Back to Journals » Drug Design, Development and Therapy » Volume 13
Valproic Acid Addresses Neuroendocrine Differentiation of LNCaP Cells and Maintains Cell Survival
Authors Giordano F, Naimo GD, Nigro A, Romeo F, Paolì A, De Amicis F, Vivacqua A , Morelli C , Mauro L, Panno ML
Received 5 September 2019
Accepted for publication 26 November 2019
Published 18 December 2019 Volume 2019:13 Pages 4265—4274
DOI https://doi.org/10.2147/DDDT.S229930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Francesca Giordano,1 Giuseppina Daniela Naimo,1 Alessandra Nigro,1 Francesco Romeo,2 Alessandro Paolì,1 Francesca De Amicis,1 Adele Vivacqua,1 Catia Morelli,1 Loredana Mauro,1 Maria Luisa Panno1
1Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Cosenza 87036, Italy; 2Pathologic Anatomy Unit, Annunziata Hospital, Cosenza, Italy
Correspondence: Maria Luisa Panno
Department of Pharmacy and Health and Nutrition Sciences, University of Calabria, Rende, Cosenza 87036, Italy
Tel +39 984 492927
Email [email protected]
Purpose: Neuroendocrine differentiation of prostate cancer, induced by androgen deprivation therapy, is mainly related to advanced disease and poor clinical outcome. Genetic and epigenetic alterations are the key elements of the prostate carcinogenesis. A group of compounds able to induce changes in this sense is inhibitors of histone deacetylase, to which it belongs valproic acid (VPA). In the present paper, we evaluated the role of this molecule on the neuroendocrine differentiation of LNCaP cells together with the effect on proliferation and survival signals.
Methods: Cell growth was analyzed by MTT and flow cytometry, while expression of proteins through Western blot analysis.
Results: Our results have documented that VPA in LNCaP cells reduces cell proliferation, decreases the S phase and Cyclin A, and up-regulates the cyclin-dependent kinase inhibitors p21waf and p27. The acquisition of androgen-independent condition is consistent with an induction of β-III Tubulin and gamma Enolase, both markers of neuroendocrine phenotype. However, all these features cease with the removal of valproate from the culture medium, demonstrating the transitory nature of the epigenetic event. The VPA treatment does not compromise the survival phosphorylated signals of Akt, ERK1/2 and mTOR/p70S6K that remain up-regulated. Consistently, there is an increase of phospho-FOXO3a, to which corresponds the decreased expression of the corresponding oncosuppressor protein.
Conclusion: Overall, our findings indicate that VPA in LNCaP prostate tumor cells, although it reduces cell proliferation, is able to drive neuroendocrine phenotype and to maintain the survival of these cells. Keeping in mind that neuroendocrine differentiation of prostate cancer appears to be associated with a poor prognosis, it is necessary to develop new treatments that do not induce neurodifferentiation but able to counteract cell survival.
Keywords: prostate cancer, cell proliferation, cell cycle, neuroendocrine tumor
Introduction
Androgenic milieu represents an important sustaining factor for prostate cancer development and progression. Although in many cases the prostatic cancer cells grow slowly, they can disseminate through the body and, over the years, the tumor may pose serious risks for the patient survival. For such reason, androgen deprivation, based on the reduction of circulation androgens or receptor antagonists, is a pivot of the classical therapeutic care.1
Unfortunately, with the disease progression, this approach is no longer successful as the tumor overcomes the hormonal ablation by recurring as castration-resistant cells.2
Multiple mechanisms have been proposed as responsible for this transition: mutation, amplification,3,4 and expression of alternative-splice variants of the androgen receptor (AR),5 which lead cancer cells hypersensitive to low levels of androgens during the time of hormone-ablation. Simultaneously, prostate cancer cells become more aggressive and they undergo a transdifferentiation process to convert into Neuro-Endocrine (NE)-like cells. With the final evolution phase, the androgenic markers are lost, while, at the same time, neuroendocrine peptides begin to be progressively produced. The new peptide hormones support and forward tumor growth to condition the evolution towards a more aggressive and metastatic prostate cancer disease.6,7
Although the origin of NE cells in the tumor gland is still debated, it has been evidenced that these cells came from the cancerous luminal epithelial compartment. Indeed, the NE cell population in the normal prostate is very small and it is thought that these cells, through their secretions, support both basal and luminal cells during normal development as well as in carcinogenesis. During this last process, the number of NE cells increases in the prostate gland and it is likely that they can play a relevant role in the development of resistance.4
A body of evidence has indicated that different pharmacological agents and/or conditions might induce the neuroendocrine transdifferentiation of prostate cells: the androgen withdrawal, the radiation therapy, the interleukin stimuli and the cAMP-elevating agents.8–11 The molecular process through which human prostate cancer cells undergo a transdifferentiation process to a NE cell-like includes changes in the expression of different proteins and genes.
The secretory products derived from these cells have paracrine stimulatory effects on the growth of the adjacent cells by promoting the activation of many signaling pathways, such as the c-Src/Ras/ERKs, the Wnt/β-catenin, the PI3K/JAK/STAT or the PKA/CREB.12–14
During the stage of cancer progression, the cells become more aggressive and exhibit the reactivation of developmental programs that are associated with epithelial-mesenchymal plasticity and acquisition of stem-like cell properties.15
Genetic and epigenetic alterations are the key elements of the prostate carcinogenesis. Concerning the latter modifications, it is well known how alterations in the chromatin structure activate a dynamic mechanism through which gene expression can be regulated. A class of agents that modulate chromatin dynamics are the HDAC inhibitors (HDCAi).
HDCAi are classified into different subgroups based on their structure: benzamides (mocetinostat and entinostat), cyclic peptides (romidepsin, largazole), hydroxamic acids (trichostatin A, vorinostat/suberoylanilide hydroxamic acid, belinostat, panobinostat), and aliphatic acids (phenylbutyrate, sodium butyrate, and valproic acid).16
Valproic acid (VPA), a member of the latter group of compounds, widely used as an antiepileptic drug, has recalled great attention due to its antitumor activity.17 In fact, many studies have demonstrated that VPA is able to decrease cell proliferation, angiogenesis and migration of prostate cancer cells by inducing apoptosis.17–20
On the other hand, VPA has reported to reduce AR and Prostatic Specific Antigen (PSA) and to induce neuroendocrine differentiation of human prostatic cancer cells.21 It is clearly documented that androgen-ablation therapy reduces tumor growth and tends to enhance the NE differentiation.19 Unexpectedly, the expression of AR has been shown to suppress this transdifferentiation process associated with tumor progression.8,22
In this study, we have evaluated the action of VPA on the transdifferentiation process of human LNCaP cells to NE cell-like phenotype and the effect on cell survival.
Materials and Methods
Cell Culture
LNCaP cell line was grown in RPMI 1640 medium supplemented with L-glutamine, penicillin G and 10% fetal bovine serum (FBS) (Life Technologies, Monza MB, Italy), at 37°C in a humidified atmosphere containing 95% air and 5% CO2. Subconfluent cell culture, synchronized for 12 hrs in RPMI without phenol red and serum (PRF-SFM RPMI), was used for all experiments. LNCaP cell line was kindly given by Dr R. Baserga, Philadelphia (USA), and used within 6 months after frozen aliquots resuscitations (less than 30 passages).
The authentication of human cell lines was achieved through the use of the GenePrint 10 System (Promega) kit which allows the co-amplification of nine STR loci plus the amelogenin for sex identification (TH01, TPOX, vWA, amelogenin, CSF1PO, D16S539, D7S820, D13S317, D5S818 and D21S11). The amplification products were analyzed on an automatic sequencer and the data obtained were analyzed using the GeneMapper software, version 4.0. The obtained STR data were compared with ATCC database. The LNCaP cell line used has the approval of the institutional review board.
Cell Viability Assays
Cell viability was determined with the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium (MTT, Sigma) assay. LNCaP cells (2 × 104 cells/mL) were grown in 96well plates and not exposed or exposed to 1 mM of VPA for 24, 48, 72 and 96 hrs, in phenol red-free RPMI. The MTT assay was performed as the following: 100µL MTT stock solution in PBS (2mg/mL) was added into each well and incubated at 37°C for 2 hrs followed by media removal and solubilization in 100µL DMSO. After shaking the plates for 15 mins, the absorbance at 570 nm was measured in each well, including the blanks, in Beckman Coulter Spectrophotometer.
DNA Flow Cytometry
LNCaP cells were untreated or treated with 1mM of VPA for 48, 72 and 96 hrs. The latter condition was reproduced in triplicate, two of these plates were replaced by drug-free media (RM) and harvested after 72 and 96 hrs. Replacement of media was also performed for the corresponding control.
To determine cell cycle distribution analysis, LNCaP cells were harvested by trypsinization, fixed and stained with propidium iodide (100µg/mL) after treatment with RNase A (20µg/mL). The DNA content was measured using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) and the data acquired using CellQuest software. Cell cycle profiles were determined using Mod-Fit LT.
Immunoblotting Analysis
LNCaP cells were grown to 70–80% confluence and treated in PRF-SFM RPMI as indicated in the figure legends. At the end of each treatment, cells were lysed in 500µL of 50mM Tris–HCl, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 2mM sodium fluoride, 2mM EDTA, 0.1% SDS, containing a mixture of protease inhibitors (aprotinin, phenylmethylsulfonyl fluoride, and sodium orthovanadate; Sigma-Aldrich) for total protein extraction. Equal amounts of proteins were resolved on 8% SDS-polyacrylamide gel, transferred to a nitrocellulose membrane and probed with androgen receptor, PSA, γ-enolase, antibodies from DAKO; phospho FOXO3a (Ser 253), FOXO3a, phospho mTOR (Ser 2448), mTOR, phospho p70S6K (Thr 389), p70 antibodies from Cell Signaling; Cyclin D1, Cyclin A, p21, p27, β-tubulin III, phospho Akt (Ser 473), Akt, phospho ERK 1/2 (Thr 42–44), ERK 1/2, GAPDH antibodies from Santa Cruz, Biotechnology. The antigen-antibody complex was detected by incubation of the membranes with peroxidase-coupled goat anti-mouse or goat anti-rabbit antibodies and revealed using the ECL System (Amersham Pharmacia).
Statistical Analysis
Each datum point represents the mean ± SD of at least three independent experiments. Data were analyzed by Student t test using the GraphPad Prism 4 software program (GraphPad Software). P < 0.05 was considered as statistically significant.
Results
Effects of VPA on the Proliferative Activity of LNCaP Cells
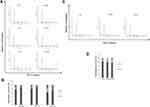
First of all, we aimed to evaluate the effect of VPA administration on cell proliferation, by treating LNCaP cells, at different times, with 1mM of VPA. As shown in Figure 1, VPA starts to significantly influence proliferative activity at 48 hrs, with a greater inhibitory effect at 72 and 96 hrs.
Cell cycle progression, evaluated under VPA stimulus, at the same time where significant effects had been noted, shows an increase in the percentage of cells distributed into the G0/G1 phase compared to control cells, with a drastic reduction of the S phase [Figure 2A and B].
The evaluation of the Cyclins after 48 and 72 hrs of VPA treatment showed no substantial changes in the expression of Cyclin D1, whereas, Cyclin A, necessary for the transition from G1 to S phase, resulted to be significantly down-regulated by the drug [Figure 3]. In support of these data, the expression of Cyclin-Dependent Kinase Inhibitors p21 and p27 increased in LNCaP-treated cells at both 48 and 72 hrs. Since the VPA action, as HDAC inhibitor, is epigenetic in nature and therefore dynamic, we wanted to evaluate the possible reversibility feature of the treatment. To this aim, the culture medium of LNCaP cells treated with VPA for the longest exposure time (96)h, was replaced with serum containing medium and after 72 and 96 hrs of incubation, we have re-examined the functional parameters above mentioned. Thus, we have found that the cells, maintained in the refreshed medium for 72 and 96 hrs, displayed a net gain of the S phase and a decrease of G0/G1 phase, compared to cells treated with VPA [Figure 2C and D]. This demonstrates that the cells lose the memory of the previously performed drug treatment.
Analogously, the analysis of Western blot on sample undergone to VPA for 72 hrs and subsequently subjected to drug-free media (-VPA) for 72 and 96 hrs has shown that, Cyclin D1 and Cyclin A levels were substantially recovered, while, as expected, p21 and p27 protein expressions were decreased, compared to the corresponding drug-treated conditions [Figure 3].
VPA Addresses Neuroendocrine Features in LNCaP Cells
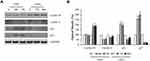
Next, in LNCaP cells under VPA stimulus, we analyzed the expression of androgen receptor, which is critical for maintaining prostate cancer growth.
The results shown in Figure 4A indicated that VPA reduced the expression of AR and, concomitantly, of the androgen-dependent protein PSA. At this stage, the prostate cancer cell loses the androgen-dependent condition, to drive itself towards the androgen-refractoriness. However, renewal of the culture medium strongly regained the expression of both proteins [Figure 4A].
To assess whether the VPA-induced change overlapped with the acquisition of the neuroendocrine phenotype, as postulated by some authors,8–11,21 we determined the expression of the markers β-III Tubulin and γ-Enolase, together with the observation of microscopic cellular morphology. As shown in Figure 4C, VPA altered LNCaP cell morphology leading to neuroendocrine transdifferentiation. In particular, the cell body assumed a more compact form with the appearance of subtle cytoplasmic extensions that resemble similar –axonal figures (see arrows in Figure 4C).
β-III Tubulin and γ-Enolase protein levels increased under treatment with VPA, and they were kept up-regulated for the entire observation time. In addition, in this case, the removal of valproate from the culture medium tended to reduce the expression of β-III Tubulin and γ-Enolase, compared to VPA-treated conditions, respectively [Figure 4A and B].
The Acquisition of the Neuroendocrine Phenotype Does Not Compromise the Survival Signals
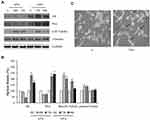
To investigate whether the transition of the prostatic cells towards the NE phenotype resulted in changes in cell survival, attention was paid to the phosphorylated signals of Akt, ERK1/2 and mTOR/p70S6K. As reported in Figure 5A, VPA induced an early increase in the phosphorylation of ERK1/2, Akt, mTOR, and p70S6K, which remained high up to 12 hrs. Consistently with this result, there is the up-regulation of phospho-FOXO3a under VPA, to which corresponds the decreased expression of the FOXO3a protein, and thus the reduced oncosuppressor activity [Figure 5A and B].
Nevertheless, the evaluation of the phosphorylative signals, mainly involved in the regulation of cell survival, performed at the same time previously used for other proteins (48 and 72 hrs), showed that they remained sustained under VPA even at longer times [Figure 5C and D].
Discussion
Currently, the androgen ablation therapy is the major approach used for the treatment of prostate cancer. However, progression of cancer cells to androgen-refractory state occurs with a negative outcome of the disease since prostate tumor tends to evolve towards a neuroendocrine transdifferentiation and this represents a challenge to develop new effective therapies. Importantly, these cells, through their secreted factors, can play effects on the regulation of cell growth and on the disease progression6,21 In this study, we have reproduced, using the deacetylase inhibitor VPA, the experimental conditions that direct prostate tumor cells to acquire the neuroendocrine phenotype. In this regard, first of all, our results have been shown that VPA treatment in LNCAP cells induces a decrease of cell proliferation, which corresponds to a significant drop of the S phase and a gain of G0/G1. This event is accompanied by a reduced expression of Cyclin A that, as is it well known, plays a critical role during the S phase through the phosphorylation of components of the DNA replication machinery.23
On the other hand, the unchanged levels of Cyclin D1, limited to our experimental conditions, prove the fact that this Cyclin, instead, is important to support the beginning of the S phase and the progression of the cell cycle, events that in our case are instead blocked by the treatment.
As evidence of this, by removing the drug and refreshing the medium from the plates, whichcoincides with the resumption of the proliferative cycle, the cyclin D1protein levels are increased.24 These results well fit with cell cycle analysis.
This also justifies the increased expression of the cyclin-dependent kinase inhibitors p21waf and p27. Indeed, both proteins are able to inhibit the activity of cyclin/CDK1/2 complexes and in such a way, the cycle is blocked at the transition from G1 to S phase.19
The up-regulation of p21 and p27, here reported, is consistent with other previous studies in which VPA treatment caused cell cycle arrest of prostate cancer tumors.19,25,26
The growth inhibition of VPA has been addressed together with an alteration of multiple pathways including cell cycle arrest, apoptosis, angiogenesis and senescence.26 Indeed, HDAC inhibitors, by affecting the expression of several genes, interrupt the tumor cell signaling pathways, provoke cell growth arrest in vitro and in vivo models, and have been shown to induce cell differentiation.16,27–30 In the same sense, the VPA decreases the levels of the androgen receptor and androgen-dependent PSA protein, important to sustain cancer cell growth. For this reason, the down-regulation of AR represents one of the main goals of the therapeutic approaches for prostate tumor. However, the continuous use of anti-androgens, prolonged over time, has its counterweight since the tumor acquires resistance and develops a neuroendocrine phenotype associated with tumor progression.
In our study, all the features correlated with the impairment of mitotic activity of LNCaP cells, ceases following the withdrawal of VPA from the culture media. Analogously, the recovery of the S phase during the cell cycle, with renewal of media, is very significant, together with the changes of other related proteins. This confirms that the dynamic shifts induced by the drug are epigenetic in nature.
During the valproate treatment, the LNCaP cells assume the neurogenic differentiation with typical protrusions and long cell prolongations as revealed by microscopy observation.
In support of this, we have shown an increase in the expression of protein makers of neuroendocrine phenotype such as β-III Tubulin and γ-Enolase. β-III Tubulin is a sensitive marker for early neuroendocrine transdifferentiation in prostate cancer cell lines and its up-regulation has been revealed under HDAIs in LNCaP cells.31,32
Additionally, overexpression of β-III Tubulin has been associated with tumor aggressiveness in prostate cancer patients, as well as with recurrence after treatment.33
Of note, the acquisition of androgen-independent condition, given by the decrease of AR and PSA, influences the evolution of the same tumor. In fact, the gain of the neuroendocrine phenotype does correlate with hormone-independent prostate carcinoma. These new NE cells, through the secretion of neuropeptides, are able to support the proliferation of adjacent carcinoma cells that become more aggressive, with a poor tumor prognosis.34,35
Furthermore, unexpectedly, the VPA treatment induced an activation of some survival signals such as Akt/mTOR/ERK1, 2, which begin to increase early, and remain high at longer times.
Thus, the maintenance of these signals, even at the time in which a proliferative block and the increase of neuroendocrine markers (as β-III Tubulin and γ-Enolase) has been evidenced, indicates that the new transdifferentiated LNCaP cells retain a good survival and this might be important for tumor progression.
Another marker here evaluated, the p70S6 kinase, important regulator for protein synthesis, cell growth and survival, resulted to be increased by the treatment associated with the sustained activation of pAkt/mTOR signal. In fact, p70S6 kinase is well known as effector of the above-mentioned signal, mostly activated in tumor cells with aggressive clinical behavior. However, as reported by previous observations, in addition to being involved in cell survival, p70S6 kinase can regulate other cellular functions such as chemotaxis, cell motility, tumor progression and invasion.36,37
On the other hand, we have observed an increase in the phosphorylation of the oncosuppressor FOXO3a, which corresponds to a decrease in protein levels. Indeed, many evidences have reported that FOXO proteins are activated by various kinases, such as Akt or ERK1/2, that, through the phosphorylation, induce the ubiquitination and degradation of these proteins.38,39
In particular, the increase of phospho-FOXO3a, here evaluated, overlaps the up-regulation of phospho-Akt and phospho ERK1/2. The sustained activity of Akt, together with other kinases, such as ERK1/2 and mTOR, plays an important role in maintaining cell tumorigenesis. Similarly, valproate treatment in rats acts as a neurotrophic factor since it promotes neurite growth and cell reemergence through the induction of ERK pathway.40
Previous studies have shown that activation of PI3K and MAPK was involved in the differentiation process of LNCaP cells. Acquisition of neuroendocrine phenotype in these cells, along with an up-regulation of the above signaling, was identified under treatment with Dovitinib, a pan receptor tyrosine kinase inhibitor.41 Although the mechanistic bases of this differentiation are unclear, this perhaps is a way through which the tumor develops resistance and maintains the differentiation process. Supporting these observations, it seems that VPA, in prostate tumors, may retain a neurotrophic action able to sustain the survival of the cells. Even if HDACIs have been shown to induce cancer cell growth arrest it cannot be excluded that the enrichment of the new cell population consistently drives tumor progression towards a more resistant phenotype.
Conclusion
On the basis of these data, it emerges that VPA in prostate cancer cells: 1) inhibits proliferation; 2) induces neuroendocrine differentiation; and 3) maintains cell survival. Keeping in mind that neuroendocrine differentiation of prostate cancer appears to be associated with a poor outcome, it is necessary to identify new therapeutic approaches that prevent neurodifferentiation and the survival of these cells.
Acknowledgment
We thank Dr Sturino D for the English revision, Professor of English, University of Calabria, Cosenza, Italy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi:10.1038/35094009
2. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15(12):701–711. doi:10.1038/nrc4016
3. Saraon P, Jarvi K, Diamandis EP. Molecular alterations during progression of prostate cancer to androgen independence. Clin Chem. 2011;57(10):1366–1375. doi:10.1373/clinchem.2011.165977
4. Devlin HL, Mudryj M. Progression of prostate cancer: multiple pathways to androgen independence. Cancer Lett. 2009;274(2):177–186. doi:10.1016/j.canlet.2008.06.007
5. Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107(39):16759–16765. doi:10.1073/pnas.1012443107
6. Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6(4):503–519. doi:10.1677/erc.0.0060503
7. Nelson EC, Cambio AJ, Yang JC, Ok JH, Lara PN
8. Yuan TC, Veeramani S, Lin FF, et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13(1):151–167. doi:10.1677/erc.1.01043
9. Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology. 2004;145(2):613–619. doi:10.1210/en.2003-0772
10. Wang Q, Horiatis D, Pinski J. Interleukin-6 inhibits the growth of prostate cancer xenografts in mice by the process of neuroendocrine differentiation. Int J Cancer. 2004;111(4):508–513. doi:10.1002/(ISSN)1097-0215
11. Bang YJ, Pirnia F, Fang WG, et al. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. 1994;91(12):5330–5334. doi:10.1073/pnas.91.12.5330
12. Spiotto MT, Chung TDK. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate. 2000;42(2):88–98. doi:10.1002/(SICI)1097-0045(20000201)42:2<88::AID-PROS2>3.0.CO;2-P
13. Gutiérrez-Cañas I, Juarranz MG, Collado B, et al. Vasoactive intestinal peptide induces neuroendocrine differentiation in the LNCaP prostate cancer cell line through PKA, ERK, and PI3K. Prostate. 2005;63(1):44–55. doi:10.1002/pros.20173
14. Uysal-Onganer P, Kawano Y, Caro M, et al. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer. 2010;9:55. doi:10.1186/1476-4598-9-55
15. Harris KS, Kerr BA. Prostate cancer stem cell markers drive progression, therapeutic resistance, and bone metastasis. Stem Cells Int. 2017;2017:8629234. doi:10.1155/2017/8629234
16. Graça I, Pereira-Silva E, Henrique R, Packham G, Crabb SJ, Jerónimo C. Epigenetic modulators as therapeutic targets in prostate cancer. Clin Epigenetics. 2016;8:98. doi:10.1186/s13148-016-0264-8
17. Chateauvieux S, Morceau F, Dicato M, Diederich M. Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol. 2010;2010:479364. doi:10.1155/2010/479364
18. Blaheta RA, Cinatl J. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002;22(5):492–511. doi:10.1002/(ISSN)1098-1128
19. Sidana A, Wang M, Shabbeer S, et al. Mechanism of growth inhibition of prostate cancer xenografts by valproic acid. J Biomed Biotechnol. 2012;2012:180363. doi:10.1155/2012/180363
20. Witt D, Burfeind P, von Hardenberg S, et al. Valproic acid inhibits the proliferation of cancer cells by re-expressing cyclin D2. Carcinogenesis. 2013;34(5):1115–1124. doi:10.1093/carcin/bgt019
21. Valentini A, Biancolella M, Amati F, et al. Valproic acid induces neuroendocrine differentiation and UGT2B7 up-regulation in human prostate carcinoma cell line. Drug Metab Dispos. 2007;35:968–972. doi:10.1124/dmd.107.014662
22. Frigo DE, McDonnell DP. Differential effects of prostate cancer therapeutics on neuroendocrine transdifferentiation. Mol Cancer Ther. 2008;7(3):659–669. doi:10.1158/1535-7163.MCT-07-0480
23. Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59(8):1317–1326. doi:10.1007/s00018-002-8510-y
24. Stacey DW. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr Opin Cell Biol. 2003;15(2):158–163. doi:10.1016/S0955-0674(03)00008-5
25. Xia Q, Sung J, Chowdhury W, et al. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006;66(14):7237–7244. doi:10.1158/0008-5472.CAN-05-0487
26. Shabbeer S, Kortenhorst MS, Kachhap S, Galloway N, Rodriguez R, Carducci MA. Multiple molecular pathways explain the anti-proliferative effect of valproic acid on prostate cancer cells in vitro and in vivo. Prostate. 2007;67:1099–1110. doi:10.1002/(ISSN)1097-0045
27. Hoshikawa Y, Kwon HJ, Yoshida M, Horinouchi S, Beppu T. Trichostatin A induces morphological changes and gelsolin expression by inhibiting histone deacetylase in human carcinoma cell lines. Exp Cell Res. 1994;214(1):189–197. doi:10.1006/excr.1994.1248
28. Yoshida M, Hoshikawa Y, Koseki K, Mori K, Beppu T. Structural specificity for biological activity of trichostatin A, a specific inhibitor of mammalian cell cycle with potent differentiation-inducing activity in Friend leukemia cells. J Antibiot. 1990;43(9):1101–1106. doi:10.7164/antibiotics.43.1101
29. Nakano K, Mizuno T, Sowa Y, et al. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem. 1997;272(35):22199–22206. doi:10.1074/jbc.272.35.22199
30. Sambucetti LC, Fischer DD, Zabludoff S, et al. Histone deacetylase inhibition selectively alters the activity and expression of cell cycle proteins leading to specific chromatin acetylation and antiproliferative effects. J Biol Chem. 1999;274(49):34940–34947. doi:10.1074/jbc.274.49.34940
31. Katsetos CD, Herman MM, Mork SJ. Class III β-tubulin in human development and cancer. Cell Motil Cytoskeleton. 2003;55(2):77–96. doi:10.1002/cm.v55:2
32. Katsetos CD, Kontogeorgos G, Geddes JF, et al. Differential distribution of the neuron-associated class III h-tubulin in neuroendocrine lung tumors. Arch Pathol Lab Med. 2000;124(4):535–544. doi:10.1043/0003-9985(2000)124<0535:DDOTNA>2.0.CO;2
33. Ploussard G, Terry S, Maillé P, et al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010;70(22):9253–9264. doi:10.1158/0008-5472.CAN-10-1447
34. Cussenot O, Villette JM, Cochand-Priollet B, Berthon P. Evaluation and clinical value of neuroendocrine differentiation in human prostatic tumors. Prostate Suppl. 1998;8:43–51.
35. Grobholz R, Griebe M, Sauer CG, Michel MS, Trojan L, Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005;36(5):562–570. doi:10.1016/j.humpath.2005.02.019
36. Lehman JA, Gomez-Cambronero J. Molecular crosstalk between p70S6k and MAPK cell signaling pathways. Biochem Biophys Res Commun. 2002;293(1):463–469. doi:10.1016/S0006-291X(02)00238-3
37. Pon YL, Zhou HY, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase promotes epithelial to mesenchymal transition through snail induction in ovarian cancer cells. Cancer Res. 2008;68(16):6524–6532. doi:10.1158/0008-5472.CAN-07-6302
38. Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011;1813(11):1961–1964. doi:10.1016/j.bbamcr.2011.01.007
39. Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278(14):12361–12366. doi:10.1074/jbc.M213069200
40. Hao Y, Creson T, Zhang L, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24(29):6590–6599. doi:10.1523/JNEUROSCI.5747-03.2004
41. Yadav SS, Li J, Stockert JA, et al. Induction of neuroendocrine differentiation in prostate cancer cells by dovitinib (TKI-258) and its therapeutic implications. Transl Oncol. 2017;10(3):357–366. doi:10.1016/j.tranon.2017.01.011
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.