Back to Journals » Cancer Management and Research » Volume 12
Validity of serum amyloid A and HMGB1 as biomarkers for early diagnosis of gastric cancer
Authors Ghweil AA, Osman HA , Hassan MH , Sabry AMM , Mahdy RE, Ahmed ARH , Okasha A, Khodeary A , Ameen HH
Received 10 March 2019
Accepted for publication 11 June 2019
Published 8 January 2020 Volume 2020:12 Pages 117—126
DOI https://doi.org/10.2147/CMAR.S207934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Ali A Ghweil,1 Heba A Osman,1 Mohammed H Hassan,2 Abeer MM Sabry,3 Reem E Mahdy,4 Ahmed RH Ahmed,5 Ahmed Okasha,6 Ashraf Khodeary,7 Hesham H Ameen8
1Tropical Medicine and Gastroenterology Department, Faculty of Medicine, South Valley University, Qena, Egypt; 2Department of Medical Biochemistry, Faculty of Medicine, South Valley University, Qena, Egypt; 3Internal Medicine and Gastroenterology Department, Faculty of Medicine, Helwan University, Helwan, Egypt; 4Internal Medicine Department, Faculty of Medicine, Assiut University, Assiut, Egypt; 5Pathology Department, Faculty of Medicine, Sohag University, Sohag, Egypt; 6Radiology Department, Faculty of Medicine, South Valley University, Qena, Egypt; 7Clinical Pathology Department, Faculty of Medicine, Sohag University, Sohag, Egypt; 8Clinical Pathology Department, Faculty of Medicine, Al-Azhar University (Assiut Branch), Assiut, Egypt
Correspondence: Mohammed H Hassan
Department of Medical Biochemistry, Faculty of Medicine, South Valley University, Qena 83523, Egypt
Tel +20 109 847 3605
Email [email protected]
Background and aim: Gastric carcinomais a frequent neoplasm with poor outcome, and its early detection would improve prognosis. This study was designed to evaluate the possible use of new biomarkers, namely SAA and HMGB1, for early diagnosis of gastric cancer.
Methods: A total of 100 patients presenting with gastric symptoms were included. All patients underwent upper endoscopic evaluation, histopathological diagnosis and serum CEA, SAA, and HMGB1 measurements.
Results: Patients were classed endoscopically with neoplastic, inflammatory, and normal-appearing gastric mucosa: 50, 25, and 25 patients, respectively. Histologically, half the patients had chronic gastritis and the remaining cases gastric carcinoma of diffuse (n=28) or intestinal (n=22) type. SAA at cutoff of 18.5 mg/L had the best validity to differentiate gastritis from gastric carcinoma, with AUC, sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) of 0.99, 98%, 100%, 100%, and 98%, respectively, followed by HMGB1 at cutoff of 14.5 pg/μL, with AUC, sensitivity, specificity, PPV, and NPV of 0.91, 70%, 96%, 94.6%, and 76.2%, respectively. Sensitivity, specificity, PPV, and NPV of serum CEA at cutoff of 2.9 ng/mL to differentiate gastritis from gastric carcinoma were 42%, 72%, 60%, and 55.4%, respectively, with AUC of 0.53. Nonetheless, higher serum levels of both SAA and HMGB1 reflected higher tumor grade (P=0.027 and P=0.016, respectively) and advanced tumor stage (P-OBrk-0.001 for both).
Conclusion: Serum levels of both SAA and HMGB1 could be of great value for early diagnosis of gastric carcinoma, comparable to the diagnostic role of serum CEA, which is not valid for early diagnosis of gastric cancer.
Keywords: gastric carcinoma, early detection, SAA, HMGB1, CEA
Introduction
Gastric carcinoma is a frequently occurring aggressive malignant tumor worldwide, with poor 5-year survival even after surgical intervention, mainly because the majority of cases are asymptomatic till reaching an advanced stage.1,2To date, the currently used diagnostic and prognostic biomarkers for gastric carcinoma have exhibited low sensitivity and specificity. and diagnosis basically depends on invasive upper digestive endoscopic examination. Therefore, there is a huge need for minimally invasive or noninvasive biomarkers with high specificity in screening and diagnosis of gastric carcinoma.3
Chronic inflammation is postulated to be one of the predisposing lesions of gastric carcinoma, particularly the intestinal subtype. The Correa hypothesis states that gastric carcinoma passes through sequential stages of chronic gastritis, gastric atrophy, intestinal metaplasia, epithelial dysplasia, and lastly development of gastric carcinoma (gastritis–dysplasia–carcinoma sequence). Several cytokines that conduct downstream signals play integral roles in the progression of these sequential stages of epithelial gastric change.4,5
HMGB1, also known as amphoterin, binds to several receptors, including RAGE, with subsequent activation of certain key cell-signaling pathways (eg, NFκB, p38, and p44/42 MAPKs) leading to cancer progression and metastasis.6–8 Many studies have emphasized the relationship between ove-expression of HMGB1 and progression and invasion of several epithelial tumors.9–13 Although during tumorgenesis, HMGB1 may have pro- or antitumor activity via enhancing or suppressing tumor angiogenesis, growth, invasion, and metastasis, current knowledge regarding the role of HMGB1 in the development of tumors is still not explicit.14As a proinflammatory cytokine, HMGB1 can create a chronic inflammatory status that subsequently predisposes to development of epithelial malignancies.15 In addition, it has been claimed that overexpression of HMGB1 promotes metastatic potential of gastric carcinoma.16
High SAA, another cytokine, has been studied in many human malignancies, but to date a consistent perspective has not yet been established.17 It has been reported as a potential biomarker for predicting survival and postoperative follow-up of patients with gastric cancer.18 SAA is a major member of acute-phase reactants, which are raised in chronic inflammatory or neoplastic conditions. It has been shown that SAA is raised in several malignant epithelial tumors, including lung, ovarian, colon, esophageal, and gastric cancers.19–23 Sasazuki et al4 reported that people with high serum SAA had a higher risk of developing gastric cancer.
CEA is a well-known tumor-associated molecule that arises in cases of gastric and colonic tumors.24 In this study, the validity of serum levels of both HMGB1 and SAA for detection of gastric carcinoma was evaluated and compared to diagnostic accuracy of the well-known serum tumor marker — the CEA molecule.
Methods
Recruited patients and endoscopic evaluation
The current case–control study was conducted on 100 patients who attended the endoscopic unit of the Tropical Medicine and Gastroenterology Department, Qena University Hospital from January 2017 to October 2018. Patients who presented with upper gastrointestinal tract symptoms, including epigastric pain, vomiting, hematemesis, and melena were included in the study. Patients with a history of cardiac disease, diabetes mellitus, autoimmune disease, coagulation disorders, malignant tumors elsewhere, and those had undergone gastric surgery or used acid-suppressor therapy during the previous 60 days were excluded. The study was approved by the local Ethics Committee of Medical Research of the Faculty of Medicine, South Valley University, Qena, Egypt, and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects included in the study.
Patients included were subjected to detailed history-taking, full clinical examination, and complete blood count. Theey underwent upper endoscopic examination using an Olympus GIF-XQ260 instrument under sedation with intravenous midazolam 5 mg, and endoscopic punch biopsies were obtained for histopathological evaluation. Subjects were classified endoscopically into three categories: neoplastic, inflammatory, and normal-appearing gastric mucosa. After histopathological examination, patients were classified into two groups, neoplastic and inflammatory, depending on biopsy findings.
Biochemical evaluation of serum levels of SAA, HMGB1, and CEA
Assessment of SAA, HMGB1, and CEA was performed prior to endoscopic evaluation or any therapeutic intervention, without knowledge of endoscopic data or histopathological diagnosis. Venous blood (5 mL) was obtained after skin cleaning with 70% ethyl alcohol. Samples were divided in two tubes: 2 mL in an EDTA-containing Vacutainer for complete blood count and 3 mL in a plain Vacutainer for assessment of CEA, SAA, and HMGB1. The plain tube was left till the blood had clotted, then centrifuged (3,500 rpm for 10 minutes) and the separated serum was transferred into 1 mL cryotubes and stored at −80°C for later biochemical analysis. Complete blood counts were Taken with a Celtac automated hematology analyzer (Nihon Kohden).
The serum level of CEA was evaluated with a Mini Vidas system (Biomérieux, France), which is a fully automated immunoassay machine. Vidas CEA is a quantitative test employing an enzyme-linked fluorescent assay for determination of CEA level. For quantitative measurement of human SAA and HMGB1, manual ELISA kits were used (supplied by Abcam, and MyBioSource, respectively), using a microplate ELISA reader (EMR-500).
Histopathological and immunohistochemical evaluation of gastric biopsies
For histopathological evaluation of gastric biopsies, standard formalin-fixed, paraffin-embedded tissue sections were prepared and stained with H&E. Gastric tumors classified histologically as diffuse gastric carcinomas were evaluated further for expression of cytokeratin using a Dako automated immunostaining system. Antigen retrieval, incubation with primary antibody, and secondary chromogen detection were performed according to manufacturer protocols. The staining procedure was performed at room temperature, and all steps were separated by washing twice in tris-buffered saline–Tween, pH 7.6. Normal mucosal glands encountered worked as positive internal controls.
Multidetector computed tomography
Multidetector computed tomography is the modality of choice for gastric carcinoma staging, due to its ability to detect tumor spread (especially gastric wall invasion), lymph node-involvement, or metastasis through multiple-phase imaging. Patients were examined after fasting for 4–8 hours. Contrast agents, eg, water or effervescent granules producing gas (CO2), were used. Additional gastric distension using scopalomine N-butyl bromide or intravenous/intramuscular glucagon injection enabled assessment of the thickness of the gastric wall and proper differentiation of gastric walls and lumen.
Statistical analysis
SPSS version 13.0 was used for data support and analysis. CEA, HMGB1 and SAA values are expressed as means with 25%–75% SD. Independent-sample t-tests were performed to compare means of CEA, HMGB1, SAA between two groups and one-way ANOVA used to compare means among three or more groups with multiple comparisons by the post hoc Scheffé method. ROC curves were plotted to determine the best cutoff for gastric cancer screening for each value, and relevant sensitivity and specificity were calculated. P<0.05 was considered statistically significant.
Results
Demographics and endoscopic findings
A total of 100 patients with upper gastrointestinal symptoms were recruited for this study: 71 males and 29 females. Patient ages ranged 37–65 years (mean 52.2±8.2 years). Leading symptoms were chronic epigatsric pain/dyspepsia, repeated vomiting, and attack(s) of bleeding (hematemesis and/or melena) in 84%, 67%, and 41%, respectively. On initial clinical evaluation, 59% of patients looked cachectic, and none was hypertensive, diabetic, or had a history of systemic inflammatory or autoimmune disease.
Endoscopic evaluation revealed involvement of the gastric fundus in 20 patients, body/antrum in 44, and diffuse gastric involvement in eleven. Lesions identified by endoscopy were mucosal erythema, erosion, ulcers, wall thickening, and gastric mass in 78.7%, 13.3%, 26.7%, 13.3%, and 10.7%, respectively. As for patients with chronic gastritis, 12.0% had endoscopic features suggestive of Helicobacter pylori infection in the form of mosaic patterns, erythema, erosion, or antral nodularity. On the other hand, 25 patients had no remarkable changes on endoscopic evaluation. Based on endoscopic findings, gastric lesions were classified as malignant, inflammatory (chronic gastritis), or normal-appearing gastric mucosa in 50, 25, and 25 cases, respectively (Table 1, Figure 1).
 |
Table 1 Descriptive data of all studied patients |
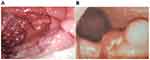 |
Figure 1 Endoscopy of chronic gastritis (A) and malignant polypoid mass (B). |
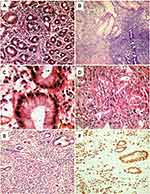
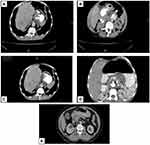
The final diagnosis of gastric lesions was established by histopathological evaluation of gastric endoscopic biopsies. Half the patients had chronic gastritis (Figure 2, A and B), classified as mild, moderate, or severe in 25, 16, and nine cases, respectively. Helicobactercolonies were identified histologically in 23 patients (Figure 2C). Gastric carcinomas were confirmed histologically in 50 patients and classified as diffuse and intestinal subtypes (Figure 2, D and E) in 28 and 22 cases, respectively. The epithelial origin of diffuse gastric carcinomas was confirmed by expression of cytokeratin (AE1/AE3) molecules (Figure 2F). Ttumors were graded 1, 2, 3, and 4 in four (8%), eleven (22%), 19 (38%), and 16 (32%) cases, respectively, and tumor stage 0, 1, 2, 3, and 4 in five (10%), ten (20%), 14 (28%), 13 (26%), and eight (16%) cases, respectively, based on radiological evaluation (Figure 3).
Serum levels of HMGB1, SAA, and CEA
Serum levels of HMGB1, SAA, and CEA ranged 0.3–50 pg/μL, 0.6–150 mg/L, and 0.5–54 ng/mL, respectively, with mean values of 14.8±14.1, 42.9±44.8, and 6.5±10.9, respectively. The association of these serum molecules with different endoscopic and pathological parameters is summarized in (Table 2). There was a significant difference in serum levels of the three investigated biomarkers among patients with gastric cancer compared to patients with chronic gastritis. Nonetheless, high-grade tumors tended to be significantly associated with high serum levels of HMGB1 and SAA but not CEA compared to low-grade tumors. Tumors with advanced stages were associated with significantly high serum levels of the three molecules.
 |
Table 2 Association of serum HMGB1, SAA, and CEA levels with endoscopic and pathological parameters |
Diagnostic validity of serum levels of SAA and HMBG1
Serum levels of SAA and HMBG1 were significantly high in cases of gastric carcinoma (Table 2). The validity of these two molecules for differentiation of chronic gastritis from gastric carcinoma was measured and compared to the diagnostic validity of serum levels of CEA. Different cutoffs for serum levels of the two molecules were tested statistically to identify the best point at which malignancy is strongly predicted. SAA >18.5 mg/L had the strongest diagnostic performance for gastric carcinoma, followed by HMBG1 >14.5 pg/μL. Both molecules had better sensitivity, specificity, positive predictive values, and negative predictive values than standard serum levels of CEA, with AUC of 99% for SAA, 91% for HMBG1, and 53% for CEA (Table 3, Figure 4).
 |
Table 3 Diagnostic performance of SAA and HMBG1 compared to serum CEA to discriminate gastric carcinoma form chronic gastritis |
 |
Figure 4 ROC curve of SAA (A), HMBG1 (B), and CEA (C) for discrimination of gastric carcinoma from chronic gastritis. |
Discussion
Early diagnosis and posttreatment follow-up of patients with malignant tumors requires finding serum markers that can reflect tumor-cell growth in early stages. Gastric cancer is believed at least partially to follow a hypothesis of being initiated by persistent chronic inflammation.25 Accordingly, serum levels of inflammation-associated biomarkers could be of great value for prediction of malignant changes in gastric epithelia. In this context, the ability of the inflammation-associated molecules SAA and HMGB1 to detect early gastric carcinogenesis was investigated and compared to the diagnostic validity of CEA serum levels.
We first investigated the three biomarkers used in the different types of gastric cancer in our patients, ie, diffuse and intestinal. There was no statistical significance between the two types in terms of malignancy. Therefore, we decided to consider that patients with gastric cancer represented one group of 50 patients, to be analyzed alongside 50 patients with chronic gastritis, and a control group was added to the latter after inflammation had been confirmed on histopathology.
SAA is known to be elevated in inflammation, trauma, and neoplasm.26–28 It is also increased significantly in metastatic disease over early stages,29 so it can indicate progression and relapse.30 Chan et al concluded that SAA is raised significantly in gastric cancer, but with no relation to tumor size or clinical stage of the disease.19 This was in concordance with our study in its first part, including its increase in relation to gastric cancer (P<0.001) based on means in the different groups. However, this was not the case for the ability of SAA to differentiate between diffuse gastric carcinoma and intestinal gastric carcinoma groups. SAA showed high statistical significance (P<0.001) when comparing gastric cancer and chronic gastritis. This was the case when studying different stages of gastric cancer, where it showed high statistical significance (P<0.001). This was similar to results obtained by Liu et al, where SAA peaks were positively correlated with the course of gastric carcinoma, and peak intensity gradually increased with aggravation of the patient’s condition.31
SAA discriminating between gastric cancer and chronic gastritis showed sensitivity of 98%, specificity, PPV of 100%, and NPV of 98%. Its ability to discriminate between early and late stages of gastric cancer was significant (P<0.001), though it did not show a significant difference between diffuse and intestinal gastric cancer (P=0.7). This means that it can detect the early and invasive stages of gastric cancer, but has nothing to do with the type of tissue pathology. The sensitivity of SAA in our study was much higher than Chan et al report19 (74%), who investigated serum SAA from 96 gastric cancer patients, 32 patients with gastric ulcers, and 52 healthy subjects. This difference could attributed to sample size, ethnic and geographic differences, and differences in the number of gastric cancer grades and stages included.
Next, we studied HMGB1 and its ability to diagnose early gastric cancer. HMGB1 has a significant role in tumor genesis and invasion.16,32,33 It can be detected in the serum of patients diagnosed with malignancy, because it either reaches the serum passively from dead tumor cells or is actively released from immune cells in extracellular space.18,34,35 We measured HMGB1 levels in sera of the control group, patients with gastritis, and those with gastric cancer. Significant differences were found among the groups, mean increases of 2.07±1.7 in the control group, 7.9±4.2 reaching 24.7±13.6 in the gastric cancer group (P<0.001). This was consistent with Kauniyasu et al.16 The same results were obtained by Chung et al.36 Zahang et al and Yue et al reported significantly increased positive expression of HMBG1 in gastric adenocarcinoma tissue samples in comparison with samples from adjacent noncancerous tissue using immunohistochemical staining, suggesting that increased HMBG1 expression is associated with tumor development and progression via the NFκB pathway and may serve as a potential therapeutic target for gastric adenocarcinoma.37,38 Additionally, Chung et al reported increased serum HMBG1 in gastric cancer carcinogenesis, reaching a maximum before macroscopic metastasis occurred, implying its role in gastric cancer micrometastasis.39
Validation of HMGB1 for discrimination between gastric cancer and chronic gastritis showed sensitivity of 70%, specificity of 96%, PPV of 94.6%, and NPV of 76.2% (P<0.001). For discrimination between early and late stages of gastric cancer, HMGB1 showed significance (P<0.001). HMGB1 did not show a significant difference between intestinal and diffuse gastric cancer (P=0.6), which was in accordance with Kauniyasu et al and Chung et al.16,36
CEA results were not encouraging, although it is a known marker for malignancy, where it showed sensitivity of 42%, specificity of 72%, PPV of 60%, and NPV of 55.4% (P=0.57) when discriminating between gastric cancer and chronic gastritis, indicating that CEA is significantly inferior to SAA or HMGB1 as a gastric cancer biomarker. This finding was near the results obtained by other studies comparing CEA with other biomarkers, such as pepsinogen and high-sensitivity CRP.40–43 Additionally, Dolscheid-Pommerich et al44 reported nonsignificant differences in the CEA serum concentrations between patients with benign vs malignant gastric tumors (AUC=0.61). Our CEA results were better when discriminating between early and late gastric cancer (P=0.002). This means that it can work as a follow-up marker in differentiating between early and invasive stages, but cannot detect early stages.
Study limitation
Our study lacked the follow-up to analyze relationships between serum concentrations of the biomarkers studied with the prognosis or recurrence of gastric carcinoma. This could be implemented in future studies. Also, we did not construct our study to compare the biomarkers in various types of cancer, so further research is required to identify and compare the serum profiles of these biomarkers among patients with different types of cancers versus gastric cancers to ensure their specificity for gastric carcinoma.
Conclusion
In the current study we demonstrated that both serum SAA and HMGB1 can be used as important, informative, and excellent biomarkers for early detection of gastric cancer, as evidenced by the high AUC (˃0.9) obtained, with nearly equally high specificity and much higher sensitivity for SAA than HMGB1.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma review and considerations for future directions. Ann Surg. 2005;241(1):27–39. doi:10.1097/01.sla.0000149300.28588.23
2. Matsuoka T, Yashiro M. Biomarkers of gastric cancer: current topics and future perspective. World J Gastroenterol. 2018;24(26):2818–2832. doi:10.3748/wjg.v24.i26.2818
3. Necula L, Matei L, Dragu D, et al. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25(17):2029–2044. doi:10.3748/wjg.v25.i17.2029
4. Sasazuki S, Inoue M, Sawada N, et al. Japan Public Health Center-Based Prospective Study Group. Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan Public Health Center-based prospective study. Carcinogenesis. 2010;31:712–718. doi:10.1093/carcin/bgq010
5. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–first American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740.
6. Kuniyasu H, Yano S, Sasaki T, Sasahira T, Sone S, Ohmori H. Colon cancer cell-derived high mobility group 1/amphoterin induces growth inhibition and apoptosis in macrophages. Am J Pathol. 2005;166:751–760. doi:10.1016/S0002-9440(10)62296-1
7. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi:10.1146/annurev.immunol.021908.132603
8. Zhao CB, Bao JM, Lu YJ, et al. Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. Am J Cancer Res. 2014;4(4):369–377.
9. Flohr AM, Rogalla P, Meiboom M, et al. Variation of HMGB1 expression in breast cancer. Anticancer Res. 2001;21:3881–3885.
10. Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi:10.1002/pros.20219
11. Takada M, Hirata K, Ajiki T, Suzuki Y, Kuroda Y. Expression of receptor for advanced glycation end products (RAGE) and MMP-9 in human pancreatic cancer cells. Hepatogastroenterology. 2004;51:928–930.
12. Ellerman JE, Brown CK, de Vera M, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–2848. doi:10.1158/1078-0432.CCR-06-1953
13. Sasahira T, Akama Y, Fujii K, Kuniyasu H. Expression of receptor for advanced glycation end products and HMGB1/amphoterin in colorectal adenomas. Virchows Arch. 2005;446:411–415. doi:10.1007/s00428-005-1210-x
14. He SJ, Cheng J, Feng X, Yu Y, Tian L, Huang Q. The dual role and therapeutic potential of high-mobility group box 1 in cancer. Oncotarget. 2017;8(38):64534–64550. doi:10.18632/oncotarget.17885
15. Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L, Bucci E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: is there any evidence? Oral Oncol. 2004;40:120–130.
16. Kuniyasu H, Oue N, Wakikawa A, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–170. doi:10.1002/path.1031
17. Zhou J, Sheng J, Fan Y, et al. Association between serum amyloid A levels and cancers: a systematic review and meta-analysis. Postgrad Med J. 2018;94(1115):499–507. doi:10.1136/postgradmedj-2018-136004
18. Cheng BQ, Jia CQ, Liu CT, et al. Serum high mobility group box chromosomal protein 1 isassociated with clinicopathologic features in patients with hepatocellular carcinoma. Dig Liver Dis. 2008;40:446–452. doi:10.1016/j.dld.2007.11.024
19. Chan DC, Chen CJ, Chu HC, et al. Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol. 2007;14:84–93. doi:10.1245/s10434-006-9091-z
20. Sung HJ, Ahn JM, Yoon YH, et al. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J Proteome Res. 2011;10:1383–1395. doi:10.1021/pr101154j
21. Wang JY, Zheng YZ, Yang J, et al. Elevated levels of serum amyloid A indicate poor prognosis in patients with esophageal squamous cell carcinoma. BMC Cancer. 2012;12:365.
22. Moshkovskii SA, Vlasova MA, Pyatnitskiy MA, et al. Acute phase serum amyloid A in ovarian cancer as an important component of proteome diagnostic profiling. Proteomics. Clin Appl. 2007;1:107–117. doi:10.1002/prca.200600229
23. Gutfeld O, Prus D, Ackerman Z, et al. Expression of serum amyloid A, in normal,dysplastic, and neoplastic human colonic mucosa: implication for a role in colonic tumorigenesis. J Histochem Cytochem. 2006;54:
24. Deng K, Yang L, Bing H, Hao W, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10(4):e0124151. doi:10.1371/journal.pone.0124151
25. Vannella L, Lahner E, Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: a critical reappraisal. World J Gastroenterol. 2012;18(12):1279–1285. doi:10.3748/wjg.v18.i12.1279
26. Cho WC, Yip TT, Ngan RK, et al. Protein chip array profiling for identification of disease- and chemotherapy-associated biomarkers of nasopharyngeal carcinoma. Clin Chem. 2007;53:241–250. doi:10.1373/clinchem.2005.065805
27. Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–435.
28. Kokubun M, Imafuku Y, Okada M, et al. Serum amyloid A (SAA) concentration varies among rheumatoid arthritis patients estimated by SAA/CRP ratio. Clin Chim Acta. 2005;360:92–102. doi:10.1016/j.cccn.2005.04.006
29. Weinstein PS, Skinner M, Sipe JD, Lokich JJ, Zamcheck N, Cohen AS. Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol. 1984;19:193–198.
30. Yokoi K, Shih LC, Kobayashi R, et al. Serum amyloid A as a tumor marker in sera of nude mice with orthotopic human pancreatic cancer and in plasma of patients with pancreatic cancer. Int J Oncol. 2005;27:1361–1369.
31. Liu C, Pan C, Shen J, Wang H, Yong L. Identification of serum amyloid A in the serum of gastric cancer patients by protein expression profiling. Oncol Lett. 2012;3:1259–1262. doi:10.3892/ol.2012.664
32. Oue N, Aung PP, Mitani Y, Kuniyasu H, Nakayama H, Yasui W. Genes involved in invasion and metastasis of gastric cancer identified by array-based hybridization and serial analysis of gene expression. Oncology. 2005;69(Suppl 1):17–22. doi:10.1159/000086627
33. Xiang YY, Wang DY, Tanaka M, et al. Expression of high-mobility group-1 mRNA in human gastrointestinal adenocarcinoma and corresponding non-cancerous mucosa. Int J Cancer. 1997;74:1–6.
34. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi:10.1038/nri1594
35. Palumbo R, Sampaolesi M, De Marchis F, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi:10.1083/jcb.200304135
36. Chung HW, Lee SG, Kim H, et al. Serum high mobility group box-1 (HMGB1) is closely associated with the clinical and pathologic features of gastric cancer. J Transl Med. 2009;7:38. doi:10.1186/1479-5876-7-38
37. Zhang J, Kou YB, Zhu JS, Chen WX, Li S. Knockdown of HMGB1 inhibits growth and invasion of gastric cancer cells through the NF-κB pathway in vitro and in vivo. Int J Oncol. 2014;44(4):1268–1276. doi:10.3892/ijo.2014.2285
38. Yue Y, Zhou T, Gao Y, et al. High mobility group box 1/toll-like receptor 4/myeloid differentiation factor 88 signaling promotes progression of gastric cancer. Tumour Biol. 2017;39(3):1010428317694312. doi:10.1177/1010428317694312
39. Chung HW, Jang S, Kim H, Lim JB. Combined targeting of high-mobility group box-1 and interleukin-8 to control micrometastasis potential in gastric cancer. Int J Cancer. 2015;137(7):1598–1609. doi:10.1002/ijc.29539
40. Nakopoulou L, Zinozi M, Theodoropoulos G, Papacharalampous N. Carcinoembryonic antigen detection by immunocytochemical methods in carcinomas of the colon and stomach. Dis Colon Rectum. 1983;26:269–274. doi:10.1007/BF02562496
41. Victorzon M, Haglund C, Lundin J, Roberts PJ. A prognostic value of CA 19-9 but not of CEA in patients with gastric cancer. EurJ Surg Oncol. 1995;21:379–384. doi:10.1016/S0748-7983(95)92450-7
42. Chung HW, Kim JW, Lee JH, et al. Comparison of the validity of three biomarkers for gastric cancer screening: carcinoembryonic antigen, pepsinogens, and high sensitive C-reactive protein. J Clin Gastroenterol. 2009;43:19–26. doi:10.1097/MCG.0b013e318135427c
43. Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi:10.1136/gut.44.5.693
44. Dolscheid-Pommerich RC, Manekeller S, Walgenbach-Brünagel G, et al. Clinical performance of CEA, CA19-9, CA15-3, CA125 and AFP in gastrointestinal cancer using LOCI™-based assays. Anticancer Res. 2017;37(1):353–359. doi:10.21873/anticanres.11329
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.