Back to Journals » Cancer Management and Research » Volume 13
Value of 18F-FDG Hybrid PET/MR in Differentiated Thyroid Cancer Patients with Negative 131I Whole-Body Scan and Elevated Thyroglobulin Levels
Authors Li H, Chen X, Zhang Y, Wang K, Gao Z
Received 20 November 2020
Accepted for publication 2 February 2021
Published 29 March 2021 Volume 2021:13 Pages 2869—2876
DOI https://doi.org/10.2147/CMAR.S293005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xueqiong Zhu
Hongyan Li,1,2 Xiaomin Chen,1,2 Yajing Zhang,1,2 Kun Wang,1,2 Zairong Gao1,2
1Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan, 430022, People’s Republic of China
Correspondence: Zairong Gao
Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1277 Jiefang Avenue, Wuhan, 430022, People’s Republic of China
Tel/Fax +86-27-85726426
Email [email protected]
Purpose: To evaluate the diagnostic performance of 18F-FDG PET/MR in detecting recurrent or metastatic disease in patients with differentiated thyroid cancer (DTC) who have increased thyroglobulin (Tg) levels but a negative 131I whole-body scan (WBS). The relationship between 18F-FDG PET/MR and serum Tg levels was explored. We also evaluated the therapeutic impact of PET/MR on patient clinical management.
Patients and Methods: Twenty-nine DTC patients with a negative 131I-WBS of the last post-therapeutic and increased Tg levels under thyroid-stimulating hormone suppression treatment who underwent 18F-FDG PET/MR examination were retrospectively analyzed.
Results: Of those 29 patients, 18F-FDG PET/MR findings were true positive, true negative, false positive, and false negative in 18, 7, 2, and 2 patients, respectively. The overall sensitivity, specificity, and accuracy were 90.0%, 77.8%, and 86.2%, respectively. We noticed significant differences in serum Tg levels between the PET/MR-positive and PET/MR-negative patient groups (P=0.049). Receiver operating characteristic curve analysis showed that a Tg level of 2.4 ng/mL was the optimal cut-off value for predicting PET/MR results. The sensitivity, specificity, and accuracy of PET/MR were higher in patients with Tg levels greater than 2.4 ng/mL than in patients with lower levels. By detecting recurrent or metastatic disease, 18F-FDG PET/MR altered the clinical management in 7 patients (24.1%) of the overall population.
Conclusion: 18F-FDG PET/MR has high diagnostic accuracy for detecting recurrent or metastatic diseases in DTC patients and is useful for clinical management.
Keywords: 18F-FDG, PET/MR, differentiated thyroid cancer, thyroglobulin
Introduction
Thyroid cancer is one of the most common endocrine malignancies, and its incidence has increased worldwide over recent decades.1,2 Differentiated thyroid carcinoma (DTC) is the most common type of thyroid cancer and has a good overall prognosis.3 The effective treatment strategies for DTC patients are total thyroidectomy followed by radioactive iodine (RAI) treatment and thyroid-stimulating hormone (TSH) suppression therapy in most cases. After thyroid remnant ablation, serum thyroglobulin (Tg) is a sensitive marker for detecting recurrent or metastatic disease. The most accepted follow-up imaging methods are neck ultrasound and 131I whole-body scan (WBS) for localizing recurrent disease.4 Ultrasound examination is an operator-dependent technique, and 131I-WBS has limited value for tumors that have lost the features of differentiation.5 Besides, an early diagnosis of iodine-negative tumors appears to be important because the prognosis of RAI-refractory disease is poor compared with other DTC cases.6 Therefore, it is necessary to seek the optimal imaging modality for accurate disease restaging and treatment decision making.
Growing evidence has indicated that 18F-FDG PET/CT can be used as a reliable diagnostic tool for detecting local and distant metastatic diseases in dedifferentiated thyroid cancer.7–10 Moreover, magnetic resonance imaging (MRI) has become a major imaging modality for soft tissue tumors, as it has superior soft-tissue contrast and functional imaging abilities.11,12 Because MRI does not use ionizing radiation, the radiation exposure of PET/MR is lower than that of PET/CT, which is beneficial for cancer patients undergoing a series of examinations for disease monitoring. Additionally, RAI contrast agents are not used in PET/MR, thus avoiding potential delays in situations requiring RAI treatment. PET/MR is a hybrid modality that has demonstrated promising results in head and neck cancers.13,14 There are currently few studies with a limited number of cases on the application of 18F-FDG PET/MR in DTC patients.15,16 These studies revealed that PET/MR performed equivalently to PET/CT. However, PET/MR appeared to be less sensitive than PET/CT in assessing lung nodules.15 More research is needed to evaluate the application value of PET/MR in DTC patients.
In the present study, we aimed to evaluate the diagnostic ability of hybrid PET/MR for detecting recurrent or metastatic disease in DTC patients who have increased Tg levels but a negative 131I-WBS. The relationship between PET/MR findings and serum Tg levels was investigated. We also evaluated the therapeutic impact of PET/MR on patient clinical management.
Patients and Methods
Patients
Between November 2017 and December 2019, twenty-nine DTC patients were retrospectively screened at our PET center. All selected patients previously underwent total/near-total thyroidectomy, and all underwent 131I treatment. All patients had a negative 131I-WBS of the last post-therapeutic and increased Tg levels (>1 ng/mL) under TSH suppression treatment, and patients were referred to our center for 18F-FDG PET/MR scans.
18F-FDG PET/MR was performed with a median period of 2.9 months after the negative post-therapy 131I-WBS. Serum Tg levels were measured in patients within 2 weeks before the PET/MR examination.
PET/MR Imaging Acquisition
Whole-body PET/MR images were acquired using a hybrid PET/MR scanner (SIGNA PET/MR, GE Healthcare, Waukesha, WI, USA). All participants were instructed to fast for at least 6 hours, and the blood glucose level was confirmed to be ≤200 mg/dL. Subsequently, all patients were intravenously injected with a dose of 0.1 mCi/kg (3.7 MBq/kg) 18F-FDG. After approximately 60 minutes, all patients underwent PET/MR scans.
PET scanning included five beds covering the body from head to thigh. The scanning time for the chest and abdomen was 6 min per bed due to the need for respiratory motion correction, while the time for the other locations was 4 min per bed. The parameters for scanning and reconstruction were as follows: axial length/bed, 24 cm, and FOV, 60×60 cm2. The ordered subset expectation maximum (OSEM) algorithm with the TOF technique was used for PET reconstruction, with the following parameters: matrix, 192 × 192; filter cutoff, 5.0 mm; subsets, 28; and iterations, 3. The attenuation correcting of PET was based on MRI and then combined the Dixon water-fat separation technique and template matching methods.
During PET scanning, the MRI was acquired simultaneously. For the first bed, the MRI sequences were as followed: axial T2 weighted imaging; axial T2 FLAIR; axial T1 weighted imaging; axial diffusion-weighted imaging (DWI); and axial apparent diffusion coefficient (ADC). For the other four beds, there were four MRI sequences for every bed as follows: axial T2 weighted imaging with fat suppression (FS); three dimension liver acceleration volume acquisition (LAVA) T1 weighted image; axial DWI; and axial ADC.
After the whole-body scanning, another 10 min of local PET/MR of the neck was performed to observe the thyroid and neck lymph nodes in detail, which required only one bed. The corresponding PET parameters were consistent with the description above. The MRI sequences were as follows: axial T1 weighted imaging; axial T2-FS; axial DWI; axial ADC; coronal T2-FS. All MRIs were reconstructed using Fast Fourier Transform Algorithm.
PET/MR Image Analysis
The images were reviewed by two board-certified nuclear medicine physicians and two board-certified radiologists. All readers had information on the patient’s history of thyroid cancer but were blinded to the follow-up results. In MRI evaluation, the morphological criteria suspicious for lymph metastases were as follow: (1) central necrosis; (2) loss of a fatty hilus sign; (3) round shape; (4) focally increased cortical thickness; and (5) irregular external contour.17 At least two suspicious morphological criteria were regarded as signs of malignancy. In addition, long T1 and long T2 signal images, signs of increased signal intensity on DWI images and a low signal on the corresponding ADC map were considered indications of malignancy.15 During the PET imaging evaluation, a focal tracer uptake (SUVmax≥2.5) was considered abnormal if it was different from physiological background. For the PET/MR imaging evaluation, MR imaging abnormalities or increased tracer uptake were suspicious for malignancy.
Follow-Up
During the follow-up period, serum Tg and TgAb levels under TSH suppression treatment and imaging examinations, such as ultrasonography, CT, and MRI, were evaluated every 6 months. The diagnosis of recurrent or metastatic thyroid cancer was based on pathology findings (cytological reports, histopathologic examinations) or clinical follow-up (range, 12–37 months; mean, 31.6 months). Moreover, an increasing Tg trend of more than 6 months was considered a biochemical indication of disease. The impact of PET/MR on clinical management were investigated.
Statistical Analysis
Statistical analyses were conducted using SPSS software 21.0 (IBM, Chicago, IL, USA). The continuous data are shown as the mean ± standard deviation, and the categorical data are presented as percentages. The sensitivity, specificity, accuracy, and positive and negative predictive values were recorded in a patient-based approach. Fisher’s exact test was used to compare the sensitivity and specificity of the MRI and PET/MR imaging. Comparison of serum Tg levels between PET/MR-positive and PET/MR-negative patient groups were performed using Mann–Whitney U-test. A receiver operating characteristic (ROC) curve was used to assess the cut-off value of serum Tg level with optimal sensitivity and specificity. P values < 0.05 were considered significant.
Results
Patient Characteristics
This study was approved by the ethics committee of Tongji Medical College of Huazhong University of Science and Technology and in accordance with the Declaration of Helsinki. The ethics committee waived the requirement for informed consent because of the retrospective nature of the study.
A total of 29 patients were included in this retrospective study (20 women, 9 men; mean age, 40.3 ± 11.8 years). All recruited patients had elevated serum Tg levels with TSH suppression therapy (mean, 18.6 ng/mL; range, 1.1–202.0 ng/mL). All patients had undergone at least one 131I therapy: five patients underwent one treatment; 18 patients underwent two treatments; three patients underwent three treatments; two patients underwent four treatments; and one patient underwent six treatments. The detailed clinical and imaging characteristics of those 29 patients are presented in Table 1.
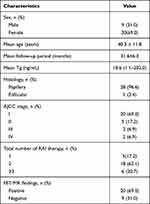 |
Table 1 Pertinent Characteristics of the Patients |
18F-FDG PET/MR Findings
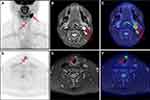
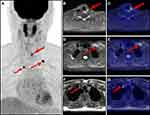
In the overall population, the PET/MR imaging results were positive in 20 patients (69.0%). The distribution of positive lesions in PET/MR was as follows: neck lymph nodes in 11 patients (Figure 1); thyroid bed lesion and cervical lymph nodes in three patients; pulmonary lesions in three patients; lung lesions and thyroid bed lesion in one patient; thyroid bed lesion, supraclavicular and mediastinal lymph nodes in one patient (Figure 2); and cervical and axillary lymph nodes in one patient. After a full assessment, 10 patients were true positive (TP) as confirmed by pathology results. Of the remaining 10 patients, two patients were identified by neck ultrasound with enlarged neck lymph nodes and elevated levels of serum Tg, two patients were identified by lung CT with enlarged pulmonary nodules and elevated levels of serum Tg, and four patients were confirmed by persistently/progressively increased serum Tg levels (TP). One patient had no evidence of disease (FP), and this patient could be explained by inflamed lymph nodes in the follow-up. One patient had a suspicious lymph node on MRI without a correlate tracer uptake, and disease recurrence or metastasis was not found in the follow up (FP).
PET/MR scans were negative in nine patients (31.0%). Of those patients with negative PET/MR findings, one patient was a false negative (FN) according to pathology finding. In the remaining 8 patients identified by clinical follow-up, disease recurrence or metastasis was not found in 7 patients (TN). One patient was confirmed by neck lymph nodes metastases during clinical follow-up (FN).
In the total 29 patients, 14 cases underwent lung CT and PET/MR examinations at the same time, and five cases were negative on both examination images. Eight patients with lung nodules were detected by CT only (diameter range, 2–4 mm), and two patients were finally diagnosed with cervical lymph nodes and lung metastases in the follow-up. One patient had multiple lung nodules (diameter range, 0.5–1.1 cm), which was found on both CT and PET/MR.
Diagnostic Impact of MRI, PET/MR and Serum Tg Levels
Of the 29 patients, MRI findings were TP, TN, FP, and FN in 12, 8, 1, and 8 patients, respectively. MRI demonstrated a sensitivity, specificity, and accuracy of 60.0, 88.9, 69.0%, respectively, for detecting recurrent or metastatic DTC in the patient-based analysis. MRI failed to detect metastatic lymph nodes in six patients and lung nodules in two patients. PET/MR findings were TP, TN, FP, and FN in 18, 7, 2, and 2 patients, respectively. We observed a sensitivity, specificity, and accuracy of 90.0, 77.8, and 86.2% for PET/MR (Table 2). The sensitivity of PET/MR was higher than that of MRI. Conversely, the specificity of PET/MR was lower than that of MRI. However, there was no significant difference in the sensitivity or specificity between the two methods (P=0.147 and P=0.222, respectively). PET/MR was able to identify metastatic cervical lymph nodes in four patients classified as benign lesions by MRI and pulmonary metastases in two patients missed by MRI.
 |
Table 2 18F-FDG PET/MR Findings Compared with Serum Tg Levels |
We noticed significant differences in serum Tg levels between the PET/MR-positive and PET/MR-negative patient groups (median, 7.8 ng/mL vs 2.1 ng/mL, P = 0.049, Figure 3). To assess the potential ability of serum Tg levels to predict PET/MR results, ROC analysis was employed, and the results showed that a Tg level of 2.4 ng/mL was the optimal cut-off value with 80.0% sensitivity and 66.7% specificity (area under the curve:0.733, P = 0.048, Figure 4). The sensitivity and specificity of PET/MR scans were 94.1% and 100%, respectively, in patients with Tg levels ≥ 2.4 ng/mL. Among patients with lower Tg levels, the sensitivity and specificity were 66.7% and 71.4%, respectively (Table 2).
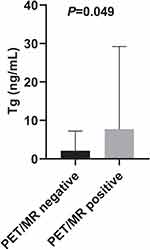 |
Figure 3 Distribution of serum Tg levels in patients according to positive and negative 18F-FDG PET/MR findings. |
 |
Figure 4 Receiver operating characteristic curve analysis demonstrates Tg cut-off value (2.4 ng/mL, arrow) under TSH suppression therapy. |
Change in Treatment After PET/MR
Among the 20 patients with positive PET/MR results, PET/MR guided the surgical strategy for seven patients with local relapse or neck node metastases. PET/MR showed unresectable metastases in 4 patients, and all patients were treated with TSH suppression therapy. Nine patients unwilling to undergo surgical intervention, and all of them with TSH suppression therapy. By detecting recurrent or metastatic disease, 18F-FDG PET/MR changed the treatment plan in 7 patients (24.1%) of the overall population.
Discussion
PET/MR combines the advantages of the high soft-tissue contrast of MRI with the metabolic imaging of PET. However, there are currently limited studies on the application of 18F-FDG PET/MR in DTC patients. In the present study, we enrolled 29 DTC patients who had elevated serum Tg levels but negative 131I-WBS scan undergoing 18F-FDG PET/MR examination. PET/MR demonstrated a sensitivity, specificity, and accuracy of 90.0, 77.8, and 86.2%, respectively, in patient-based analysis. In ROC analysis, a Tg level of 2.4 ng/mL was the optimal cut-off value for predicting the PET/MR results. Compared with patients who had lower Tg levels, PET/MR had higher sensitivity and specificity in patients with serum Tg levels ≥ 2.4 ng/mL. Moreover, 18F-FDG PET/MR provided additional information and changed the treatment management in 7 patients (24.1%) of the overall population.
As PET/MR is a relatively new imaging modality, only a few studies have evaluated PET/MR in thyroid cancer patients using 18F-FDG or 124I as a tracer. Binse et al18 reported that 124I PET/MR was superior to 124I PET/CT in the detection of iodine-positive lesions of the neck. Vrachimis et al15 evaluated the diagnostic ability of 18F-FDG PET/MR compared with that of 18F-FDG PET/CT in dedifferentiated thyroid cancer patients, and the results showed that 96% of patients with dedifferentiated thyroid cancer were correctly identified by both PET/CT and PET/MR in patient analysis, and as expected, PET/MR appeared less sensitive in assessing lung metastases. Similarly, a recent study showed that PET/MR brought only slight improvements over PET/CT numerically.16 In this study, we did not compare the diagnostic ability of PET/MR and PET/CT because none of the patients had PET/CT scans at the same time. In the present study, PET/MR was able to identify metastatic cervical lymph nodes in four patients classified as benign lesions by MRI, and pulmonary metastases in two patients missed by MRI. However, there was no significant difference in sensitivity or specificity between the two methods, which may be due to the sample in the present study was relatively small. One FP finding with elevated tracer uptake was observed on the reactive cervical lymph nodes. Because of the overlap between the SUVmax values of the TP and FP findings, the SUVmax value should not be used as the sole discriminating factor for differential diagnosis. Eight patients showed pulmonary nodules (diameter, 2–4 mm), which were found on CT only, and two patients were finally diagnosed with cervical lymph nodes and lung metastases in the follow-up. PET scans failed to find small lung metastases due to partial volume effects and respiratory motion. MRI has difficulty detecting lung nodules that are smaller than 4 mm.19 Therefore, PET/MR combined with non-contrast CT scans of the lung may provide more valuable information in thyroid cancer patients.15 PET/MR had a lower radiation dose delivered to the patient than PET/CT. The mean effective dose from 18F-FDG was estimated at 9.0±1.6 mSv. For all standard PET/CT patients, the mean effective dose from CT was 5.0±1.0 mSv. For the diagnostic PET/CT modality, the mean CT effective dose was 15.4 ± 5.0mSv.20 PET/MR is a beneficial technique for performing serial examinations for disease monitoring in DTC patients, who have an excellent prognosis.
The American Thyroid Association guidelines recommend that 18F-FDG PET/CT examination should be performed when DTC patients have stimulated Tg >10 ng/mL.3 Other studies have reported different cut-offs of serum Tg levels for predicting PET/CT results.5,21 Klain et al16 reported that PET/MR was positive in 10/22 patients (45%) with serum Tg ≥ 2 ng/mL and in only 1/18 patients (5%) with serum Tg < 2 ng/mL. To our knowledge, there has been no study concerning serum Tg levels for predicting PET/MR results. In the present study, we noticed significant differences in serum Tg levels between the PET/MR-positive and PET/MR-negative patient groups. A serum Tg level of 2.4 ng/mL was the optimal cut-off value for predicting PET/MR results, and compared with patients who had lower Tg levels, PET/MR had higher sensitivity and specificity in patients with serum Tg levels ≥ 2.4 ng/mL. Similar to previous studies concerning 18F-FDG PET/CT examination, PET/CT had high sensitivity rates in patients with increasing Tg levels.22,23
PET/MR guided surgical intervention in seven patients (24.1%). 18F-FDG PET/MR offered important diagnostic information and accurately localized recurrent or metastatic disease for these patients. The proportion of patients guided to surgical intervention by PET/MR appeared relatively low. This finding may be due to some patients being unable or unwilling to undergo surgical intervention. The results are in line with those of studies concerning 18F-FDG PET/CT, and a review reported that 18F-FDG PET/CT changes the clinical management in 14%-78% of DTC patients.24
It cannot be overlooked that there are several limitations in this study. The retrospective nature of the study and relatively small sample size are the main limitations. Furthermore, we assessed the diagnostic performance of PET/MR on patient-based analysis and not on lesion-based analysis because of the lack of pathological results for all lesions. Therefore, large-scale prospective studies are needed to verify our results.
Conclusion
Taken together, our findings showed that 18F-FDG PET/MR may be a valuable imaging modality for detecting recurrent or metastatic disease and useful for the clinical management of DTC patients who have increased Tg levels and negative 131I-WBS results. However, there was a relatively small sample size in this study, and further studies are needed to verify our results.
Data Sharing Statement
All relevant raw data used and analyzed of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We truly appreciate the great help and support of the two radiologists in Wuhan Union Hospital.
Funding
This study was supported in part by grant 81771866 from the National Natural Science Foundation of China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–617. doi:10.1056/NEJMp1604412
2. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16(1):17–29. doi:10.1038/s41574-019-0263-x
3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
4. Fatourechi V, Hay ID. Treating the patient with differentiated thyroid cancer with thyroglobulin-positive iodine-131 diagnostic scan-negative metastases: including comments on the role of serum thyroglobulin monitoring in tumor surveillance. Semin Nucl Med. 2000;30(2):107–114. doi:10.1053/nm.2000.4600
5. Giovanella L, Ceriani L, De Palma D, et al. Relationship between serum thyroglobulin and 18FDG-PET/CT in 131I-negative differentiated thyroid carcinomas. Head Neck. 2012;34(5):626–631. doi:10.1002/hed.21791
6. Fugazzola L, Elisei R, Fuhrer D, et al. 2019 European thyroid association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer. Eur Thyroid J. 2019;8(5):227–245. doi:10.1159/000502229
7. Nascimento C, Borget I, Al Ghuzlan A, et al. Postoperative fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography: an important imaging modality in patients with aggressive histology of differentiated thyroid cancer. Thyroid. 2015;25(4):437–444. doi:10.1089/thy.2014.0320
8. Oh JR, Byun BH, Hong SP, et al. Comparison of 131I whole-body imaging, 131I SPECT/CT, and 18F-FDG PET/CT in the detection of metastatic thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38(8):1459–1468. doi:10.1007/s00259-011-1809-x
9. Qichang W, Lin B, Gege Z, et al. Diagnostic performance of 18F-FDG-PET/CT in DTC patients with thyroglobulin elevation and negative iodine scintigraphy: a meta-analysis. Eur J Endocrinol. 2019;181(2):93–102. doi:10.1530/eje-19-0261
10. Piccardo A, Trimboli P, Foppiani L, et al. PET/CT in thyroid nodule and differentiated thyroid cancer patients. The evidence-based state of the art. Rev Endocr Metab Disord. 2019;20(1):47–64. doi:10.1007/s11154-019-09491-2
11. Debois M, Oyen R, Maes F, et al. The contribution of magnetic resonance imaging to the three-dimensional treatment planning of localized prostate cancer. Int J Radiat Oncol Biol Phys. 1999;45(4):857–865. doi:10.1016/s0360-3016(99)00288-6
12. Del Grande F, Subhawong T, Weber K, et al. Detection of soft-tissue sarcoma recurrence: added value of functional MR imaging techniques at 3.0 T. Radiology. 2014;271(2):499–511. doi:10.1148/radiol.13130844
13. Platzek I, Beuthien-Baumann B, Schneider M, et al. PET/MRI in head and neck cancer: initial experience. Eur J Nucl Med Mol Imaging. 2013;40(1):6–11. doi:10.1007/s00259-012-2248-z
14. Queiroz MA, Hüllner M, Kuhn F, et al. PET/MRI and PET/CT in follow-up of head and neck cancer patients. Eur J Nucl Med Mol Imaging. 2014;41(6):1066–1075. doi:10.1007/s00259-014-2707-9
15. Vrachimis A, Burg MC, Wenning C, et al. [(18)F]FDG PET/CT outperforms [(18)F]FDG PET/MRI in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016;43(2):212–220. doi:10.1007/s00259-015-3195-2
16. Klain M, Nappi C, Nicolai E, et al. Comparison of simultaneous (18)F-2-[18F] FDG PET/MR and PET/CT in the follow-up of patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2020;47(13):3066–3073. doi:10.1007/s00259-020-04938-0
17. Schaarschmidt BM, Grueneisen J, Stebner V, et al. Can integrated 18F-FDG PET/MR replace sentinel lymph node resection in malignant melanoma? Eur J Nucl Med Mol Imaging. 2018;45(12):2093–2102. doi:10.1007/s00259-018-4061-9
18. Binse I, Poeppel TD, Ruhlmann M, et al. Imaging with (124)I in differentiated thyroid carcinoma: is PET/MRI superior to PET/CT? Eur J Nucl Med Mol Imaging. 2016;43(6):1011–1017. doi:10.1007/s00259-015-3288-y
19. Biederer J, Ohno Y, Hatabu H, et al. Screening for lung cancer: does MRI have a role? Eur J Radiol. 2017;86:353–360. doi:10.1016/j.ejrad.2016.09.016
20. Quinn B, Dauer Z, Pandit-Taskar N, et al. Radiation dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med Imaging. 2016;16(1):41. doi:10.1186/s12880-016-0143-y
21. Bertagna F, Bosio G, Biasiotto G, et al. F-18 FDG-PET/CT evaluation of patients with differentiated thyroid cancer with negative I-131 total body scan and high thyroglobulin level. Clin Nucl Med. 2009;34(11):756–761. doi:10.1097/RLU.0b013e3181b7d95c
22. Shammas A, Degirmenci B, Mountz JM, et al. 18F-FDG PET/CT in patients with suspected recurrent or metastatic well-differentiated thyroid cancer. J Nucl Med. 2007;48(2):221–226.
23. Vural GU, Akkas BE, Ercakmak N, et al. Prognostic significance of FDG PET/CT on the follow-up of patients of differentiated thyroid carcinoma with negative 131I whole-body scan and elevated thyroglobulin levels: correlation with clinical and histopathologic characteristics and long-term follow-up data. Clin Nucl Med. 2012;37(10):953–959. doi:10.1097/RLU.0b013e31825b2057
24. Ciarallo A, Marcus C, Taghipour M, et al. Value of fluorodeoxyglucose PET/computed tomography patient management and outcomes in thyroid cancer. PET Clin. 2015;10(2):265–278. doi:10.1016/j.cpet.2014.12.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.