Back to Journals » Medical Devices: Evidence and Research » Volume 10
Variability in delivered dose and respirable delivered dose from nebulizers: are current regulatory testing guidelines sufficient to produce meaningful information?
Authors Hatley RHM, Byrne SM
Received 19 October 2016
Accepted for publication 5 December 2016
Published 1 February 2017 Volume 2017:10 Pages 17—28
DOI https://doi.org/10.2147/MDER.S125104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ross HM Hatley, Sarah M Byrne
Respironics Respiratory Drug Delivery (UK) Ltd, a business of Philips Electronics UK Limited, Chichester, UK
Background: To improve convenience to patients, there have been advances in the operation of nebulizers, resulting in fast treatment times and less drug lost to the environment. However, limited attention has been paid to the effects of these developments on the delivered dose (DD) and respirable delivered dose (RDD). Published pharmacopoeia and ISO testing guidelines for adult-use testing utilize a single breathing pattern, which may not be sufficient to enable effective comparisons between the devices.
Materials and methods: The DD of 5 mg of salbutamol sulfate into adult breathing patterns with inhalation:exhalation (I:E) ratios between 1:1 and 1:4 was determined. Droplet size was determined by laser diffraction and RDD calculated. Nine different nebulizer brands with different modes of operation (conventional, venturi, breath-enhanced, mesh, and breath-activated) were tested.
Results: Between the non-breath-activated nebulizers, a 2.5-fold difference in DD (~750–1,900 µg salbutamol) was found; with RDD, there was a more than fourfold difference (~210–980 µg). With increasing time spent on exhalation, there were progressive reductions in DD and RDD, with the RDD at an I:E ratio of 1:4 being as little as 40% of the dose with the 1:1 I:E ratio. The DD and RDD from the breath-activated mesh nebulizer were independent of the I:E ratio, and for the breath-activated jet nebulizer, there was less than 20% change in RDD between the I:E ratios of 1:1 and 1:4.
Conclusion: Comparing nebulizers using the I:E ratio recommended in the guidelines does not predict relative performance between the devices at other ratios. There was significant variance in DD or RDD between different brands of non-breath-activated nebulizer. In future, consideration should be given to revision of the test protocols included in the guidelines, to reflect more accurately the potential therapeutic dose that is delivered to a realistic spectrum of breathing patterns.
Keywords: nebulizer, inhalation:exhalation (I:E) ratio, breathing pattern, delivered dose (DD), respirable delivered dose (RDD), testing guidelines
Introduction
The design of nebulizers has been the subject of considerable development activity in recent years. This includes both compressor-based jet-nebulizer systems and more portable mesh-based device designs. The focus of the development activity has been geared toward making the devices more patient/carer-friendly (eg, faster, quieter, and less environmental contamination with drug). There have been a number of publications on the variability of nebulizer–compressor combinations with respect to delivered dose (DD),1–3 but both the mode of operation of the nebulizer itself and the additional variability that could be introduced by the patient have received less attention.
In the four main guidelines for nebulizer testing (European Committee for Standardization [CEN] EN 13544-1,4 United States Pharmacopoeia [USP] chapter 1601,5 European Pharmacopoeia [EP] chapter 2.9.44,6 and International Organization for Standardization [ISO] 27427:2013[E]),7 test methods for nebulizers are described in terms of DD, output rate, and mass median aerodynamic diameter. It is of note that the USP contained such guidance up to USP 37,8 but in recent updates all guidance relating to parameters to report and how to calculate them has been removed.9
The ISO 27427:2013(E) standard states that its objective is “to ensure that the results of the various tests declared by the manufacturer are meaningful to the users and buyers of nebulizers”.7 The test methods for DD (the total amount of drug that leaves the nebulizer and is delivered into inhalation) and DD-output rate (the amount of drug delivered into inhalation during a minute) use a single standardized breathing pattern of 500 mL tidal volume, 1:1 inhalation:exhalation (I:E) ratio, and 15 breaths per minute (BPM) frequency. The standards represent a good basis for the direct comparison of nebulizers under in vitro lab conditions for quality-control purposes. They are, however, limited in that the respirable DD (RDD; the amount of drug contained in droplets of a size suitable for penetration into the lungs,10 ie, in the respirable range <5 µm) leaving the nebulizer mouthpiece during inhalation is not directly reported. In the ISO 27427:2013(E) standard, it is stated that “the percentage of fill volume emitted is an important value to be disclosed to the user, because it can influence the decisions of dosage intended for delivery in terms related to the expected amount of drug given to the patient”.7 This statement shows an attempt to address clinically relevant parameters in the standard, but the omission of RDD or use of different patient-relevant breathing patterns could lead to incorrect decisions in terms of the expected amount of clinically effective drug delivered to the patient from a device. The results of the test methods in the standards are thus limited in their clinical usefulness.
Within publications and manufacturer literature, a number of different terms are used to describe the performance of nebulizers, but the meanings of the terms can vary from publication to publication, eg, in some publications, the term “delivered dose” has been used for everything that leaves the nebulizer gravimetrically (ie, solution delivered into exhalation and into inhalation, as well as the sum of the mass of solution from within the reservoir solution that is lost to evaporation),11,12 while in other publications it has been used to refer to the drug that is delivered to inhalation only.13–15 The terms used in this article are detailed in Table 1.
  | Table 1 Explication of terms used in the text |
The study reported in this article was conducted in three parts. The protocols recommended in the guidelines are used in Part 1 to assess DD, as well as additional parameters significant to nebulizer performance, such as RDD. Outside the laboratory, patient breathing patterns vary considerably, and the dose delivered to the 1:1 I:E ratio laboratory pattern may not reflect the actual dose delivered to the patient. In some studies,16,17 a 1:2 I:E ratio has been used, which is more representative of a healthy adult breathing ratio.18 It has also been reported that patients with obstructive lung function can have I:E ratios of in excess of 1:4.19–22 Although other factors may affect real-life device use, eg, peak inspiratory flow and tidal volume, to enable an identification of the effects of the highly significant I:E ratio without the complication of having to resolve effects from the other variables, I:E ratio was as far as possible isolated as a single variable. I:E ratios between 1:1 and 1:4 are used in Part 2 to determine the effect on DD and RDD, and the contrast with emitted dose (ED) and respirable ED (RED), which are often quoted by manufacturers and in publications. Breath-activated nebulizers deliver only into inhalation, and thus in theory DD should be independent of I:E ratio3 and DD and ED should be equivalent. Therefore, the effect of I:E ratio on the performance parameters of breath-activated nebulizers that are claimed to be breathing pattern-independent is examined in Part 3.
Although parameters in the breathing pattern besides I:E ratio, such as peak inspiratory flow and tidal volume, also potentially affect DD and RDD, the scope of this investigation was limited to change the I:E ratio only, to enable a clear separation of the varying effects of this on the nebulizers of different modes of operation, without the complication of other variables.
Types of nebulizer evaluated
A summary of the modes of operation of nebulizers that are currently available for use in aerosol therapy is presented in Table 2. It is clear that there are a number of different modes of operation, and even within a mode of operation there can be subtypes, eg, jet nebulizers can be conventional, venturi, or breath-enhanced. The focus of the study reported in this article was on jet and mesh nebulizers, to examine the effect of the mode of operation and breathing pattern on DD and RDD.
  | Table 2 Modes of operation of nebulizers |
It is useful to visualize the effect of the different modes of operation of different nebulizer types upon the delivered drug. A typical means of doing this is via a tidal breathing flow-time graph. The principles of operation of the three subtypes of jet nebulizer are illustrated in Figure 1. A conventional nebulizer uses a driving gas flow (typically 6 L/min) to generate aerosol. It delivers an approximately equal volume of aerosol into inhalation and exhalation when a breathing pattern with a 1:1 I:E ratio is employed. The addition of a venturi to the nebulizer allows additional air to be entrained, and thus the same 6 L/min driving flow is complemented by entrained air to give a total output of aerosol-laden air of around 16 L/min (Figure 1B). This makes delivery faster, but still produces equal delivery into inhalation and exhalation. The breath-enhanced design of the nebulizer includes a valve that allows the venturi effect to be exploited during inhalation; however, the valve closes during exhalation, thus reducing aerosol delivery during this part of the breathing cycle. This enables more drugs to be delivered to inhalation and reduces the amount of drug lost to exhalation (Figure 1C).
The I-neb Adaptive Aerosol Delivery (AAD) System and the AeroEclipse II are breath-activated devices that deliver only into inhalation (Figure 1D and E). The I-neb AAD System is a mesh-based nebulizer system that delivers aerosol using sensors and electronics, which run a breathing-pattern algorithm. The I-neb AAD System nebulizer can be run in two different modes of operation – tidal breathing mode (TBM) and target inhalation mode – and only TBM is relevant to this article. TBM operates when a patient inhales spontaneously during tidal breathing, during which the I-neb AAD System nebulizer monitors the inspiratory flow rate and length of the inhalation. Aerosol is then pulsed during the first 50%–80% of the inhalation, depending upon the specific characteristics of the breathing pattern. The duration of each pulse of aerosol is determined by the patient’s breathing pattern and varies for each subsequent breath, depending on a rolling average of the preceding three breaths. These features eliminate waste during exhalation, provide precise dose delivery independently of I:E ratio, and give the patient feedback on performance.23 The AeroEclipse II is a jet nebulizer with a mechanical mechanism that allows the production of aerosol only when the inhalation airflow exceeds a certain flow rate. This provides control over the portion of the breath into which the aerosol is delivered, but is dependent on the inhalation flow of the patient. This can result in aerosol being delivered into the last part of the breath. On exhalation, this aerosol would be exhaled and wasted into the environment. Nine different brands of nebulizers were used in this study, and are shown in Table 3.
  | Table 3 Nebulizers used in the study |
Materials and methods
Droplet size
Droplet-size distributions of salbutamol sulfate aerosols produced by the nebulizers were evaluated in terms of volume median diameter (VMD) and fine-droplet fraction (FDF) by laser diffraction. Laser diffraction was chosen, as salbutamol sulfate is a homogeneous solution, and thus the drug is distributed equally in the droplets; therefore, the reported values of droplet size represent the drug distribution and allow a greater number of measurements in a reasonable time frame compared with the more time-consuming determination of mass median aerodynamic diameter using an impactor. Values are reported as VMD, but due to the homogeneous distribution of the drug, this equates to mass median diameter. The nebulizers were filled with 2.5 mL salbutamol sulfate solution (2 mg/mL, Salamol Steri-Neb; Ivax Pharmaceuticals, Castleford, UK). The aerosols were evaluated using a Mastersizer S laser-diffraction particle-size analyzer (Malvern Instruments Ltd., Malvern, UK). The Mastersizer S was operated using an open flow-cell arrangement used to present aerosol to the laser, and included a 10 L/min shroud airflow introduced into this cell and a 30 L/min extraction airflow set at the exit of this cell. The nebulizers were attached at the entrance to the Mastersizer S flow cell and sealed. Droplet-size measurements were made after 20 seconds of priming. Three measurements were determined, and there was a 20-second delay between each measurement. The nebulizers were then stopped after the third determination. For each nebulizer brand, three nebulizers were each tested in triplicate. The I-neb AAD System nebulizer was run in an engineering test mode that allows continuous operation without the need for a simulated breathing pattern to be applied.
Part 1: Standard test protocol with 1:1 I:E ratio
The methods used to determine the interbrand DD variability between the seven non-breath-activated devices were based on methodology stated in the CEN EN 13544-1 guideline.4 Each of the seven nebulizers was weighed, filled with 2.5 mL of 2 mg/mL salbutamol, reweighed, and connected via a filter (Filtrete; 3M, Maplewood, MN, USA) to an ASL 5000 breathing simulator (IngMar Medical, Pittsburgh, PA, USA) set to generate the CEN standard test pattern (tidal volume 500 mL, I:E ratio 1:1, BPM 15) (Figure 2). The connection between the filter and nebulizer mouthpiece was sealed with Parafilm (Alcan Packaging, Neenah, WI, USA). The nebulizers were run until 60 seconds after the onset of sputter (detected audibly by the operator) for the jet nebulizers, and until the end of aerosol generation for the mesh nebulizers. The jet nebulizers were driven by 6 L/min wall air. The nebulizers were reweighed at the end of nebulization to determine the residual mass and gravimetric ED. The drug collected on the filter was eluted for quantification by high-performance liquid chromatography to give the DD. As with the droplet-size tests, three of each brand of nebulizer were tested in triplicate and washed in warm soapy water, rinsed, and dried in a drying cabinet between the tests.
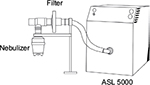  | Figure 2 Assessment of emitted dose. |
Part 2: Delivered dose variability between non-breath-activated nebulizers when tested with different I:E ratios
To determine the DD variability between the seven non-breath-activated devices with different I:E ratios, the same methods were employed as those used to determine the interbrand DD variability between the devices, using three additional breathing patterns, shown in Table 4 as B, C, and D.
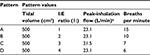  | Table 4 Summary of breathing patterns used in the study Note: aDetermined using a flow meter during pattern verification at the start of testing. Abbreviation: I:E, inhalation:exhalation. |
Part 3: Delivered dose variability between mechanical electronic breath-activated nebulizers when tested with different I:E ratios
The methods employed to determine the DD variability between the breath-activated devices with different I:E ratios were the same as those previously described, using the three additional breathing patterns shown in Table 4. The I-neb AAD System nebulizer was fitted with a 0.5 mL dosing chamber to ensure that the DD was within the range of the non-breath-activated nebulizers, and filled with the same fill volume as the other nebulizers.
Results
Droplet size
There was a considerable range in droplet sizes across the different brands of nebulizer, ranging from 3.27 µm to 7.35 µm (Table 5). This droplet-size variability is reflected in the FDF, the amount of drug in the respirable range, which across the nebulizers ranged from 30% to 73%. The laboratory humidity conditions during droplet-size analysis were relatively constant, ranging from 46% to 50% relative humidity.
Part 1: Standard test protocol with 1:1 I:E ratio
The DD between the non-breath-activated nebulizers ranged from ~750 µg to ~1,900 µg salbutamol. This is an almost threefold difference in the amount of salbutamol delivered. As expected, the conventional and venturi nebulizers delivered less to inhalation than the breath-enhanced nebulizers, due to greater delivery efficiency of aerosol into the inhalation portion of the breath by the breath-enhanced nebulizers (Figure 1). The greatest DD was obtained from the MicroAir U22 mesh nebulizer.
The RDD was calculated by multiplying the DD by the FDF. The amount of salbutamol delivered by the different non-breath-activated nebulizers varied from ~210 µg to ~980 µg; this is a more than a fourfold difference. It is also notable that although the DD from the Salter 8900 and SideStream nebulizers was similar (Figure 3A), the difference in droplet size and FDF resulted in a significant difference between the two nebulizers when RDD was considered (Figure 3B).
The nonrespirable DD (NRDD) was calculated by subtracting the RDD from the DD. This represents the amount of drug delivered through the nebulizer that is potentially too large to reach the lung and is deposited in the oral cavity or throat. There was a nearly fivefold difference in NRDD between the nebulizers (Figure 3C), ranging from ~210 µg to 960 µg salbutamol.
The results of residual solution remaining in the nebulizers following nebulization are illustrated in Figure 3D. A residual between 1.45 g and 1.84 g solution was observed for most of the non-breath-activated nebulizers, regardless of whether they were conventional, venturi, breath-enhanced, or mesh nebulizers. However, when compared with the other non-breath-activated nebulizers, the residual of the MicroAir U22 nebulizer was much lower (0.62 g solution), which would account for its considerably higher DD when tested with the same fill volume as the other nebulizers.
Part 2: Effect of I:E ratio on dose and treatment time for non-breath-activated nebulizers
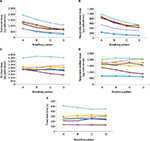
The DD from all the non-breath-activated nebulizers tested reduced as the I:E ratio changed from 1:1. The DD varied from ~1,900 µg salbutamol with the MicroAir U22 nebulizer at a 1:1 I:E ratio down to ~200 µg salbutamol with the Salter 8900 nebulizer at a 1:4 I:E ratio (Figure 4A). As was found with the comparison of nebulizers at a fixed 1:1 I:E ratio, the expression of the dose as RDD rather than DD resulted in wider variability in the dose delivered by the nebulizers. The RDD varied from ~1,000 µg with the MicroAir U22 nebulizer at an I:E ratio of 1:1, to ~100 µg with the Salter 8900 nebulizer at an I:E ratio of 1:4 (Figure 4B), and thus a tenfold difference in RDD was observed.
The ED represents all of the mass of liquid leaving the nebulizer into inhalation, exhalation, and due to evaporation. Since the nebulizers were run continuously, this would be expected to be independent of I:E ratio. This was the case for most of the non-breath-activated nebulizers; however, the ED results for the eFlow Rapid nebulizer showed some breathing-pattern dependence (Figure 4C).
As with ED, RED would also be expected to be independent of I:E ratio. As with RDD, the addition of the FDF into the calculation resulted in a change in the relative dose from each of the brands of non-breath-activated nebulizer (Figure 4D). As shown in Figure 4E, treatment times for each of the non-breath-activated nebulizers were independent of I:E ratio, but between the brands there were considerable differences, ranging from ~140 seconds to 500 seconds.
Overall, the variability increased as the output measurement moved from simple ED to other performance factors. DD included the variability of the nebulizers in the ratio of drug delivered to the mouthpiece and that delivered to exhalation, and RDD further included the FDF, a measure of the quality of the aerosol generated by the nebulizer. For all the non-breath-activated nebulizers that operate continuously, with the exception of the MicroAir U22 nebulizer, EDs (measured gravimetrically) were very similar, clustered around 2,500 µg salbutamol (Figure 4C), ie, 50% of the dose was retained within the device. When DD was considered, significant variability was apparent between the brands, as DDs ranged from ~700 µg for the conventional and venturi jet nebulizers, ~1,200 µg for the breath-enhanced jet nebulizers, and ~1,900 µg for the MicroAir U22 mesh nebulizer.
Part 3: Effect of I:E ratio on dose and treatment time for mechanical and electronic breath-activated nebulizers
The DD from the I-neb AAD System nebulizer was independent of I:E ratio, as it delivered ~1,000 µg salbutamol across the four breathing patterns. The AeroEclipse II nebulizer delivered on average ~1,500 µg salbutamol across the four breathing patterns, but showed a small dependence of dose on I:E ratio, with less being delivered as the I:E ratio changed from 1:1 to 1:4 (Figure 5A).
The average RDD from the I-neb AAD System nebulizer was ~600 µg, and from the AeroEclipse II nebulizer it was ~1,100 µg, although as with DD, there was a small change in the amount delivered across the four breathing patterns (Figure 5B). The RDD from the AeroEclipse II nebulizer was approximately twice that from the other jet nebulizers at a 1:1 I:E ratio (Figure 4B). This would be expected, as the AeroEclipse II nebulizer delivers all of the drug from a standard fill into inhalation rather than into both inhalation and exhalation, as with the non-breath-activated nebulizers.
The ED and RED from the I-neb AAD System nebulizer remained constant throughout the four breathing patterns (Figure 5C and D). However, as shown in Figure 5C, there appeared to be a decrease in the ED from the AeroEclipse II nebulizer as the I:E ratio increased. The reason for this is not clear, but it reflects the similar trend found with the DD and RDD.
As expected, there was an increase in treatment time for both of the breath-activated nebulizers as the I:E ratio increased (Figure 5E), reflecting the greater time spent in exhalation per breath and thus the greater time during which aerosol was not being produced. Due to its improved overall efficiency, the I-neb AAD System nebulizer is metered to provide an equivalent DD to that provided by the jet nebulizers. As a consequence of the smaller total amount of drug that needs to be nebulized to give an equivalent DD to that from a non-breath-activated nebulizer, nebulization is faster with the I-neb AAD System nebulizer (200 seconds compared with up to 300 seconds with the jet nebulizers). The treatment time for the AeroEclipse II nebulizer was 620 seconds, but being unmetered it provided a considerably higher DD of 1,600 µg salbutamol (since the aerosol that would normally go to exhalation was delivered).
Discussion
Variability between the nebulizers when tested with 1:1 I:E ratio as used in standard test protocols
Consistently with the findings of others,1,2 significant differences in ED, DD, and RDD between the nebulizers of different brands were found in this study. In this study, a single nebulizer driving a flow rate of 6 L/min was used for all nebulizers, but the results showed almost threefold variability in DD. Smaldone et al2 reported on a similar study in variability of DD, but used different compressors with a different flow rate. The variability in their study was sixfold, indicating that both the nebulizer and compressor contribute to variability in DD.
For the RDD (Figure 3B), the amount of drug that would be expected to penetrate into the lungs was taken into account. Due to the range of VMDs between the nebulizer brands (Table 5), the range of respirable doses delivered expanded further; overall, there was a fivefold difference between the lowest RDD and highest RDD. Therefore, a low FDF resulted in a lowered RDD. An additional consequence of a low FDF was that much of the ED was not in the respirable range and the NRDD was higher for nebulizers with low FDFs compared with those that produced a higher quality aerosol with a higher FDF (Figure 3C). This NRDD would be deposited in the oral cavity or throat, and would subsequently be swallowed.
The regulatory guidelines provide international standards that enable a direct comparison of DD between different nebulizers. In all three guidelines mentioned earlier in this article, the DD pattern used for adult simulation is a single breathing pattern with a 1:1 I:E ratio, 500 mL tidal volume, and 15 BPM frequency. The data in Figure 3A were obtained using this breathing pattern, allowing for a comparison to be made. Such a comparison would conclude that the Salter 8900 and SideStream nebulizers may underdose and that the MicroAir U22 nebulizer may overdose. However, as discussed, DD includes both the potentially clinically effective RDD and the wasted NRDD, and thus may not represent the best comparison. The comparison of RDD (Figure 3B) showed that although the Salter 8900 nebulizer still underdosed, the doses delivered by the SideStream and MicroAir U22 nebulizers were closer to the nominal delivery of the other nebulizers. All four standards also include a measurement of the particle size and FDF, so the data needed to calculate the RDD are available, but additional calculation is required, as the numbers are reported individually in the manufacturer literature. If the intent of the standards is to provide data that are meaningful to the users and buyers of nebulizers, then quotation of the more clinically relevant parameter (RDD) should be required, rather than relying on the expert knowledge of users and buyers.
Effect of changing I:E ratio on nebulizer performance
With a constant 1:1 I:E ratio, there were considerable differences in the performances of the non-breath-activated nebulizers. However, a 1:1 I:E ratio is not typical of the ratio in either healthy or patient populations. A typical healthy adult has an I:E ratio typically closer to 1:2,24 while in such patient groups as those with chronic obstructive pulmonary disease, this can extend to 1:525 and tends to increase as the condition progresses.26,27 It would be expected that with different I:E ratios, as exhibited with some patient groups, differences in ED, RED, DD, and RDD could become even greater.4
The ED is the measure of the total mass leaving the nebulizer, and as shown in Figure 4C was independent of I:E ratio for all nebulizers except the eFlow Rapid. The variability in ED between the nebulizers was due to differences in residual rather than any nebulizer aerosol-generation performance criteria. The much-higher dose from the MicroAir U22 nebulizer was a consequence of the very low residual mass, which made an additional 1,500 µg of salbutamol available for emission from the nebulizer. Although ED was independent of I:E ratio, as the ED does not give any information about the dose received by the patient, this independence is of limited value.
The DD is of more relevance, as it is the total dose available to the patient and is the quoted figure that clinicians would be expected to use to determine an appropriate dose to be delivered; however, from examination of Figure 4A, it is apparent that the DD was substantially dependent on the I:E ratio, with a percentage change of 40%–60% across the breathing patterns tested, depending on the nebulizer tested. Therefore, the effect of increased I:E ratio upon the DD would need to be accounted for in sicker patients. The decline in DD with increasing I:E ratio was fairly consistent for each nebulizer, so it is possible that the dose calculations could be adjusted. However, as there was an appreciable difference in DD between the nebulizers tested and across the breathing patterns (eg, between the MicroAir U22 nebulizer and the Salter 8900 nebulizer), nebulizers would need to be individually characterized to allow appropriate adjustment.
The RDD also reduced significantly (Figure 4B) as the I:E ratio extended and more time was spent on exhalation, and as with DD, the decline was fairly consistent for each nebulizer. The greatest percentage change was seen with the conventional nebulizer, for which with an increasing I:E ratio the RDD reduced to 40% of that delivered with a 1:1 I:E ratio breathing pattern. In the case of the breath-enhanced nebulizers, a reduction also occurred, as ~55% of the RDD with a 1:1 I:E ratio was delivered with a 1:4 I:E ratio breathing pattern. The MicroAir U22 nebulizer, which appeared to be the best performing of the non-breath-activated nebulizers at a 1:1 I:E ratio, delivered a comparable respirable dose to the SideStream Plus nebulizer at a 1:4 I:E ratio. With RDD, there is the added advantage that the variation in individual nebulizer performance due to FDF is already accounted for, so a clinician using RDD to make dosing decisions would have fewer variables to deal with in setting or adjusting an appropriate dose. Given that the patients with the most severe disease are the ones most likely to suffer extended I:E ratios of 1:4, be prescribed a nebulizer, and be most in need of efficient drug delivery, these results have significant implications for device selection and the standards used to generate the data upon which the selection decisions are made. The results suggest that breath-enhanced nebulizers will deliver proportionally more drug to sicker patients than conventional nebulizers. Therefore, the standards should incorporate test parameters that cover patients from across the spectrum of disease severity to ensure that prescribing decisions are based on representative data.
Not surprisingly, the DD results for the breath-activated nebulizers did not show the same breathing-pattern dependence that was observed with the non-breath-activated nebulizers. The I-neb AAD System nebulizer results showed no trend in dose reduction with an increasing I:E ratio, and thus the DDs and RDDs were independent of I:E ratio across the breathing patterns (Figure 5A and B). However, a slight reduction in dose of approximately 10% was observed for the AeroEclipse II nebulizer across the breathing patterns, and the reason for this is not clear. As these devices are designed to be independent of breathing pattern, testing using the standard pattern is a reliable indicator of performance across the range of breathing patterns.
We have investigated the effect of changes in I:E ratio on the performance parameters of non-breath-activated and breath-activated nebulizers. The I:E ratio, although a major factor, is just one of the parameters of the breathing pattern that could affect dose. It would also be beneficial to explore the relationship between mode of operation of the nebulizer and DD using simulated patient breathing patterns with different peak-inhalation flows and tidal volumes to define fully the potential variability in dose delivered to patients, both within a single nebulizer brand/type and between the brands/types. In addition, it may be beneficial to compare between nebulizers with particle sizes ≤2 µm, as they may be more efficient at reaching small respiratory bronchioles, which is of significance for some disease conditions, so a topic for further study would be to examine whether the range of doses expanded even further for aerosol delivered in these very small particles.
Changing nebulizer types, eg, from a compressor-driven venturi nebulizer to a breath-enhanced nebulizer or portable mesh nebulizer, or vice versa, may need to be carefully considered; indeed, even changing between different brands of nebulizer with the same mode of operation should be carefully considered. For drugs with wide therapeutic ranges, this may not be a great concern, but where the drug has a narrower therapeutic range, it could be a cause for concern.
In this study, the effect of changes in I:E ratio on variability in DD from nine nebulizers was examined. To provide a more comprehensive understanding of the relationship between device mode of operation and DD to patients, additional studies with simulated breathing patterns with I:E ratios typically found with different disease conditions that address the effects of other breathing parameters, such as peak inspiratory flow and tidal volume, would be beneficial.
Conclusion
Based on the results of this study, it can be seen that there is a variance in performance between different brands of nebulizers. Consequently, standards exist to recommend test protocols to evaluate performance. The degree of the variance depends on the measure used to determine nebulizer output performance (ED, DD, or RDD).
The methods for the in vitro determination of DD in the four regulatory guidelines examined in this study use a 1:1 I:E ratio; however, as shown in this study, DDs with I:E ratios that are more representative of patient use were much lower than found with the 1:1 I:E ratio. Consideration should be given to this when selecting the appropriate drug-dosing regimen with individual nebulizer brands, particularly as the I:E ratio could change as the disease progresses. Results obtained using the 1:1 I:E ratio, 500 mL tidal volume, and 15 BPM breathing pattern described in the regulatory guidelines may not provide the most appropriate selection criteria for nebulizers, as these methods do not reflect actual breathing patterns and are limited by measuring ED, rather than the clinically more significant RDD. This may lead to inappropriate device selection for the sickest patients, if the relative performance of nebulizers with different modes of operation is based solely on measurements obtained with a 1:1 I:E ratio standardized adult laboratory test pattern, rather than test patterns resembling the actual breathing patterns of such patients. In future revisions of the guidelines, testing protocols that more accurately reflect the potential therapeutic dose delivered to patients should be considered.
Potentially, the RDD is the parameter of greatest clinical significance, as this represents the dose that reaches the patient’s lung. For the nebulizers that operate continuously, as the I:E ratio increased, the RDD decreased. The RDD across these nebulizer brands and across the I:E ratios tested varied between 100 µg and 1,000 µg salbutamol. With the breath-activated nebulizers, there was a much more consistent dose across the I:E ratios. However, due to the nature of these nebulizers delivering only into inhalation, the DD and RDD from a fill with a standard ampoule can be significantly higher than from a continuous nebulizer, unless the breath-activated nebulizer has a dosimetric function to compensate for the extra dose delivered to inhalation. The variability of nebulizer performance between different brands, modes of operation, and I:E ratios limits their applicability to deliver active drugs with narrow therapeutic ranges. For such active drugs, the use of such devices as the I-neb AAD System nebulizer, which produces a consistent DD irrespective of I:E ratio, would be more appropriate.
This study has demonstrated that regulatory guidelines for nebulizer-aerosol testing, although providing quality-control data, provide little information on the dose received by a potential patient. This stems from the differences between patient breathing patterns, different modes of operation of devices, and the way they are affected by changes in breathing pattern and the dose measure quoted compared with standardized laboratory testing. The original CEN 13544-1 standard was developed in the 1990s as an approach to standardize comparative laboratory nebulizer testing. In this era, most nebulizers were conventional jet nebulizers, and thus the testing parameters were suitable for the time and scope. The guidelines have developed over the years, but the basic aerosol-test methods and reported values have not evolved. The information generated using these test methods is published in the device instructions for use, and this information can be used to compare devices and make dosing decisions. As it is often used as part of the sourcing process by health authorities in Europe, it is suggested that the nebulizer guidelines are revised to provide greater information under patient-relevant conditions, as has been proposed for inhalation devices in general.28 This would allow informed decisions to be made regarding potential patient dosing with the great variety of different nebulizers with different modes of operation. Two potential revisions could include the use of more realistic patient breathing patterns and the inclusion of RDD.
Acknowledgments
The authors acknowledge Danielle Jeffrey and Ben Woodington for performing the technical work; during the time of the study, both were employed by Respironics Respiratory Drug Delivery (UK) Ltd, a business of Philips Electronics UK Limited, Chichester, West Sussex, UK. The authors also acknowledge Sophia Kuperman (PS5 Consultants Ltd, Portsmouth, Hampshire, UK) for her editorial assistance.
Disclosure
RHMH and SMB are employees of Respironics Respiratory Drug Delivery (UK) Ltd, a business of Philips Electronics UK Limited, Chichester, West Sussex, UK. The authors report no other conflicts of interest in this work.
References
Berg EB, Picard RJ. In vitro delivery of budesonide from 30 jet nebulizer/compressor combinations using infant and child breathing patterns. Respir Care. 2009;54(12):1671–1678. | ||
Smaldone GC, Cruz-Rivera M, Nikander K. In vitro determination of inhaled mass and particle distribution for budesonide nebulizing suspension. J Aerosol Med. 1998;11(2):113–125. | ||
Hess DR. Nebulizers: principles and performance. Respir Care. 2000;45(6):609–622. | ||
European Committee for Standardization. CEN: EN 13544-1: 2007+A1:2009: Respiratory Therapy Equipment: Nebulizing Systems and Their Components. London: British Standards Institute; 2010. | ||
US Pharmacopoeia. Chapter 1601: Products for nebulization – characterization tests. In: United States Pharmacopeia (USP) 35. Rockville (MD): USP; 2012. | ||
European Pharmacopoeia. Chapter 2.9.44: Preparations for nebulisation – characterisation. In: European Pharmacopeia (EP) 7.3. Strasbourg: European Directorate for the Quality of Medicines and Healthcare; 2010. | ||
International Organization for Standardization. ISO 27427:2013(E): Anaesthetic and Respiratory Equipment: Nebulizing Systems and Components. Geneva: ISO; 2013. | ||
US Pharmacopoeia. Chapter 1601: Products for nebulization – characterization tests. In: United States Pharmacopoeia (USP) 37. Rockville (MD): USP; 2014. | ||
US Pharmacopoeia (USP). Chapter 1601: Products for nebulization – characterization tests. In: United States Pharmacopeia (USP) 40. Rockville (MD): USP; 2017. | ||
Heyder J, Gebhart J, Rudolf G, Schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range of 0.005-15 µm. J Aerosol Sci. 1986;17(5):811–825. | ||
Bauer A, McGlynn P, Bovet LL, Mims PL, Curry LA, Hanrahan JP. The influence of breathing pattern during nebulization on the delivery of arformoterol using a breath simulator. Respir Care. 2009;54(11):1488–1492. | ||
Gatnash AA, Chandler ST, Connolly CK. A new method for measuring aerosol nebulizer output using radioactive tracers. Eur Respir J. 1998;12(2):467–471. | ||
Smaldone GC, Sangwan S, Shah A. Facemask design, facial deposition, and delivered dose of nebulized aerosols. J Aerosol Med. 2007;20(Suppl 1):S66–S77. | ||
Schüepp KG, Jauernig J, Janssens HM, et al. In vitro determination of the optimal particle size for nebulized aerosol delivery to infants. J Aerosol Med. 2005;18(2):225–235. | ||
Nikander K, Denyer J, Everard M, Smaldone GC. Validation of a new breathing simulator generating and measuring inhaled aerosol with adult breathing patterns. J Aerosol Med. 2000;13(2):139–146. | ||
Skaria S, Smaldone GC. Omron NE U22: comparison between vibrating mesh and jet nebulizer. J Aerosol Med Pulm Drug Deliv. 2010;23(3):173–180. | ||
Ari A, de Andrade AD, Fink JB. Performance comparisons of jet and mesh nebulizers with mouthpiece, aerosol mask, and valved mask in simulated spontaneously breathing adults. Chest. 2014;145(Suppl 3):548A. | ||
Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns – 1: normal subjects. Chest. 1983;84(2):202–205. | ||
Carter R, Tashkin D, Djahed B, Hathaway E, Nicotra MB, Tiep BL. Demand oxygen delivery for patients with restrictive lung disease. Chest. 1989;96(6):1307–1311. | ||
Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns – 2: diseased subjects. Chest. 1983;84(3):286–294. | ||
Loring SH, Garcia-Jacques M, Malhotra A. Pulmonary characteristics in COPD and mechanisms of increased work of breathing. J Appl Physiol. 2009;107(1):309–314. | ||
Díaz O, Villafranca C, Ghezzo H, et al. Breathing pattern and gas exchange at peak exercise in COPD patients with and without tidal flow limitation at rest. Eur Respir J. 2001;17(6):1120–1127. | ||
Denyer J, Dyche T. The adaptive aerosol delivery (AAD) technology: past, present, and future. J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 1):S1–S10. | ||
Nikander K, Denyer J. Breathing patterns. Eur Respir Rev. 2000;10(76):576–579. | ||
Vitacca M, Clini E, Porta R, Foglio K, Ambrosino N. Acute exacerbations in patients with COPD: predictors of need for mechanical ventilation. Eur Respir J. 1996;9(7):1487–1493. | ||
Bauerle O, Chrusch CA, Younes M. Mechanisms by which COPD affects exercise tolerance. Am J Respir Crit Care Med. 1998;157(1):57–68. | ||
Vogiatzis I, Stratakos G, Athanasopoulos D, et al. Chest wall volume regulation during exercise in COPD patients with GOLD stages II to IV. Eur Respir J. 2008;32(1):42–52. | ||
Mitchell JP. Why clinically appropriate in vitro inhaler testing? Introducing the patient experience into the picture. Available from: http://www.fda.gov/downloads/forindustry/userfees/genericdruguserfees/UCM398876.pdf. Accessed December 3, 2015. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




 ), SideStream (
), SideStream ( ), LC Plus (
), LC Plus ( ), SideStream Plus (
), SideStream Plus ( ), LC Sprint (
), LC Sprint ( ), MicroAir U22 (
), MicroAir U22 ( ), and eFlow Rapid (
), and eFlow Rapid ( ) non-breath-activated nebulizers, tested with four breathing patterns (n=9; pattern A = 1:1 I:E ratio, pattern B = 1:2 I:E ratio, pattern C = 1:3 I:E ratio, and pattern D = 1:4 I:E ratio). (A) Average delivered dose to filter; (B) average respirable delivered dose; (C) average emitted dose; (D) average respirable emitted dose; (E) average treatment time.
) non-breath-activated nebulizers, tested with four breathing patterns (n=9; pattern A = 1:1 I:E ratio, pattern B = 1:2 I:E ratio, pattern C = 1:3 I:E ratio, and pattern D = 1:4 I:E ratio). (A) Average delivered dose to filter; (B) average respirable delivered dose; (C) average emitted dose; (D) average respirable emitted dose; (E) average treatment time.
 ) and I-neb AAD System (
) and I-neb AAD System ( ) breath-activated nebulizers, tested with four breathing patterns (n=9; pattern A = 1:1 I:E ratio, pattern B = 1:2 I:E ratio, pattern C = 1:3 I:E ratio, and pattern D = 1:4 I:E ratio). (A) Average delivered dose to filter; (B) average respirable delivered dose; (C) average emitted dose; (D) average respirable emitted dose; (E) average treatment time.
) breath-activated nebulizers, tested with four breathing patterns (n=9; pattern A = 1:1 I:E ratio, pattern B = 1:2 I:E ratio, pattern C = 1:3 I:E ratio, and pattern D = 1:4 I:E ratio). (A) Average delivered dose to filter; (B) average respirable delivered dose; (C) average emitted dose; (D) average respirable emitted dose; (E) average treatment time.