Victoria Infirmary 2009–2013.
Abbreviations: BC, board certified; ER, estrogen receptor; HER2, human epidermal growth receptor 2; LA, local anethesia; MDT, multidisciplinary team; PET, primary endocrine therapy.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




