Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Validation and Functional Relevance of the Short Form of the Perceived Deficits Questionnaire for Depression for Japanese Patients with Major Depressive Disorder
Authors Sumiyoshi T, Uchida H, Watanabe K , Oosawa M , Ren H, Moriguchi Y , Fujikawa K, Fernandez J
Received 11 July 2022
Accepted for publication 19 October 2022
Published 2 November 2022 Volume 2022:18 Pages 2507—2517
DOI https://doi.org/10.2147/NDT.S381647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Tomiki Sumiyoshi,1 Hiroyuki Uchida,2 Koichiro Watanabe,3 Masato Oosawa,4 Hongye Ren,5 Yoshiya Moriguchi,6 Keita Fujikawa,4 Jovelle Fernandez4
1Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan; 2Department of Neuropsychiatry, Keio University School of Medicine, Tokyo, Japan; 3Department of Neuropsychiatry, Kyorin University School of Medicine, Tokyo, Japan; 4Japan Medical Office, Takeda Pharmaceutical Company Limited, Osaka, Japan; 5Medical Affairs, H. Lundbeck A/S, Copenhagen, Denmark; 6Medical Affairs, Lundbeck Japan KK, Tokyo, Japan
Correspondence: Tomiki Sumiyoshi, Department of Preventive Intervention for Psychiatric Disorders, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan, Tel +81-42-341-2711 ext. 6234, Fax +81-42-346-1979, Email [email protected]
Purpose: To validate the five-item version of the Perceived Deficits Questionnaire for Depression (PDQ-D-5) for assessing subjective cognitive function in Japanese patients with major depressive disorder (MDD) using data from the PERFORM-J study.
Patients and Methods: A total of 518 Japanese outpatients diagnosed with MDD were assessed on severity of depressive symptoms, cognitive function, social and work function, and quality of life (QoL) over 6 months following initiation of antidepressant therapy. This post hoc analysis evaluated the internal consistency and convergent validity of the PDQ-D-5 in relation to the original PDQ-D-20. Correlations of scores on these measures were examined at each time point and over time. The same set of analyses was explored between PDQ-D-5 and the Patient Health Questionnaire–nine-item (PHQ-9), Montgomery–Asberg Depression Rating Scale (MADRS), Digit Symbol Substitution Test (DSST), five-level version of EQ-5D (EQ-5D-5L), Sheehan Disability Scale (SDS), and Work Productivity and Activity Impairment (WPAI) questionnaire.
Results: PDQ-D-5 scores showed good internal consistency. Strong positive correlations were observed between PDQ-D-5 and PDQ-D-20 at each time point (correlation coefficient: baseline, 0.94; month 1, 0.94; month 2, 0.96; month 6, 0.96) and over time (0.92) (all p < 0.0001). Longitudinally, there were positive correlations between PDQ-D-5 scores versus those on the PHQ-9, MADRS, and SDS. Similarly, negative correlations were noted between PDQ-D-5 scores and EQ-5D-5L and DSST scores to a variable degree. There were moderate positive correlations over time between PDQ-D-5 and all WPAI subscale scores except those on absenteeism.
Conclusion: PDQ-D-5 scores rated in Japanese patients with MDD were found to adequately represent scores on the PDQ-D-20. The short version also showed associations with several measures of functional outcome, depression severity and QoL. This validates the PDQ-D-5 as a feasible and clinically reliable tool to assess subjective experience on cognition, which is applicable to time-limited consultations.
UMIN Clinical Trials Registry for Primary Study: UMIN000024320.
Keywords: PDQ-D-20, PDQ-D-5, cognition, functional impairment, major depressive disorder
Corrigendum for this paper has been published.
Introduction
Major depressive disorder (MDD) is a common mental disorder with an estimated 12-month prevalence of 2.2% in Japan.1 MDD may negatively impact cognitive function, quality of life (QoL), and work productivity.2 Although cognitive disturbances are related to poor social function and reduced work capacity, they are only evaluated on limited occasions owing to a lack of simple assessment scales appropriate for the short consultation times typically seen in Japan.3
The Perceived Deficits Questionnaire for Depression (PDQ-D) is a self-reported scale for monitoring cognitive dysfunction in patients with MDD. The PDQ-D is a modified version of the original PDQ, which was developed to assess cognitive symptoms in patients with multiple sclerosis.4 The original PDQ-D (PDQ-D-20) consists of 20 questions covering the cognitive domains of attention/concentration, retrospective memory, prospective memory, and planning/organization.5 Convergent validity of the PDQ-D-20 has been established based on significant correlations with scores on the Sheehan Disability Scale (SDS; self-rated psychosocial function), the Work Productivity and Activity Impairment (WPAI) questionnaire (self-rated impact of disease on productivity), and the Patient Health Questionnaire–nine-item (PHQ-9; self-rated depression severity).6 The PDQ-D-5 is an abbreviated (five-item) version of the PDQ-D-20, and has been proposed as a more feasible measure to assess cognitive dysfunction in the limited time available in daily clinical practice.7,8 Limited analyses have been conducted in Europe to assess the validity of the PDQ-D-5 and investigate its association with other functional outcome measures in patients with MDD.9,10 To our knowledge, the clinical characteristics of the PDQ-D-5 based on the Japanese population have not yet been examined.
PERFORM-J (Prospective Epidemiological Research on Functioning Outcomes Related to MDD in Japan; UMIN000024320) was a 6-month, prospective, multicenter, observational study on MDD-related functional outcomes, conducted in 48 psychiatric institutions (mainly clinics) located in various regions of Japan.11,12 Eligible patients were assessed for severity of depressive symptoms, cognitive function (including the PDQ-D-20), social and work function, and QoL at four visits over 6 months. Using the PERFORM-J cohort data, the primary aim of this post hoc study was to determine the correlation between the PDQ-D-5 and the PDQ-D-20 to assess the convergent validity of the two scales and evaluate the internal consistency of the PDQ-D-5 in comparison with the PDQ-D-20. We also aimed to assess the relationship between the PDQ-D-5 and the other physician-rated or patient-reported functionality scores used in the PERFORM-J study. Further, the present study evaluated whether the PDQ-D-5 scores, a measure of subjective cognition, are correlated with performance on a test of attention/processing speed, a domain of objective cognition. We hypothesized that the PDQ-D-5 would provide a feasible and valid assessment tool to examine subjective cognitive function in patients with MDD.
Materials and Methods
Study Design and Patient Population
PERFORM-J enrolled 523 Japanese patients with MDD between September 2016 and June 2017. After new antidepressant monotherapy was initiated, patients were monitored for physician-rated and patient-reported outcomes over a 6-month period. Detailed methods for the PERFORM-J study have been published previously.13 The present report describes a post hoc analysis of data from the PERFORM-J study, which aimed to validate the PDQ-D-5 against the PDQ-D-20, and examine its associations with other functional outcome measures.
The analysis population for the current study was the 518 patients enrolled in the PERFORM-J study (five patients were excluded post enrollment).11,12 Briefly, eligible participants were Japanese outpatients aged 18–65 years with a recurrent or new diagnosis of MDD (according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision).14 The diagnosis was confirmed using the Mini International Neuropsychiatric Interview.15 In these patients, new antidepressant monotherapy was started either as a first-line treatment or a switch of medication, as part of their routine medical care. Demographics and baseline characteristics of participants have been described previously.12 The mean (standard deviation [SD]) age was 37.3 (11.2) years, and 55.6% were female. Mean (SD) baseline Montgomery–Asberg Depression Rating Scale (MADRS; physician-rated depression severity), Digit Symbol Substitution Test (DSST; psychomotor speed), and PDQ-D total scores were 27.0 (8.8), 72.4 (19.2), and 32.2 (15.8), respectively.11,12
Outcome Assessments
In the PERFORM-J study, assessments were made at baseline and at month 1, month 2, and month 6. For this post hoc analysis, primary outcomes were the PDQ-D-5 and PDQ-D-20 total and subscale scores. The PDQ-D-20 scale comprises 20 items in four cognitive domains: attention/concentration, retrospective memory, prospective memory, and organization/planning.4 Patients report on each of the 20 items over the past 7 days, on a scale of 0 (never) to 4 (very often – more than once a day). As each domain consists of five items, the combined subscales result in a total score of 0–80. Items for the PDQ-D-5 were selected from the PDQ-D-20, as previously described.4 The total score for the PDQ-D-5 is the sum of the raw scores for these five items, and ranges from 0 to 20. For both scales, higher scores reflect a worse perceived cognitive deficit. The PDQ-D-20 scores were used as a quantitative variable at each study visit.
The participants also took part in the following assessments: the MADRS, DSST, five-level version of EuroQol five-dimension quality of life questionnaire (EQ-5D-5L; self-rated QoL), SDS, WPAI, and PHQ-9. The MADRS comprises 10 items (reported sadness, apparent sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts, and suicidal thoughts) with a 7-point scale for each, from 0 (normal; not at all ill) to 6 (extremely ill), giving a total score of 0–60.16 The MADRS item and total scores were used as continuous variables at each visit. The DSST was used to measure speed of psychomotor performance requiring visual perception, spatial decision-making, and motor skills. Its continuous scores range from 0 to 133, with a higher score indicating better performance. The DSST assessments were conducted at baseline, month 2, and month 6. The EQ-5D-5L consists of a descriptive system of five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). These dimensions and the utility index of the EQ-5D-5L were used as continuous variables at each visit. The SDS includes three self-rated domains designed to measure work/school, social life/leisure activities, and evaluate whether family life/home duties are impaired by current psychiatric symptoms. Each domain corresponds to a discretized 10-point visual analog scale (VAS) from 0 (no disability) to 10 (extreme disability), with the sum of scores providing a single measure of global functional impairment (ranging from 0 [unimpaired] to 30 [highly impaired]). The three subscale scores and the total score were used as continuous variables at each visit. The WPAI consists of six questions related to the number of hours missed from work and usual activities, as well as the degree to which work or regular daily activities were limited over the past 7 days. The questionnaire yields quantitative scores for absenteeism, presenteeism, overall work impairment, and impairment of general activity, which are expressed as a percentage of impairment. The subscales of the WPAI were used as continuous variables at each visit. The PHQ-9 questionnaire has nine items (little interest or pleasure in doing things; feeling down, depressed, or hopeless; trouble falling or staying asleep or sleeping too much; feeling tired or having little energy; poor appetite or overeating; feeling bad about yourself — or that you are a failure or that you have let yourself or your family down; trouble concentrating on things, such as reading the newspaper or watching television; moving or speaking so slowly that other people could have noticed, or being so fidgety or restless that you have been moving a lot more than usual; and thoughts that you would be better off dead or of hurting yourself in some way). Ratings are reported by patients, with scores ranging from 0 (not at all) to 3 (nearly every day). The total score ranges from 0 (absence of depression) to 27 (severe depression), with the total score studied as a continuous outcome. The PHQ-9 was rated at all visits.
Statistical Analyses
Overall, 518 participants were included in this analysis; this provides 80% power to detect a Pearson correlation of at least 0.12 (weak correlation) between the PDQ-D-20 and PDQ-D-5. Standard descriptive statistics were used to characterize the distribution of PDQ-D-20 and PDQ-D-5 scores at each time point. All statistical comparisons were made using two-sided tests at α = 0.05 significance level (unless stated otherwise), with the null hypothesis of no difference between groups. The repeated measures correlation (rmcorr) calculation was performed using R version 4.0.5 (R Core Team and R Foundation for Statistical Computing, Vienna, Austria), and SAS (version 9.4 or later) (SAS Institute, North Carolina, USA) was used to analyze data.
Validity of the PDQ-D-5 vs the PDQ-D-20
The correlation of the PDQ-D-20 with the PDQ-D-5 at each time point was assessed using Pearson correlations, where a correlation coefficient with an absolute value > 0.7 indicates a strong correlation, 0.4–0.7 indicates a moderate correlation, and < 0.4 indicates a weak correlation. To integrate all visits in the analyses, rmcorr was used to account for non-independence among repeated measures, where the rmcorr coefficient is bounded by −1 to 1 and represents the strength of the linear association between two variables.
Reliability of the PDQ-D-5 and the PDQ-D-20
Respective measures of the reliability of the PDQ-D-5 and PDQ-D-20 were computed at each time point using the Cronbach’s alpha coefficients (where < 0.7 indicates poor reliability, 0.7–0.8 indicates moderate reliability, 0.8–0.9 indicates good reliability, and > 0.9 indicates excellent reliability).
Validity of the PDQ-D-5 by Comparison with Other Measures
Pearson correlations were used to explore the direction and magnitude of associations between PDQ-D-5 total scores and those of other assessment scores at each time point. Correlations between PDQ-D-5 scores and those of the MADRS, EQ-5D-5L, SDS, DSST, and PHQ-9 were calculated and placed in a 6×6 matrix. To integrate the longitudinal nature of the data, rmcorr was used to account for non-independence among repeated measures. Scores for each WPAI subscale were calculated as follows: absenteeism, 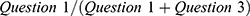 ; presenteeism,
; presenteeism, 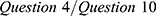 ; overall work impairment,
; overall work impairment, 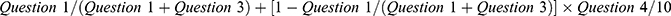 ; impairment of general activity,
; impairment of general activity, 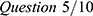 , where WPAI questions were as follows (based on the past 7 days) (condensed version): Question 1: How many hours did you miss from work because of your health problems? Question 2: How many hours did you miss from work because of any other reason? Question 3: How many hours did you actually work? Question 4: How much did your health problems affect your productivity while you were working? Question 5: How much did your health problems affect your ability to do your regular daily activities, other than work at a job?
, where WPAI questions were as follows (based on the past 7 days) (condensed version): Question 1: How many hours did you miss from work because of your health problems? Question 2: How many hours did you miss from work because of any other reason? Question 3: How many hours did you actually work? Question 4: How much did your health problems affect your productivity while you were working? Question 5: How much did your health problems affect your ability to do your regular daily activities, other than work at a job?
Results
Validity of the PDQ-D-5 vs the PDQ-D-20
Figure 1 shows the median and distribution of the PDQ-D-5 and PDQ-D-20 scores at baseline and at 1, 2, and 6 months after the initiation of antidepressant medication. For both assessments, the scores (median) and variability (Q1, Q3) were greater at baseline (PDQ-D-5, 8.0 [5.0, 11.0]; PDQ-D-20, 32.0 [20.0, 42.0]) than at month 6 (PDQ-D-5, 4.0 [2.0, 7.0]; PDQ-D-20, 17.0 [9.0, 28.0]), indicating an improvement in subjective cognitive function over time. At each time point, strong positive correlations were observed between total scores on the PDQ-D-5 and those on the PDQ-D-20 (correlation coefficient: baseline, 0.94; month 1, 0.94; month 2, 0.96; month 6, 0.96; all p < 0.0001) (Figure 2). A strong positive correlation was also seen when rmcorr was used to integrate all visits in the analyses (rmcorr, 0.92, p < 0.0001). Together, these results indicate that the PDQ-D-5 scores are highly correlated with those of the PDQ-D-20.
Reliability of the PDQ-D-5 and the PDQ-D-20
From baseline to month 6, the PDQ-D-20 data showed excellent internal consistency (Cronbach’s alpha: baseline, 0.94; month 1, 0.95; month 2, 0.96; month 6, 0.96). The PDQ-D-5 scores also showed good internal consistency (Cronbach’s alpha: baseline, 0.80; month 1, 0.83; month 2, 0.85; month 6, 0.87), with consistency scores from both assessments improving over the 6-month period (data not shown).
Validity of the PDQ-D-5 by Comparison with Other Measures
The PDQ-D-5 correlated most strongly with the PHQ-9 (rmcorr, 0.69, p < 0.0001; Table 2). There were also moderate positive correlations between PDQ-D-5 scores and the MADRS (rmcorr, 0.60, p < 0.0001) and SDS (rmcorr, 0.56, p < 0.0001) scores. A moderate negative correlation was found between PDQ-D-5 and EQ-5D-5L scores (rmcorr, −0.57, p < 0.0001), while the correlation between PDQ-D-5 and DSST scores was weakly negative (rmcorr, −0.37, p < 0.0001). PDQ-D-5 scores showed a moderate correlation with the presenteeism (rmcorr, 0.56, p < 0.0001), overall work impairment (rmcorr, 0.58, p < 0.0001), and impairment of general activity (rmcorr, 0.63, p < 0.0001) subscale scores on WPAI, while the correlation with absenteeism scores was weak (rmcorr, 0.21, p < 0.004) (Table 1). As shown in Tables 1 and 2, the degree of association between the PDQ-D-5 and other measures tested in this study increased from baseline over the 6-month observation period.
 |
Table 1 Pearson Correlation Coefficients Between WPAI Subscale Scores and PDQ-D-5 Total Scores |
 |
Table 2 Correlation Matrices for PDQ-D-5, MADRS, EQ-5D-5L, SDS, DSST, and PHQ-9 Scores |
Discussion
In the cohort of Japanese patients with MDD, the PDQ-D-5 showed strong convergent validity with the PDQ-D-20, supporting its prospective use as a clinically relevant and time-saving tool to assess subjective cognitive function.
Reported associations between modified PDQ scores and both subjective and objective measures of depressive symptoms in Japanese patients have indicated the importance of diagnosing both aspects of clinical symptoms when assessing patients with MDD.12,17,18 It is also important to assess the patient’s condition with both self-reported and physician-rated scales in terms of suicidality.19 The correlations between the PDQ-D-5 and other subjective and objective measures of depressive symptoms presented in this study are consistent with these previous observations. The correlations seen between the PDQ-D-5 and the other clinical indices tested (eg, the MADRS and the PHQ-9) tended to become stronger over the 6-month study period. This may be due to a greater variance of data (eg, depressive symptoms) among participants at later time points.
The relationship between cognitive function and QoL in patients with MDD has been discussed previously.2,11,18,20,21 In our study, PDQ-D-5 scores showed negative correlations with EQ-5D-5L scores at all time points. This finding is similar to results from previous studies, including the PERFORM-J study, which also reported negative associations between PDQ-D-20 scores and EQ-5D-5L scores.11,18,21 Thus, data from the current study support an association between self-reported QoL and subjective cognitive function in depressed patients.
The negative impacts of MDD on work outcomes have been well described.9,11,20–23 High PDQ-D-20 or PDQ-5 scores have been associated with decreased work productivity,9,12,21 while other studies have suggested that cognitive impairment has a detrimental impact on work outcomes (eg, leading to increased presenteeism) in patients with MDD.22,23 In the present study, PDQ-D-5 scores were moderately correlated with the presenteeism, overall work impairment, and impairment of general activity subscale scores of the WPAI; there was only a weak correlation with the absenteeism subscale. This may be due to the fact that permission to be absent from work is regulated not only by the severity of depressive and cognitive symptoms, but also by the employment environment, as has been proposed.12 The link between PDQ-D-5 scores and most WPAI subscale scores reported here may help explain the impact of cognition on work efficiency in patients with MDD.
Cognitive function has been suggested to provide an estimate of current, as well as future, social consequences in patients with MDD.9,11,21 Consistent with this concept, PDQ-D-5 scores were moderately correlated with SDS scores at month 1 in this study. Similar correlations have been reported with the Functioning Assessment Short Test, another indicator for social function.24,25 Therefore, cognitive deficits measured by the PDQ-D-5 may predict several aspects of psychosocial function in patients with MDD.
Correlations between PDQ-D-5 and DSST scores were weak at all time points. Similarly, in a study with Chinese patients with MDD, there was no correlation between PDQ-D-20 scores and performance on the DSST.26 The limited degree of correlation between these two functionality scores suggests that subjective and objective assessments evaluate different aspects of cognitive ability. This discordance highlights the importance of a comprehensive evaluation of cognitive difficulties associated with MDD, as patients with subjective, but not objective cognitive problems may underestimate their own cognitive ability, while patients with the opposite presentation may require greater support from caregivers.
There are limitations in this study. The study was uncontrolled and observational in nature, which does not enable the evaluation of cause-and-effect relationships. The study also recruited only outpatients, which may limit the generalization of the results to the wider population. The PDQ-D-5 results used in this study were based on items extracted from the PDQ-D-20, and did not reflect answers from an independent PDQ-D-5 questionnaire. Future evaluations should be conducted using a questionnaire consisting solely of the PDQ-D-5. In addition, as the PDQ-D-5 is part of the PDQ-D-20, this will have influenced the high correlation between the two measures and future assessments should correlate the PDQ-D-5 with questionnaires that do not include the PDQ-D-5 items. Patient responses in the PERFORM-J study may also have been influenced by the completion of several other depression instruments at the same time. Finally, it was not possible to calculate the content validity index and coefficient of variation ratio using the available data from the original PERFORM-J study; such calculations would have enhanced assessment of the content validity of the PDQ-D-5.
Conclusions
In conclusion, this study reports the validity of the PDQ-D-5 as a feasible measure of cognitive function in Japanese patients with MDD. The results also suggest that PDQ-D-5 scores predict key domains of functional outcome in these patients.
Abbreviations
CC, correlations coefficient; DSST, Digit Symbol Substitution Test; EQ-5D, EuroQol five-dimension quality of life questionnaire; EQ-5D-5L, EuroQol five-dimension, five-level quality of life questionnaire; IQR, interquartile range; MDD, major depressive disorder; MADRS, Montgomery–Asberg Depression Rating Scale; PDQ-D, Perceived Deficits Questionnaire for Depression; PDQ-D-5, Perceived Deficits Questionnaire for Depression–five-item; PDQ-D-20, Perceived Deficits Questionnaire for Depression–20-item; PHQ-9, Patient Health Questionnaire–nine-item; QoL, quality of life; rmcorr, repeated measures correlation; SD, standard deviation; SDS, Sheehan Disability Scale; VAS, visual analog scale; WPAI, Work Productivity and Activity Impairment.
Data Sharing Statement
Takeda does not plan to share data supporting the results reported in this article. The study is part of a co-development program agreement which prevents Takeda from data sharing due to commercially confidential information and intellectual property.
Ethics Approval and Informed Consent
The study used anonymously processed information from PERFORM-J and was carried out in accordance with the ethical standards described in the Declaration of Helsinki and approved by the Ethics Review Committee of the Research Institute of Healthcare Data Science (Tokyo, Japan). Patients were required to provide written informed consent and were free to withdraw from the study at any time.
Acknowledgments
The authors acknowledge the following people for their contributions to this work: Izumi Mishiro and Shinji Fujimoto for planning, analysis of results, and advice; Patcharapim Takizawa for advice on the analysis plan; Maki Ueyama, Yoshie Onishi, Sylvaine Barbier, Monia Ezzalfani, Marwa Mezghani and Khaoula Aroui for data analysis; and Shigeru Sakamoto for design of the original study (PERFORM-J).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Takeda Pharmaceutical Company Limited, Japan. Medical writing support was provided by Emily Manktelow, PhD, of Oxford PharmaGenesis, Melbourne, Australia and funded by Takeda Pharmaceutical Company Limited and Lundbeck Japan KK.
Disclosure
Tomiki Sumiyoshi reports honoraria received for advisory boards/lectures/papers and/or research funding from Takeda Pharmaceutical Company Limited, Sumitomo Pharma, Boehringer Ingelheim Japan, Meiji Seika Pharma, Otsuka Pharmaceutical, Lundbeck, Ono Pharmaceutical, Novartis, Janssen Pharmaceuticals, Shionogi Pharma and VeraSci. Hiroyuki Uchida has received personal fees from Takeda Pharmaceutical Company; grants from Daiichi Sankyo, Eisai, Mochida Pharmaceutical, Otsuka Pharmaceutical, and Sumitomo Dainippon Pharma; speaker’s fees from Eisai, Janssen Pharmaceuticals, Lundbeck, Meiji Seika Pharma, Boehringer Ingelheim Japan, Otsuka Pharmaceutical, and Sumitomo Dainippon Pharma; and advisory board fees from Lundbeck and Sumitomo Dainippon Pharma. Koichiro Watanabe has received manuscript fees or speaker’s honoraria from Astellas, Boehringer Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Janssen Pharmaceuticals, Kyowa Pharmaceutical Industry, Lilly, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Otsuka Pharmaceutical, Pfizer, Shionogi, Sumitomo Dainippon Pharma, Takeda Pharmaceutical Company Limited, and Yoshitomi; has received research and grant support from Astellas, Daiichi Sankyo, Eisai, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Otsuka Pharmaceutical, Pfizer, and Shionogi; is a consultant for Eisai, Janssen Pharmaceuticals, Kyowa Pharmaceutical, Lundbeck Japan, Luye Pharma, Lilly, Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical, Pfizer, Sumitomo Dainippon Pharma, and Taisho Toyama Pharmaceutical; and is a consultant and advisory board member for Takeda Pharmaceutical Company Limited, Lundbeck Japan KK, and Viatris, outside the submitted work. Masato Oosawa and Keita Fujikawa are employees of Takeda Pharmaceutical Company Limited. Jovelle Fernandez was a previous employee of Takeda Pharmaceutical Company Limited and holds restricted shares from GSK and Takeda Pharmaceutical Company Limited. Hongye Ren was an employee of H. Lundbeck A/S until December 2021 (currently an employee of Novo Nordisk), and Yoshiya Moriguchi is an employee of Lundbeck Japan KK. The authors report no other conflicts of interest in this work.
References
1. Ishikawa H, Kawakami N, Kessler RC.; World Mental Health Japan Survey Collaborators. Lifetime and 12-month prevalence, severity and unmet need for treatment of common mental disorders in Japan: results from the final dataset of World Mental Health Japan Survey. Epidemiol Psychiatr Sci. 2016;25(3):217–229. doi:10.1017/S2045796015000566
2. Saragoussi D, Christensen MC, Hammer-Helmich L, Rive B, Touya M, Haro JM. Long-term follow-up on health-related quality of life in major depressive disorder: a 2-year European cohort study. Neuropsychiatr Dis Treat. 2018;14:1339–1350. doi:10.2147/NDT.S159276
3. Hori H, Yamato K. Assessment of current clinical practices for major depression in Japan using a web-based questionnaire. Neuropsychiatr Dis Treat. 2019;15:2821–2832. doi:10.2147/NDT.S217098
4. Fehnel SE, Forsyth BH, DiBenedetti DB, Danchenko N, Francois C, Brevig T. Patient-centered assessment of cognitive symptoms of depression. CNS Spectr. 2016;21(1):43–52. doi:10.1017/S1092852913000643
5. Lam RW, Saragoussi D, Danchenko N, Rive B, Lamy FX, Brevig T. Psychometric validation of Perceived Deficits Questionnaire-Depression (PDQ-D) in patients with Major Depressive Disorder (MDD). Value Health. 2013;16(7):A330. doi:10.1016/j.jval.2013.08.046
6. Lam RW, Lamy FX, Danchenko N, et al. Psychometric validation of the Perceived Deficits Questionnaire-Depression (PDQ-D) instrument in US and UK respondents with major depressive disorder. Neuropsychiatr Dis Treat. 2018;14:2861–2877. doi:10.2147/NDT.S175188
7. Sullivan MJ, Edgley K, Dehoux E. A survey of multiple sclerosis. Part 1. Perceived cognitive problems and compensatory strategy use. Can J Rehabil. 1990;4:99–105.
8. The Consortium of Multiple Sclerosis Centers Health Services Research Subcommittee. Multiple Sclerosis Quality of Life Inventory: A User’s Manual. New York: National Multiple Sclerosis Society; 1997.
9. Hammer-Helmich L, Haro JM, Jonsson B, et al. Functional impairment in patients with major depressive disorder: the 2-year PERFORM study. Neuropsychiatr Dis Treat. 2018;14:239–249. doi:10.2147/NDT.S146098
10. Haro JM, Hammer-Helmich L, Saragoussi D, Ettrup A, Larsen KG. Patient-reported depression severity and cognitive symptoms as determinants of functioning in patients with major depressive disorder: a secondary analysis of the 2-year prospective PERFORM study. Neuropsychiatr Dis Treat. 2019;15:2313–2323. doi:10.2147/NDT.S206825
11. Sumiyoshi T, Watanabe K, Noto S, et al. Relationship of subjective cognitive impairment with psychosocial function and relapse of depressive symptoms in patients with Major Depressive Disorder: analysis of longitudinal data from PERFORM-J. Neuropsychiatr Dis Treat. 2021;17:945–955. doi:10.2147/NDT.S288108
12. Sumiyoshi T, Watanabe K, Noto S, et al. Relationship of cognitive impairment with depressive symptoms and psychosocial function in patients with major depressive disorder: cross-sectional analysis of baseline data from PERFORM-J. J Affect Disord. 2019;258:172–178. doi:10.1016/j.jad.2019.07.064
13. Sumiyoshi T, Watanabe K, Noto S, Sakamoto S, Moriguchi Y, Okamoto S. Prospective epidemiological research on functioning outcomes related to major depressive disorder in Japan (PERFORM-J): protocol for a prospective cohort study. JMIR Res Protoc. 2018;7(6):e161. doi:10.2196/resprot.9682
14. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision).
15. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl20):22–33;quiz 34–57.
16. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi:10.1192/bjp.134.4.382
17. Sawada K, Yoshida K, Ozawa C, et al. Impact of subjective vs. objective remission status on subjective cognitive impairments in depression. J Affect Disord. 2019;246:99–104. doi:10.1016/j.jad.2018.12.049
18. Zhang BH, Feng L, Feng Y, et al. The effect of cognitive impairment on the prognosis of major depressive disorder. J Nerv Ment Dis. 2020;208(9):683–688. doi:10.1097/NMD.0000000000001180
19. Baertschi M, Costanza A, Canuto A, Weber K. The dimensionality of suicidal ideation and its clinical implications. Int J Methods Psychiatr Res. 2019;28(1):e1755. doi:10.1002/mpr.1755
20. Wang G, Tan KHX, Ren H, Hammer-Helmich L. Impact of cognitive symptoms on health-related quality of life and work productivity in Chinese patients with major depressive disorder: results from the PROACT Study. Neuropsychiatr Dis Treat. 2020;16:749–759. doi:10.2147/NDT.S230403
21. Kim JM, Chalem Y, Di Nicola S, Hong JP, Won SH, Milea D. A cross-sectional study of functional disabilities and perceived cognitive dysfunction in patients with major depressive disorder in South Korea: the PERFORM-K study. Psychiatry Res. 2016;239:353–361. doi:10.1016/j.psychres.2016.01.022
22. Clark M, DiBenedetti D, Perez V. Cognitive dysfunction and work productivity in major depressive disorder. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):455–463. doi:10.1080/14737167.2016.1195688
23. Toyoshima K, Inoue T, Shimura A, et al. Associations between the depressive symptoms, subjective cognitive function, and presenteeism of Japanese adult workers: a cross-sectional survey study. Biopsychosoc Med. 2020;14:10. doi:10.1186/s13030-020-00183-x
24. Cha DS, Carmona NE, Subramaniapillai M, et al. Cognitive impairment as measured by the THINC-integrated tool (THINC-it): association with psychosocial function in major depressive disorder. J Affect Disord. 2017;222:14–20. doi:10.1016/j.jad.2017.06.036
25. Knight MJ, Fourrier C, Lyrtzis E, et al. Cognitive deficits in the THINC-integrated tool (THINC-it) are associated with psychosocial dysfunction in patients with major depressive disorder. J Clin Psychiatry. 2018;80(1):18m12472. doi:10.4088/JCP.18m12472
26. Shi C, Wang G, Tian F, et al. Reliability and validity of Chinese version of perceived deficits questionnaire for depression in patients with MDD. Psychiatry Res. 2017;252:319–324. doi:10.1016/j.psychres.2017.03.021
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


