Back to Journals » Nature and Science of Sleep » Volume 14
Validation of the Sonographic Measurement of Lateral Parapharyngeal Wall Thickness in Childhood Obstructive Sleep Apnea
Authors Yuen HM , Lai AC , Liu EK, Lee MC, Chu WC, Chan JW, Chan NY, Wing YK , Li AM, Chan KC, Au CT
Received 8 June 2022
Accepted for publication 24 October 2022
Published 4 November 2022 Volume 2022:14 Pages 2013—2021
DOI https://doi.org/10.2147/NSS.S369727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ahmed BaHammam
Hoi Man Yuen,1 Andy CY Lai,1 Eric KH Liu,2 Ming Chung Lee,2 Winnie CW Chu,2 Joey WY Chan,3 Ngan Yin Chan,3 Yun Kwok Wing,3 Albert M Li,1,4,5 Kate C Chan,1,4,5 Chun Ting Au1,4,5
1Department of Pediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, People’s Republic of China; 2Department of Imaging and Interventional Radiology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, People’s Republic of China; 3Li Chiu Kong Family Sleep Assessment Unit, Department of Psychiatry, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, People’s Republic of China; 4Laboratory for Pediatric Respiratory Research, Li Ka Shing Institute of Health Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, People’s Republic of China; 5Hong Kong Hub of Pediatric Excellence, The Chinese University of Hong Kong, Hong Kong SAR, People’s Republic of China
Correspondence: Kate C Chan; Chun Ting Au, Department of Pediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong SAR, People’s Republic of China, Tel +852 35053515 ; +852 35052917, Fax +852 26360020, Email [email protected]; [email protected]
Background: Lateral parapharyngeal wall (LPW) thickness is a potentially useful anatomical marker of childhood obstructive sleep apnea (OSA). Measuring LPW thickness by ultrasonography (USG) is technically feasible but its use in children has not been validated. Therefore, this study aimed to assess the intra- and inter-operator reliability of the sonographic measurements of LPW thickness in children and to assess its validity against magnetic resonance imaging (MRI) measurements.
Methods: Prepubertal children aged 6– 11 years suspected to suffer from OSA were recruited. Repeated measurements of LPW thickness by USG were conducted to evaluate the intra- and inter-operator reliability, examined by intraclass correlation coefficient (ICC). LPW thickness was measured as the distance between the internal carotid artery and the echogenic surface of the pharynx in an oblique coronal plane by USG. LPW thickness was measured by MRI at the retropalatal level. The agreement between the LPW thickness measured by USG and MRI was assessed by ICC and Bland-Altman plot.
Results: Thirty-four children (mean age: 8.66 ± 1.61, 26 male) were recruited. The intra- and inter-operator reliability of the LPW thickness by USG was good (ICC = 0.84 and 0.82, respectively). The agreement between the USG-measured and MRI-measured LPW thickness was moderate (ICC = 0.72). he Bland-Altman plot demonstrated a mean difference of 0.061 cm and a 95% limit of agreement from 0.91 to 1.12 cm.
Conclusion: In this study, we demonstrated that ultrasonography is a valid and reliable method to assess LPW thickness in children. This study was supported by the Direct Grant for Research from the Research Committee of the Chinese University of Hong Kong (Project no. 2020.073).
Keywords: pediatric, ultrasound, MRI, obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) results from anatomic narrowing of the upper airway during sleep that cannot be completely compensated by the action of the pharyngeal dilator muscles. For the pediatric population, adenotonsillar hypertrophy is the most common cause of OSA and adenotonsillectomy (AT) is the first-line treatment. Although obesity is one of the emerging risk factors for childhood OSA,1–5 a substantial proportion of children even without obesity have residual disease after AT.1,6,7 Therefore, it is believed that there are other factors substantially contributing to the severity of childhood OSA.
Upper airway structures that influence airway patency play an important role in the pathogenesis of OSA. One of which is the lateral parapharyngeal wall (LPW), referring to the soft-tissue structures, namely muscles, lymphoid tissue, and fat within the region. LPW thickness is the distance between the wall of the pharynx and the internal carotid artery. Our previous study demonstrated that LPW thickness has a dose-response relationship with obstructive apnea hypopnea index (OAHI), a traditional severity index of OSA, in children.8 The association between LPW thickness and OAHI was independent of other risk factors of OSA including body mass index, neck circumference and tonsil size.8 More recently, we found that LPW thickness is a highly heritable trait that may have shared genetic variance with OAHI, suggesting that genes affecting LPW thickness may also contribute to OSA severity.9 These findings suggest that LPW thickness is a potentially useful anatomical marker of childhood OSA.
Magnetic resonance imaging (MRI) of the upper airway can be used to measure LPW thickness accurately and is considered the gold standard. However, MRI is expensive and not readily accessible, especially in resource-scarce centers. On the other hand, ultrasonography (USG) is increasingly used to identify upper airway pathology in children suspected to have OSA.8–10 In fact, LPW thickness can also be captured and measured by USG, which is more readily available, technically less demanding, more cost-effective, and allow point-of-care assessment. The ultrasound-measured LPW thickness was found to be highly correlated with that measured by MRI in adults.11 However, similar validation has not been carried out in children. In this study, we aimed to assess the intra- and inter-operator reliability of the ultrasonographic measurement of LPW thickness in children and to validate the measurement against MRI. We hypothesized that USG would provide a valid and reliable measurement of LPW thickness.
Methods
Study Design and Subjects
Part I – Test-Retest Reliability
It was a prospective cohort study that consecutively recruited prepubertal children aged 6–11 years admitted to the pediatric sleep laboratory of the Prince of Wales Hospital for polysomnography (PSG). All subjects underwent repeated sonographic measurements by two well-trained sonographers.
Part II – Concurrent Validation
It was a sub-study of an ongoing case-control study that evaluated the differences in the upper airway morphology between children with (OAHI ≥ 1 event/hour) and without OSA (OAHI < 1 event/hour). Case-control pairs matched with age, gender, and body mass index (BMI) z score were recruited. All subjects underwent sonographic measurement and MRI within a 2-hour interval on the same visit to the hospital.
All children with previous upper airway surgery, genetic or syndromal disease, and craniofacial abnormalities were excluded. Written informed consent and assent were obtained from the parents and the children, respectively. The study protocol was conducted in compliance with the Declaration of Helsinki. Ethics approval was obtained from the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (CREC no: 2021.158). All subjects participated voluntarily.
Anthropometric Measurements
Anthropometric parameters including standing height without shoes and body weight were recorded using a digital doctor scale (Tanita WB-3000, Tokyo, Japan). Z scores of BMI were calculated based on local norms.12 Obesity was defined as a BMI z-score ≥ 1.645. Tonsil size was graded according to the Brodsky scale.13 Large tonsil was defined as the average tonsil size > 2, ie tonsils occupying > 50% of the oropharyngeal width.
Sonographic Measurements of Lateral Parapharyngeal Wall (LPW) Thickness
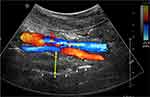
All subjects underwent sonographic assessment according to a standard protocol. An ultrasound machine Philips IU-22 (Eindhoven, The Netherlands) with C5-2 or C9-4 MHz curvilinear transducer was used. All measurements were made by two operators (EKH Liu & MC Lee). Both operators were blinded to other assessment data of the subjects. The subjects were positioned supine on the examination couch, and their neck was slightly extended with the infraorbital meatal baseline (the line joining the infraorbital margin and ear tragus) perpendicular to the scanning table. The transducer was placed longitudinally on the lateral side of the neck, just underneath the lateral border of the occipital bone, to scan the oblique coronal plane of the parapharyngeal space. With Doppler application, the long axis of the ipsilateral internal carotid artery was identified. On real-time ultrasound, the lateral wall of the pharynx appeared as an echogenic line whereas the lumen of the pharynx was obscured by gas shadowing. The distance between the internal carotid artery and the echogenic surface of the pharynx represents the LPW thickness in an oblique coronal plane. All measurements were recorded when the lateral wall of the pharynx moved farthest away from the transducer on frozen images (Figure 1). The maximum thickness of LPW on both sides was measured 3 times on 3 separate images, and the mean value was obtained for analysis. Total LPW thickness was determined by summing the mean values on both sides of the neck. The standardized methodology helped to minimize the variation in the measurements.
To assess the intra-operator and inter-operator reliability of this measurement (ie part I study), each subject underwent USG three times on the same day. The first and last scans were performed by operator 1 (EKH Liu) and the second scan was performed by operator 2 (MC Lee). The inter-operator reliability was assessed by comparing the results from the first and second scans. The intra-operator correlation was examined by comparing the results of the first and third scans.
Magnetic Resonance Imaging
MRI was performed using a 3.0 Tesla magnetic resonance imaging scanner (Achieva X-series, Philips Healthcare) on the same day as the USG scanning. Since head and neck position may alter the upper-airway soft-tissue configuration and upper-airway geometry, subjects were aligned in the Frankfort plane (a plane bisecting the soft tissue of the orbit and the tragus of the ear, perpendicular to the scanner table) before the scanning.14 Foam pads were placed between the patient’s head and each side of the receive-only volume neck coil to ensure that head movement did not occur during scanning. Throughout the study, patients remained in the supine position and were instructed to breathe normally through their nose and encouraged to refrain from swallowing. All studies were performed during wakefulness, which was ensured with frequent communication with the subjects. Each study was initiated with a 3.5-minute sagittal localizing spin echo scan [TR (repetition time) = 400.0 ms, TE (echo time) = 16.0 ms, 256×128 matrix, 1 NEX, flip angle = 90°, FOV (field of view) = 24.0 cm, slice thickness of 3.0 mm, and 1.5 mm, skip to establish the rostral and caudal margins of the upper airway (roof of the nasopharynx and the base of larynx)]. Subsequently, a 4.0-minute contiguous axial T1-weighted spin-echo data set (TR = 400.0 ms, TE = 16.0 ms, 0.5 NEX, flip angle = 90°, FOV = 24.0 cm, slice thickness of 5.0 mm and 0.0 mm skip) was acquired from the roof of the nasopharynx to the vocal cords.
The criteria for measurement of LPW thickness on MRI were adopted from previous studies which documented a good association between LPW thickness and OSA and airway narrowing.14–16 From the image with the smallest airway cross-sectional area in the retropalatal region, the transverse dimension of LPW (LPW-transverse) thickness on both sides was measured and this was defined as the distance between the medial border of parapharyngeal fat pads and the lateral edge of the airway.14 In the same image, additional measurements of the oblique transverse dimension of LPW (LPW-oblique) thickness between the internal carotid artery and lateral edge of the airway were made (Figure 2). Values on both sides of the neck were summated to determine the total LPW-transverse and LPW-oblique thickness measured by MRI. The standardized methodology aided to ensure the consistency of the measurement sites and minimize the variations. The MRI measurements were performed on OsiriX software by the author (ACY Lai) who was blinded to other assessment data of the subjects.
Polysomnography
Overnight polysomnography (PSG) was performed on all subjects at the Prince of Wales Hospital. A model SiestaTM ProFusion III PSG monitor (Compumedics Telemed, Abbotsford, Victoria, Australia) was used to record the following parameters: electroencephalogram (F4/A1, C4/A1, O2/A1), bilateral electrooculogram, electromyogram of mentalis activity and bilateral anterior tibialis. Arterial oxyhemoglobin saturation (SpO2) was measured by an oximeter with a finger probe. Respiratory airflow pressure signal was measured via a nasal catheter placed at the anterior nares and connected to a pressure transducer. An oronasal thermal sensor was used to detect the absence of airflow. Snoring was measured by a snoring microphone placed near the throat. Body position was monitored via a body position sensor. An adequate overnight PSG is defined as a recorded total sleep time of > 6 hours. Respiratory events including obstructive apneas, mixed apneas, central apneas and hypopneas were scored based on the recommendations from the AASM Manual for the Scoring of Sleep and Associated Events.17 Obstructive apnea hypopnea index (OAHI) is defined as the total number of obstructive and mixed apneas and hypopneas per hour of sleep. Subjects with an OAHI of < 1/h were defined as non-OSA, while those with an OAHI between 1/h and 5/h and ≥ 5/h were defined as having mild and moderate-to-severe (MS) OSA, respectively. The PSG scoring and reporting were performed by a registered polysomnographic technologist experienced in performing pediatric PSG (CT Au). He was blinded to other assessment data of the subjects.
Statistical Analysis
Subject characteristics were described using absolute numbers and percentages, means and standard deviations, and median and range for categorical, normal, and skewed continuous variables respectively. The inter-operator and intra-operator reliability of the sonographic measurement of the LPW thickness was assessed by the intra-class correlation coefficient (ICC) in a two-way random model.18 The resultant ICC was tested against the minimum acceptable degree of agreement of an ICC of 0.5, as the null hypothesis.
Pearson correlation between USG-measured and MRI-measured LPW thickness was performed. The agreement between the LPW thickness measured by ultrasound and that measured by MRI was also assessed by ICC in a two-way mixed model. The agreement was further checked by the Bland-Altman plot. The mean of the difference with a bias of ± 1.96 SD denotes the limits of agreement. All data were analyzed using R 3.6.2 software.
Results
USG Intra-Operator and Inter-Operator Reliabilities
Eighteen children (mean age: 8.6 ± 1.7 years, 13 males) were recruited for this part of the study. Their mean BMI z-score was 0.23 ± 0.96, with one obese subject. Their median OAHI was 3.9 events/hour (range: 0.1–59.8). Seven, five, and six of them were diagnosed with MS OSA, mild OSA, and non-OSA, respectively (Table 1).
 |
Table 1 Subject Characteristics |
The intra-operator reliability of the LPW thickness by USG was good, with an ICC = 0.84 (95% CI = 0.62–0.93, p = 0.004) (Figure 3). Good inter-operator reliability was also observed with an ICC = 0.82 (95% CI = 0.59–0.93, p = 0.007).
 |
Figure 3 Intra- and inter-operator reliabilities of the sonographic measurement of LPW thickness. |
Agreement Between USG and MRI Measurements
Thirty-four children (mean age: 8.6 ± 1.6 years, 26 males) were recruited for this part of the study. The study included subjects with a broad range of BMI z-score, from −2.71 to 2.98 (mean BMI z-score: 0.66 ± 1.31). Their median OAHI was 1.0 events/hour (range: 0–59.8). Twelve, five, and seventeen of them had MS OSA, mild OSA, and non-OSA, respectively (Table 1).
There was a good correlation between the USG-measured and MRI-measured oblique LPW thicknesses (r = 0.72, p < 0.001), while the correlation between the USG-measured and MRI-measured transverse LPW thickness was poor (r = 0.38, p = 0.025) (Figure 4).
 |
Figure 4 Correlations between USG measurements and MRI measured LPW thickness in transverse and oblique dimensions. |
The agreement between the USG-measured and MRI-measured oblique LPW thickness was moderate (ICC = 0.72, 95% CI = 0.50–0.85, p = 0.023). The Bland-Altman plot demonstrated a mean difference of 0.061 cm and a 95% limit of agreement from −0.91 to 1.12 cm (Figure 5).
 |
Figure 5 Bland-Altman plot for LPW thickness measured by USG and MRI in the oblique dimension. |
The agreement between the USG-measured LPW thickness and the transverse dimension of the MRI-measured LPW thickness was low (ICC = 0.07, 95% CI = −0.05–0.27, p = 1). The Bland-Altman plot demonstrated a mean difference of −1.66 cm and a 95% limit of agreement from −3.59 to 0.55 cm (Figure 6).
 |
Figure 6 Bland-Altman plot for LPW thickness measured by USG and MRI in the transverse dimension. |
Discussion
Our results showed good intra- and inter-operator reliability in USG measurements of LPW thickness, with both ICC greater than 0.8. We also documented a moderate agreement between USG and MRI LPW measurements in the oblique dimension.
Compared to adults, pediatric subjects can be less cooperative during a radiological examination, especially with MRI scanning which requires the subject to stay still for a long period in a confined and noisy environment. Despite pre-scanning preparation such as training and briefing which can improve the success rate,19 the usage of MRI in OSA prediction is still limited by its high cost. Ultrasonography can be used as an alternative for LPW thickness measurement, as it is more readily available and cost-effective. Our experiences will also suggest that ultrasonography is usually well-accepted by children.
For the MRI LPW measurement taken in the oblique plane, our study reported a strong correlation between the readings obtained by the two imaging techniques, albeit the correlation is less than what has been reported in adults.11 The presence of enlarged tonsils with varying shapes and sizes that affect the LPW dimensions could explain the lower agreement seen in our study.20,21 When compared to the oblique plane, a much weaker agreement was found between LPW measurements in the transverse plane. The reason for this poorer agreement relates to the different imaging planes obtained by USG and MRI in the transverse direction,11 thus the two techniques are not measuring the same dimensions. A similar finding of poor agreement in the transverse plane has been reported in a previous adult study.11
The LPW thickness is an independent risk factor for childhood OSA.8,22 LPW consists of muscular and lymphatic tissues, including hyoglossus muscle, styloglossus muscle, stylohyoid muscle, stylopharyngeus muscle, palatoglossus muscle, palatopharyngeal muscle, pharyngeal constrictor muscles, and palatine tonsils.14 The increased LPW thickness is suggested to compress the airway, narrowing and deforming the airway so that the airway collapsibility increases.11,14,23 The increased fat deposit in the parapharyngeal area further narrows the airway and reduces compliance.24 Moreover, the increased LPW thickness in OSA patients might indicate increased use of pharyngeal muscles to maintain airway patency during wakefulness and compensate for the anatomical deficit than their non-apneic counterparts.25 Although the exact mechanism behind the increased LPW thickness and OSA pathogenesis remains to be clarified, the relationship has already been demonstrated in previous studies.8,9,26 The current study supported the validity and reliability of USG on LPW assessment in children with OSA. Therefore, incorporating the LPW assessment as a routine OSA evaluation should be further explored. This measurement can be used as one of the parameters in the characterization of childhood OSA. Future studies are needed to investigate whether LPW thickness can help to predict treatment response and guide treatment strategy.
This study had a few limitations. First, the sample size was small, which was partially attributed to the COVID-19 pandemic that affected the recruitment process. However, the current sample size provided > 80% power to demonstrate that the obtained ICCs were significantly larger than the minimum acceptable agreement (ie ICC = 0.5). Second, the majority of participants were male, thus the results might be less applicable to females. However, there was no significant difference in LPW thickness between genders, according to our previously published data.8
Conclusion
The sonographic measurement of LPW thickness in children demonstrated good intra- and inter-rater reliability and moderate agreement with MRI measurement. Ultrasonography is a valid method to assess LPW thickness in children and its inclusion as part of childhood OSA assessment should be further explored.
Acknowledgments
This study was supported by the Direct Grant for Research from the Research Committee of the Chinese University of Hong Kong (Project no. 2020.073).
Disclosure
Professor Yun Kwok Wing reports personal fees from Eisai, personal fees from Lundbeck HK Ltd, outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Lee CH, Hsu WC, Chang WH, Lin MT, Kang KT. Polysomnographic findings after adenotonsillectomy for obstructive sleep apnoea in obese and non-obese children: a systematic review and meta-analysis. Clin Otolaryngol. 2016;41(5):498–510. doi:10.1111/coa.12549
2. Luz M, -álvarez A, Terán-Santos J, et al. Treatment outcomes of obstructive sleep apnoea in obese community-dwelling children: the NANOS study. Eur Respir J. 2015;46:717–727. doi:10.1183/09031936.00049615
3. Koren D, Gozal D, Bhattacharjee R, Philby MF, Kheirandish-Gozal L. Impact of adenotonsillectomy on insulin resistance and lipoprotein profile in nonobese and obese children. Chest. 2016;149(4):999. doi:10.1378/CHEST.15-1543
4. Nandalike K, Shifteh K, Sin S, et al. Adenotonsillectomy in obese children with obstructive sleep apnea syndrome: magnetic resonance imaging findings and considerations. Sleep. 2013;36(6):841–847. doi:10.5665/sleep.2708
5. Wing YK, Hui SH, Pak WM, et al. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child. 2003;88(12):1043–1047. doi:10.1136/adc.88.12.1043
6. Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676–683. doi:10.1164/rccm.200912-1930OC
7. Huang Y-S, Guilleminault C, Lee L-A, Lin C-H, Hwang F-M. Treatment outcomes of adenotonsillectomy for children with obstructive sleep apnea: a prospective longitudinal study. Sleep. 2014;37(1):71–76. doi:10.5665/SLEEP.3310
8. Au CT, Chan KCC, Liu KH, Chu WCW, Wing YK, Li AM. Potential anatomic markers of obstructive sleep apnea in prepubertal children. J Clin Sleep Med. 2018;14(12):1979–1986. doi:10.5664/jcsm.7518
9. Au CT, Chan KC, Zhang J, et al. Intermediate phenotypes of childhood obstructive sleep apnea. J Sleep Res. 2020:1–10. doi:10.1111/jsr.13191
10. Singh M, Tuteja A, Wong DT, et al. Point-of-care ultrasound for obstructive sleep apnea screening: are we there yet? A systematic review and meta-analysis. Anesth Analg. 2019;129(6):1673–1691. doi:10.1213/ANE.0000000000004350
11. Liu K-H, Chu WC, To K-W, et al. Sonographic measurement of lateral parapharyngeal wall thickness in patients with obstructive sleep apnea. Sleep. 2007;30(11):1503–1508. doi:10.1093/sleep/30.11.1503
12. Leung SSF, Cole TJ, Tse LY, Lau JTF. Body mass index reference curves for Chinese children. Ann Hum Biol. 1998;25(2):169–174. doi:10.1080/03014469800005542
13. Ng SK, Lee DLY, Li AM, Wing YK, Tong MCF. Reproducibility of clinical grading of tonsillar size. Arch Otolaryngol Head Neck Surg. 2010;136(2):159–162. doi:10.1001/archoto.2009.170
14. Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1673–1689. doi:10.1164/ajrccm.152.5.7582313
15. Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168(5):522–530. doi:10.1164/rccm.200208-866OC
16. Trudo FJ, Gefter WB, Welch KC, Gupta KB, Maislin G, Schwab RJ. State-related changes in upper airway caliber and surrounding soft- tissue structures in normal subjects. Am J Respir Crit Care Med. 1998;158(4):1259–1270. doi:10.1164/ajrccm.158.4.9712063
17. Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of SLeep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; 2017.
18. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
19. Ciet P, Tiddens HAWM, Wielopolski PA, et al. Magnetic resonance imaging in children: common problems and possible solutions for lung and airways imaging. Pediatr Radiol. 2015;45(13):1901–1915. doi:10.1007/s00247-015-3420-y
20. Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164(4):698–703. doi:10.1164/ajrccm.164.4.2101127
21. Wang JH, Chung YS, Jang YJ, Lee BJ. Palatine tonsil size and its correlation with subjective tonsil size in patients with sleep-disordered breathing. Otolaryngol Head Neck Surg. 2009;141(6):716–721. doi:10.1016/J.OTOHNS.2009.09.007
22. Lin C-Y, Chen C-N, Kang K-T, Hsiao T-Y, Lee P-L, Hsu W-C. Ultrasonographic evaluation of upper airway structures in children with obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2018;144(10):897–905. doi:10.1001/jamaoto.2018.1809
23. Leiter JC. Upper airway shape: is it important in the pathogenesis of obstructive sleep apnea? Am J Respir Crit Care Med. 1996;153(3):894–898. doi:10.1164/AJRCCM.153.3.8630569
24. Pahkala R, Seppä J, Ikonen A, Smirnov G, Tuomilehto H. The impact of pharyngeal fat tissue on the pathogenesis of obstructive sleep apnea. Sleep Breath. 2014;18(2):275–282. doi:10.1007/S11325-013-0878-4/TABLES/5
25. Svanborg E. Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir Physiol Neurobiol. 2005;147(2–3):263–272. doi:10.1016/J.RESP.2005.06.012
26. Li AM, Au CT, Chan K, Liu KH, Chu W. Sonographic measurement of lateral parapharyngeal wall thickness and severity of childhood obstructive sleep apnoea. In: 4.2 sleep and control of breathing. Eur Respir J. 2015;PA3380. doi:10.1183/13993003.congress-2015.pa3380
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.