Back to Journals » Infection and Drug Resistance » Volume 15
Vancomycin Use in Posterior Lumbar Interbody Fusion of Deep Surgical Site Infection
Authors Wang S, Yao R, Li Z, Gong X, Xu J, Yang F, Yang K
Received 2 March 2022
Accepted for publication 27 May 2022
Published 17 June 2022 Volume 2022:15 Pages 3103—3109
DOI https://doi.org/10.2147/IDR.S364432
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Shiyong Wang, Rubin Yao, Zhongjie Li, Xiangdong Gong, Jitao Xu, Fajun Yang, Kaishun Yang
Department of Spinal Surgery, First Affiliated Hospital of Dali University, Dali, Yunnan, People’s Republic of China
Correspondence: Kaishun Yang, Department of Spinal Surgery, First Affiliated Hospital of Dali University, Dali, Yunnan, People’s Republic of China, Tel +86-872-2201044, Email [email protected]
Objective: To retrospectively analyze if the use of topical intraoperative vancomycin powder reduces deep surgical site infection (DSSI) after posterior lumbar interbody fusion.
Methods: All spinal surgeries for lumbar degenerative disease and underwent posterior fixation interbody fusion between January 2013 and December 2018 were reviewed. A total of 891 patients were included, of which 527 patients (treatment group) received intraoperatively topical vancomycin powder; the others were served as control group. The primary outcomes were the overall incidence of DSSI and the effect of vancomycin on its development. The secondary outcome was risk factors for DSSI. Data on the baseline characteristics, postoperative complications, perioperative risk factors, and one-year postoperative prognoses were extracted from the medical records.
Results: A total of 20 patients met the diagnostic criteria for DSSI (2.24%), of which 7 patients (1.33%) in the treatment group and 13 patients (3.57%) in the control group. There was a significant difference in the incidence of DSSI between the groups (P = 0.026). Multivariate logistic regression analysis with stepwise backward elimination showed that the local use of vancomycin powder was an independent protective factor for DSSI (odds ratio (OR): 0.25, P = 0.01), whereas high body mass index (BMI) (OR: 1.21, P = 0.005), drinking (OR: 5.19, P = 0.005), urinary tract infections (OR: 4.49, P = 0.021), diabetes mellitus (OR: 4.32, P = 0.03), and blood transfusions (OR: 3.67, P = 0.03) were independent risk factors for DSSI.
Conclusion: The intraoperative usage of vancomycin powder could reduce effectively decreases the incidence of DSSI after posterior lumbar interbody fusion for degenerative lumbar diseases. High BMI, diabetes mellitus, drinking, and urinary tract infections were independent risk factors for DSSI, whereas the local use of vancomycin protected against these factors.
Keywords: posterior lumbar interbody fusion, vancomycin, deep surgical site infection, risk factors
Introduction
DSSI after spinal surgery is an uncommon but devastating complication with the reported incidence as high as 11.3%.1 It is well documented that the factors of risk infection are including longer operation time, posterior surgical approach, previous surgery, and application of spinal instruments.2 While many efforts has been made to control the incidence of DSSI such as the unprecedented development of antimicrobial agents, the widespread use of minimally invasive surgery, and continuous improvements in postoperative care techniques, the decrease in the incidence of SSIs is low.3–5 In clinical practice, various methods have been adopted to prevent or reduce the development of DSSI, including blood glucose control, treatment of urinary tract infections, smoking cessation, nutritional supplementation, preoperative skin debridement, antibiotic prophylaxis, while DSSI is still a substantial reason of prolonging hospital stay, increasing medical costs, rate of reoperations, pseudoarticulation formation and death.6–8 Therefore, control of DSSI after spinal surgery is urgent considering the quality of patients’ life and the financial burden.
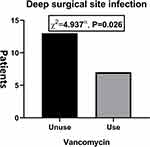 |
Figure 1 The rate of deep surgical site infection between two group. |
Recently, intraoperative application of vancomycin powder to spinal incisions has been reported effectively reducing surgical site infection rates and topical use increased vancomycin concentration without increasing drug-related side effects.9 A previous study evaluated the release characteristics in vivo and antibacterial ability of vancomycin, they found that the vancomycin concentration could be detected at 0.5 h and increased rapidly until reaching the peak at 2 h, and it could be still detected 7 days after the surgery.10 Besides, no death, local infection or other complications occurred.
The majority of the aforementioned literatures highlighting surgical site infection have historically been in the field of multiple spinal diseases and surgeries, and directly sprinkle vancomycin powder before closing the incision, but the results varied. We therefore explored if the use of topical intraoperative vancomycin powder reduces DSSI after posterior lumbar interbody fusion.
Materials and Methods
Inclusion/Exclusion Criteria and Data Collection
The study protocol was approved by the institutional review board at Spinal Surgery Department of our hospital. The inclusion criteria included patients with degenerative diseases of the lumbar spine (spondylolisthesis, spinal stenosis, disc herniation, degenerative scoliosis, discogenic low back pain, and lumbar instability); patients undergoing posterior lumbar interbody fusion; patients who were followed-up for more than one year. The exclusion criteria included lumbar fractures, spinal inflammatory diseases, patients with degenerative diseases of the lumbar spine who underwent decompression only (percutaneous endoscopic discectomy or minimally invasive transforaminal lumbar discectomy), and patients followed-up for less than one year.
A total of 891 patients with posterior lumbar interbody fusion and fixation met the inclusion criteria and finally included in this study from January 2013 to December 2018. In our cohort, 364 patients did not receive local vancomycin (control group) and 527 patients received local vancomycin (treatment group). According to the Centers for Disease Control and Prevention (CDC) criteria,11 deep surgical site infection (DSSI) is defined as an infection that occurred within 30 days (without implants) or within one year (with implants) after surgery involving deep soft tissue.
Patient demographics (age, gender, height, weight), smoking status, alcohol drinking status, presence of diabetes, hypertension, hyperuricemia, urinary tract infection (laboratory tests or urological diagnosis), surgical factors (number of spinal levels, intraoperative blood loss, duration of operation, postoperative drainage volume, number of internal fixations), preoperative hospital stay, number of blood transfusions, and catheter dwell time were recorded. All patient’s records were evaluated for signs of DSSI within one year after discharge.
Surgery and Local Use of Vancomycin
All patients underwent posterior lumbar decompression and interbody fusion after preoperative examination and exclusion of surgical contraindications. During surgery, pedicle screws were used for fixation, and autogenous bone was implanted into the intervertebral space, or one cage was implanted after bone graft (allograft bone was used in cases in which autogenous bone was insufficient). Systemic antibiotic prophylaxis with second-generation cephalosporins was performed in all patients 30–60 min before skin resection. Patients allergic to penicillin or cephalosporins received clindamycin. Antibiotics were repeated once when blood loss exceeded 1500 mL or surgical time exceeded 3 h. Antibiotics were continually administered up to 24–48 h after surgery for all patients. After discectomy and decompression, the incision and intervertebral space were washed with sterile saline. After intervertebral bone grafting and cage implantation, the incision was soaked with povidone-iodine for at least three min (except for dural rupture) and washed with 3000 mL of sterile saline. When no active bleeding was detected, two negative pressure drainage tubes were placed deep in the incision, and the incision was closed layer by layer.
In the treatment group, approximately 250 mg of vancomycin powder was mixed with the bone graft. Then, the graft was implanted into the intervertebral space and cage, and 250 mg of vancomycin powder was applied directly to the surgical bed. In cases in which more than two levels were fused, the intraoperative dose of vancomycin was increased to 1000 mg, and the other should use only 500 mg.
The drainage tubes were maintained under negative pressure after surgery to reduce the risk of hematoma formation. The drainage tubes were removed after the drainage volume was less than 50 mL/day, and radiographs and three-dimensional computed tomography (3DCT) reconstruction of the lumbar spine were reviewed.
Statistical Analysis
The effects of the local use of vancomycin powder on DSSI and its potential risk factors were analyzed. Categorical and continuous data were analyzed using the chi-square and independent-sample t-tests. In multivariate analysis, relative risk, 95% confidence intervals, and independent risk and protective factors were determined by multivariate logistic regression with stepwise backward elimination. A P-value of less than 0.05 was considered statistically significant.
Results
Patient Characteristics and the Incidence of DSSI
The demographics and clinical characteristics of two groups are shown in Table 1. There were no significant differences in demographics and clinical characteristics between patients with and without SSIs in the control group, except for BMI (25.69 ± 1.95 vs 23.20 ± 3.39 kg/m2, P = 0.001).
The incidence of DSSI was significantly lower in the treatment group (P = 0.026, chi-square test, Figure 1). A total of 20 patients (2.24%) were present with DSSI, including 13 (3.57%) in the control group and 7 (1.33%) in the treatment group (Table 2).
 |
Table 2 Effect of Intrawound Vancomycin on the Rate of Deep Surgical Site Infection |
Microbiological Analyses
Among the 20 infected patients, the bacterial culture was negative in 11 patients and positive in nine patients. In the treated group, cultures were positive for Escherichia coli (three cases) and Gram-positive cocci (one case). In the control group, cultures were positive for Gram-negative bacteria (three cases) and Gram-positive bacteria (two cases) (Table 3). No vancomycin-resistant strains were found.
 |
Table 3 Microorganisms in the Surgical Site |
Multivariate Logistic Regression Analysis of DSSI
Multivariate analysis of all patients showed that the local use of vancomycin was a protective factor for DSSI (OR: 0.25, B: –1.38, P = 0.01), whereas high BMI (P = 0.005), drinking (P = 0.005), diabetes mellitus (P = 0.03), urinary tract infection (P = 0.021), and blood transfusions (P = 0.03) were independent risk factors for DSSI.
In the subgroup analysis, the independent risk factors for DSSI in the control group were high BMI (P = 0.01), diabetes mellitus (P = 0.006), number of surgical levels (P = 0.005), postoperative drainage volume (P = 0.014). And in the treatment group were surgical duration (P = 0.025) and perioperative blood transfusion (P = 0.002) (Table 4).
 |
Table 4 Multivariate Logistic Regression Analysis of Risk Factors for Deep Surgical Site Infections |
Discussion
Spinal surgeons are focused on developing and implementing strategies to reduce and prevent the occurrence of DSSI. Staphylococcus aureus cultures in the nasal cavities, preoperative chlorhexidine showers, antibacterial treatments in the surgical sites, preoperative intravenous antibiotic prophylaxes, intraoperative temperature monitoring, intrawound vancomycin, postoperative drainage, and other measures are used to prevent DSSI.12 Intravenous vancomycin is effective in preventing infections with methicillin-resistant Staphylococcus aureus (MRSA); however, this treatment is not routinely adopted because it is associated with hypotension, ototoxicity, nephrotoxicity, and an increased risk in the development of drug-resistant microorganisms in the oropharyngeal, respiratory, and urogenital tract.13 Due to vancomycin being absorbed into the blood from the wound with very poor efficiency and not detected in the serum of most patients, the risk of development of drug-resistant microorganisms in the oropharyngeal, gastrointestinal, and respiratory tracts is low, and high concentrations of vancomycin in the surgical site, which can effectively inhibit the growth of bacteria, may help prevent DSSI.8
In our cohort, 0.5 g and 1.0 g of vancomycin were applied to patients with one/two segments and more than two segments, respectively. Armaghani’s study speculated that our study could achieve effective local vancomycin concentrations of a bacteriostatic concentration, and the serum vancomycin concentration was lower. Moreover, approximately 250 mg of vancomycin powder was mixed with autologous bone and implanted into the intervertebral space, delaying drug release into the bloodstream and increasing drug concentration in the intervertebral space, reducing the occurrence of DSSI under the condition of ensuring safety.
The effect of vancomycin on osteogenesis is a concern of many researchers and clinicians. In this study, approximately 250 mg of vancomycin powder was mixed with autologous bone, and the effect on bone graft fusion should be paid more attention to. Mendoza et al14 performed posterolateral lumbar spinal fusion in rats and found that the local administration of a standard dose (14.3 mg/kg) and a high dose (143 mg/kg) did not inhibit fusion, and the use of bone morphogenetic protein might have reduced the effect of vancomycin on bone fusion. Moreover, vancomycin (3.5 mmol/L) induced a temporary decrease in osteoblast proliferation but did not significantly affect the osteoblast metabolic activity.15 Vancomycin concentrations in our study were lower than those used in the above studies. In the cases that continued to give negative pressure suction, vancomycin applied locally would be lost some during wound irrigation, and fusion was successful in all treated patients. Therefore, the amount and method of vancomycin used in our study did not significantly affect interbody fusion.
Vancomycin affects the shape and viability of dural fibroblasts in vitro in a concentration-dependent manner.16 However, Sweet et al8 showed that vancomycin was safe to be poured into the incision when the dura was exposed. There were no cases of epidural rupture or pseudoepidural cyst formation due to local vancomycin administration in our cohort. In patients with cerebrospinal fluid leakage after dural rupture, vancomycin was not applied locally, and autologous bone mixed with vancomycin was implanted into the intervertebral space and cage. No serious complications related to cerebrospinal fluid leakage were found in the perioperative period, and the drainage tube was removed after 7–10 days. A dural tear is an independent risk factor for SSI,17 and whether or not vancomycin can be used locally for those patients, how to use it, and at what dose and its effectiveness still need to be further studied.
We also found an anomaly in the treatment group, in which a short operative time was a risk factor for DSSI. In contrast, previous studies have suggested that prolonged surgery increases the risk of SSI.18 Besides, various factors were associated with DSSI in this study, BMI, drinking, urinary tract infections, diabetes mellitus, and blood transfusions, which were independent risk factors for DSSI. The reasons are analyzed as follows: age, BMI, intraoperative blood loss, postoperative drainage volume, and the frequency of blood transfusions in infected were higher than in the uninfected patients, but the operation time was significantly shorter. This may be related to the fact that surgeons speed up surgical procedures and shorten surgical time to reduce the risk of surgery and anesthesia in elderly patients and high BMI. However, that might neglect more delicate manipulation, resulting in poor hemostasis and in increased perioperative bleeding and blood transfusions. Previous research has found that advanced age, high BMI, blood transfusions, and bleeding are risk factors for SSI.18–21 Therefore, shortening surgical times while ignoring the delicate operation and hemostatic control may be counterproductive in such patients. Besides, it has been reported that smokers presented a 3-fold greater incidence of SSI of 17.8%, patients with diabetes of 14.3% compared with 7.8% among patients who did not have diabetes, male with a 2-fold increased risk compared with female (8.8% vs 4.9%).22
The local application of vancomycin may affect the microbiota of the surgical site. In our cohort, bacteria cultured from pus in the treated group were mainly Gram-negative Escherichia coli. Several bacterial species were identified in the surgical site in the control group, including methicillin-resistant Staphylococcus epidermidis (MRSE). In this respect, a controlled study of 380 patients found that the local application of 1.0–2.0 g of vancomycin did not significantly decrease the rate of SSIs (P = 0.2) but changed the predominant bacterial species colonizing the wound.23 Therefore, the amount of vancomycin used in our study (0.5–1.0 g) was enough to change the bacterial composition in the surgical site. Although this treatment approach had no adverse clinical outcomes and decreased the incidence of infections with MRSE and MRSA, the emergence of vancomycin-resistant bacteria, SSIs with Gram-negative bacteria and fungi, and vancomycin-related side effects should be monitored. A literature review showed that using 1.0–2.0 g of vancomycin in the surgical bed reduced the risk of SSIs; however, there were 23 (0.3%) adverse events.24 Thus, further studies are necessary to find a balance between treatment efficacy and drug toxicity.
Conclusion
The results of this study have demonstrated that topical intrawound vancomycin powder administration could significantly reduce the risk of DSSI after posterior lumbar interbody fusion for degenerative lumbar diseases. However, future randomized trials are needed to confirm the present findings.
Abbreviations
DSSI, Deep surgical site infection; BMI, Body mass index; SSI, Surgical site infection; CDC, Centers for disease control and prevention; CT, Computed tomography; MRSE, Methicillin-resistant Staphylococcus epidermidis; MRSA, Methicillin-resistant Staphylococcus aureus.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of First Affiliated Hospital of Dali University. This study followed the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ojo OA, Owolabi BS, Oseni AW, Kanu OO, Bankole OB. Surgical site infection in posterior spine surgery. Niger J Clin Pract. 2016;19(6):821–826. doi:10.4103/1119-3077.183237 PMID: 27811458.
2. Yin D, Liu B, Chang Y, Gu H, Zheng X. Management of late-onset deep surgical site infection after instrumented spinal surgery. BMC Surg. 2018;18(1):121. doi:10.1186/s12893-018-0458-4
3. Hey HW, Thiam DW, Koh ZS, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine. 2017;42(4):267–274. doi:10.1097/BRS.0000000000001710
4. Marc A, Weinstein JPM, Frank P, Cammisa FP
5. Devin CJ, Chotai S, McGirt MJ, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: a multicenter analysis. Spine. 2018;43(1):65–71. doi:10.1097/BRS.0000000000001371
6. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–397. doi:10.1016/j.ajic.2008.12.010
7. Manoukian S, Stewart S, Graves N, et al. Bed-days and costs associated with the inpatient burden of healthcare-associated infection in the UK. J Hosp Infect. 2021;114:43–50. doi:10.1016/j.jhin.2020.12.027
8. Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine. 2011;36(24):2084–2088.
9. Sono T, Fujibayashi S, Izeki M, et al. Decreased rate of surgical site infection after spinal surgery with instrumentation using bundled approach including surveillance and intrawound vancomycin application. Medicine. 2018;97(34):e12010. doi:10.1097/MD.0000000000012010
10. Han J, Yang Y, Lu J, et al. Sustained release vancomycin-coated titanium alloy using a novel electrostatic dry powder coating technique may be a potential strategy to reduce implant-related infection. Biosci Trends. 2017;11(3):346–354. doi:10.5582/bst.2017.01061.
11. Mangram AJ, Pearson ML, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol. 1999;20:279–280. doi:10.1086/501620
12. White AJ, Fiani B, Jarrah R, Momin AA, Rasouli J. Surgical site infection prophylaxis and wound management in spine surgery. Asian Spine J. 2021. doi:10.31616/asj.2020.0674
13. Moise PA, Smyth DS, El-Fawal N, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61(1):85–90. doi:10.1093/jac/dkm445
14. Mendoza MC, Sonn KA, Kannan AS, et al. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine. 2016;25(2):145–153. doi:10.3171/2015.11.SPINE15536
15. Philp AM, Raja S, Philp A, Newton Ede MP, Jones SW. The effect of vancomycin and gentamicin antibiotics on human osteoblast proliferation, metabolic function, and bone mineralization. Spine. 2017;42(3):202–207. doi:10.1097/BRS.0000000000001712
16. Goldschmidt E, Rasmussen J, Chabot JD, et al. The effect of vancomycin powder on human dural fibroblast culture and its implications for dural repair during spine surgery. J Neurosurg Spine. 2016;25(5):665–670. doi:10.3171/2016.3.SPINE151491
17. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2011;93(17):1627–1633. doi:10.2106/JBJS.J.00039
18. Zhang L, Li EN. Risk factors for surgical site infection following lumbar spinal surgery: a meta-analysis. Ther Clin Risk Manag. 2018;14:2161–2169. doi:10.2147/TCRM.S181477
19. Chaichana KL, Bydon M, Santiago-Dieppa DR, et al. Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. J Neurosurg Spine. 2014;20(1):45–52. doi:10.3171/2013.10.SPINE1364
20. Aoude A, Nooh A, Fortin M, et al. Incidence, predictors, and postoperative complications of blood transfusion in thoracic and lumbar fusion surgery: an analysis of 13,695 patients from the American College of Surgeons National Surgical Quality Improvement Program Database. Global Spine J. 2016;6(8):756–764. doi:10.1055/s-0036-1580736
21. Lonjon G, Dauzac C, Fourniols E, et al. Early surgical site infections in adult spinal trauma: a prospective, multicentre study of infection rates and risk factors. Orthop Traumatol Surg Res. 2012;98(7):788–794. doi:10.1016/j.otsr.2012.07.006
22. Norris GR, Checketts JX, Scott JT, Vassar M, Norris BL, Giannoudis PV. Prevalence of deep surgical site infection after repair of periarticular knee fractures: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e199951. doi:10.1001/jamanetworkopen.2019.9951
23. Mirzashahi B, Chehrassan M, Mortazavi SMJ. Intrawound application of vancomycin changes the responsible germ in elective spine surgery without significant effect on the rate of infection: a randomized prospective study. Musculoskelet Surg. 2018;102(1):35–39. doi:10.1007/s12306-017-0490-z
24. Ghobrial GM, Cadotte DW, Williams K
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

